Abstract
TAR DNA-binding protein 43 (TDP-43) has been heavily researched in recent years due to its involvement in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD). Several studies have also sought to investigate the frequency of TDP-43 deposition in other neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, but there has been relatively little work focused on the prevalence, distribution and histopathological associations of abnormal TDP-43 deposits in the brains of cognitively normal elderly subjects. We screened thick, free-floating coronal sections of mesial temporal lobe from 110 prospectively-followed and autopsied cognitively normal subjects (age range 71–100 years) using an immunohistochemical method for phosphorylated TDP-43. We found a 36.4% prevalence of pathologic TDP-43, mostly in the form of neurites while perikaryal cytoplasmic neuronal inclusions were uncommon and intranuclear inclusions were rare. With respect to other concomitant pathologies commonly found in elderly individuals, cases with TDP-43 had a greater prevalence of argyrophilic grains (ARG) (40% vs. 18.6%) and overall ARG density (moderate vs. sparse). There were no additional associations with other concomitant pathologies, including cerebral white matter rarefaction, incidental Lewy bodies, neurofibrillary tangles or amyloid plaques. These results indicate deposition of TDP-43 occurs in a substantial subset of cognitively normal elderly subjects and is more common in those with ARG, supporting some previous studies linking pathological TDP-43 deposition with ARG and other pathological tau protein deposits.
Keywords: amygdala, hippocampus, TAR DNA binding protein, aging, neuropathology, argyrophilic grains
Introduction
TAR DNA binding protein 43 (TDP-43) is a nuclear protein implicated in exon skipping and transcriptional regulation [28]. Abnormal aggregates of TDP-43, readily identified by their abnormal phosphorylation, have been identified within ubiquitin-positive, tau- and α-synuclein-negative inclusions in subjects with frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis (ALS). Furthermore, several other neurodegenerative diseases such as Alzheimer’s disease (AD), dementia with Lewy bodies (DLB), Parkinson’s disease (PD) and hippocampal sclerosis dementia frequently have concurrent TDP-43 deposition, ranging from 25–50% in AD to 7% in PD [17,28,37,40]. However, few studies have been specifically focused on determining the prevalence, setting and significance of TDP-43 deposition in cases lacking a defined clinicopathological neurodegenerative disease, i.e. normal elderly individuals.
The prevalence of abnormal TDP-43 deposition amongst normal elderly controls has been reported as ranging between 0–29% [14,28,40]. One of these studies demonstrated an increased frequency of TDP-43 deposition with age, noting an absence of positivity in those less than 65 years of age [14]. Numerous other pathologies have been known to deposit within the brains of elderly individuals, often without clear clinical effects. Previous studies of TDP-43 deposition have not examined its relationship with such “incidental” autopsy findings. The principle abnormal protein aggregates found in AD; senile, amyloid or neuritic plaques composed of β-amyloid, as well as neurofibrillary tangles (NFTs) and neurites composed of phosphorylated tau protein, are present in most cognitively normal individuals, and the distribution and densities of these overlap those seen in subjects with clinicopathological AD [41,34,31,7,11,10,9,8,4,38]. Furthermore, the main aggregate protein in PD, α-synuclein, is detected in approximately 10–25% of clinically healthy people over the age of 60, and this prevalence increases with age [22,1,16,15]. White matter rarefaction, known in the radiological literature as white matter hyperintensities, leukoaraiosis or “small vessel ischemic injury”, is commonly found in older individuals and has been correlated with gait abnormalities [25,27]. Additionally, argyrophilic grains (ARG) are present at a frequency of 16–22% in normal elderly persons [33,21].
Understanding whether TDP-43 deposition coincides with any other common aging neuropathologies might provide further insight into its significance and the molecular mechanisms of this protein deposition.
Methods
Case Selection
All samples were obtained through autopsies of subjects enrolled in the Brain and Body Donation Program (BBDP) at Banner Sun Health Research Institute between 1998 and 2011. The BBDP recruits normal elderly subjects predominantly from the surrounding Sun Cities retirement communities. These independently living volunteer research subjects are followed prospectively with annual standardized clinical assessments for the rest of their lives. Cases (Table 1) were clinically characterized as previously described [3] through standardized periodic neurological and neuropsychological assessments and review of private medical records, self-report and telephone interviews with spouses and/or caregivers. All cases underwent autopsy and a standardized neuropathological assessment; resulting in the assignment of clinicopathological diagnoses according to published recommendations. The median postmortem interval (PMI) of all autopsied BBDP subjects is 3.2 hours [3]. This ensures the highest quality brain tissue for research purposes.
Table 1.
Subject Characteristics. All data is listed as mean ± standard deviation unless otherwise noted.
| Total series | |
|---|---|
| N (M:F) | 54:56 |
| Age at death | 86 ± 6.0 |
| Braak NFT stage- median (range) | III (I–IV) |
| CERAD plaque score- median (range) | sparse (none-frequent) |
| MMSE | 28 ± 2 |
| PMI | 3.3 ± 0.35 |
Abbreviations: M = Male, F = Female, CERAD = Consortium to Establish a Registry for Alzheimer’s disease, NFT = neurofibrillary tangles, MMSE = Mini-Mental State Exam, PMI = post mortem interval, Stdev = standard deviation
Cases were selected using strict inclusion and exclusion criteria to ensure the series consisted of non-demented elderly individual cases lacking sufficient criteria to warrant a clinicopathological diagnosis of a neurodegenerative disease. We defined elderly as subjects who were greater than 70 years of age at the time of death. A diagnosis of non-demented was based on a Mini Mental State Examination (MMSE) [12] score of 25 or greater within 2 years prior to death (Table 1) and absence of a clinical diagnosis of dementia or other defined major neuro-clinicopathologic diagnosis. Additionally, cases were excluded if the PMI was greater than 12 hours or if acute infarcts were present in the hippocampus or amygdala.
Tissue processing and histological methods
Detailed methods and neuropathological diagnostic criteria are listed in a previous publication that details our standard protocol [3]. In brief, the cerebrum was sliced in the coronal plane at the time of brain removal into 1 cm thick slices and then divided into left and right halves. Slices from the right half were frozen between sheets of dry ice while slices from the left half were fixed by immersion in buffered 4% formaldehyde for 48 hours at 4 degrees C. Paraffin-embedded sections were stained with hematoxylin and eosin while large-format, 80 μm-thick frozen sections were stained for plaques, tangles and other inclusions using Gallyas, Thioflavine-S and Campbell-Switzer methods [5]; these sections were used to assess, using a four point quantitative scale, the densities of senile plaques (all types considered together) and neurofibrillary tangles (NFT). Additionally, Braak stage for neurofibrillary degeneration was determined [6] and neuritic plaque densities were graded based on the templates provided by the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) [26]. Data from the following areas are recorded in our database: frontal, temporal, parietal, and entorhinal cortices as well as the hippocampus. Formalin-fixed, paraffin-embedded sections were used for immunohistochemistry with an antibody against phosphorylated α-synuclein peptide (1:10,000; rabbit polyclonal anti-human phosphoserine 129) with densities of pathology graded on a five point scale in 10 brain regions [30,39]. Neuropathologic assessment was performed blinded to clinical categorization and diagnosis was assigned according to published criteria [24,3,32,19,26,33]. Significant cerebral white matter rarefaction was defined as exceeding 25% of the total centrum semiovale area within one or more cerebral lobes [3]. ARG were assessed through visualization of spindle-shaped lesions on Gallyas and graded on a scale of none, mild, moderate and severe [33,21]. Lewy bodies were staged based on the Unified Staging System [2]. All cases were examined by the same neuropathologist (TGB), who was blinded to clinical and demographic status at the time of initial review.
For this study, additional hippocampal and/or amygdalar temporal lobe sections were selected from each of the 110 subjects. Immunohistochemistry was performed on 40μm free-floating sections of the mesial temporal lobe using an antibody against phosphorylated TDP-43 peptide (1:10,000 rabbit polyclonal antihuman phosphoserine 409/410, a generous gift from Dr. Haruhiko Akiyama, Department of Psychogeriatrics, Tokyo, Japan) [18,29] with nickel-enhanced 3, 3′-diaminobenzidine (DAB) as the chromogen.
Blinded primary interpretation of all cases was performed by a single observer (SJA). TDP-43 positivity, location of positivity, and morphology were recorded. Each positive case was subsequently reviewed and confirmed by the consensus of two additional investigators (BND, TGB) who were also blinded to case demographics.
Statistical analysis
Statistical analyses were performed with Sigma Plot 12.1 (Systat Software, Inc. San Jose, CA) and Microsoft Excel (Microsoft Corporation, Redmond, WA). Student’s t-tests or Mann-Whitney rank sum analysis were used to determine if group quantitative differences were significant. Chi squared analyses were used to determine whether there were proportional differences between groups. For all tests, the criterion for significance was set at p < 0.05.
Results
Of 110 cognitively normal elderly brains, 40 showed pathological TDP-43 positivity in the hippocampus and/or amygdala (36.4%), with the amygdala being the most common location for TDP-43 deposition. Of these, the deposits most often took the exclusive form of dystrophic neurites (DN); in 33/40 (82.5%) cases (figure 1). Neuronal perikaryal cytoplasmic inclusions (NCI) were present along with dystrophic neurites in 5/40 (12.5%) cases. In one case alone (2.5%), case #3, neuronal intranuclear inclusions (NII), were seen in the entorhinal cortex. Deposits were often found amongst corpora amylacea located subpially and in the subependymal region of the temporal horn of the lateral ventricle. The most frequent location was in the subpial region of the semilunar and ambient gyri of the amygdala. Deposits were also found in the subpial subiculum, fimbria and alveus. Additional descriptions of individual TDP-43 positive cases are listed in Table 2. Although the overall positivity in this series was 36.4%, a subset of these positive cases, 14/40 (35%), revealed miniscule amounts of positive staining. Many of these cases had just one or two positive neurites, associated with corpora amylacea, in the entire 40μm section. The close association of pathological TDP-43 with corpora amylacea has also been mentioned in a previous study [14].
Fig. 1.
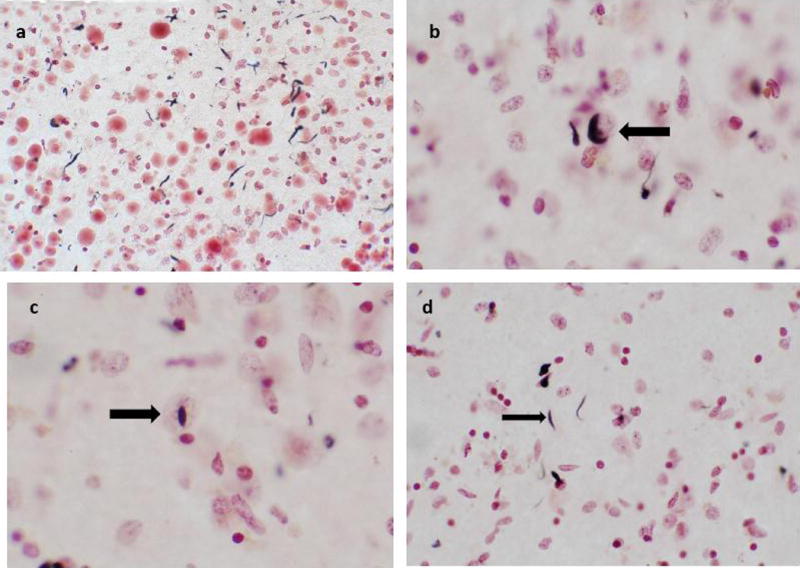
Examples of TDP-43 deposits in normal elderly subjects a Dystrophic neurites associated with corpora amylacea, subpial zone of amygdala, magnification 20X b Neuronal cytoplasmic inclusion, parahippocampal gyrus, magnification 40X c Intranuclear inclusion, hippocampus, magnification 40X d Dystrophic neurites, amygdala, magnification 40X.
Table 2.
Neuropathology of TDP-43 positive cases.
| Case # | Location of Positivity | Inclusion Type | Age at death | ARG | WMR | iLB |
|---|---|---|---|---|---|---|
| 1 | Amygdale | DN | 75 | N | N | N |
| 2 | Amygdale | DN | 77 | Y | Y | N |
| 3 | parahippocampal gyrus | DN, NII | 78 | Y | Y | Y |
| 4 | amygdala | DN | 83 | N | N | Y |
| 5 | amygdala | DN | 84 | N | N | Y |
| 6 | parahippocampal gyrus | DN | 84 | N | N | N |
| 7 | hippocampus | DN | 85 | N | Y | N |
| 8 | amygdala | DN | 86 | Y | N | Y |
| 9 | amygdala | DN | 86 | Y | N | N |
| 10 | amygdala | DN | 87 | N | N | N |
| 11 | amygdala | DN | 87 | N | Y | N |
| 12 | subiculum | DN | 88 | N | Y | N |
| 13 | amygdala | DN | 88 | N | N | Y |
| 14 | parahippocampal gyrus /amygdala | DN, NCI | 90 | Y | Y | Y |
| 15 | parahippocampal gyrus | NCI | 90 | Y | N | N |
| 16 | amygdala | DN, NCI | 90 | Y | Y | N |
| 17 | amygdala | DN | 91 | N | N | N |
| 18 | parahippocampal gyrus and amygdala | DN, NCI | 91 | Y | Y | N |
| 19 | amygdala | DN, NCI | 91 | Y | Y | N |
| 20 | amygdala | DN, NCI | 91 | Y | Y | N |
| 21 | amygdala | DN | 91 | N | N | N |
| 22 | amygdala | DN | 91 | Y | N | N |
| 23 | subiculum / parahippocampal gyrus | DN | 92 | Y | N | Y |
| 24 | amygdala | DN | 92 | N | Y | Y |
| 25 | amygdala | DN | 94 | N | Y | Y |
| 26 | amygdala | DN | 97 | N | Y | N |
| 27* | amygdala | DN | 96 | N | N | N |
| 28* | subiculum / parahippocampal gyrus | DN | 98 | Y | N | N |
| 29* | subiculum / parahippocampal gyrus | DN | 73 | N | N | N |
| 30* | subiculum / parahippocampal gyrus | DN | 88 | N | N | N |
| 31* | amygdala | DN | 89 | N | Y | N |
| 32* | parahippocampal gyrus and amygdala | DN | 91 | N | N | N |
| 33* | amygdala | DN | 82 | Y | N | N |
| 34* | hippocampus | DN | 84 | N | N | N |
| 35* | subiculum / parahippocampal gyrus | DN | 96 | N | Y | N |
| 36* | parahippocampal gyrus and amygdala | DN | 88 | N | N | N |
| 37* | subiculum / parahippocampal gyrus | DN | 85 | N | N | N |
| 38* | subiculum / parahippocampal gyrus | DN | 90 | N | Y | N |
| 39* | amygdala | DN | 91 | Y | N | Y |
| 40* | amygdala | DN | 85 | Y | N | N |
DN = dystrophic neurites, NCI = neuronal cytoplasmic inclusions, NII = intranuclear inclusions, ARG = argyrophilic grains, WMR = white matter rarefaction, iLB = incidental Lewy bodies, Y = yes, N = no.
in cases 27–40 TDP-43 deposits were very rare, having less than 5 deposits in the entire 40μm section.
There were no statistically significant differences between cases with and without TDP-43 inclusions with respect to age at death, CERAD neuritic plaque density, Braak NFT stage, and plaque and tangle densities in the entorhinal, hippocampus and temporal lobes (Table 3, Ps > 0.08). Of 40 positive subjects, 18 were female and 22 male, with no significant difference in gender ratios as compared to TDP-43-negative subjects. With respect to other concomitant pathologies commonly found in elderly individuals, cases with TDP-43 had were more likely to have ARG (40% vs. 18.6%; p = 0.03). Of these, subjects with higher ARG scores were more likely to have positive pathological TDP-43 staining than those with lower ARG scores. There were no differences between TDP-43-positive and negative cases in terms of the proportion with incidental Lewy bodies or cerebral white matter rarefaction.
Table 3.
Subject characteristics of TDP-43 positive and negative cases.
| Variable | TDP-43 Negative | TDP-43 Positive | P values |
|---|---|---|---|
| N (M:F) | 32:38 | 22:18 | 0.46 |
| Age at death yrs- mean ± SD (range) | 86 ± 6.2 (71–100) | 88 ± 5.6 (73–98) | 0.09 |
| Braak NFT stage- median (range) | III (I–IV) | III (I–IV) | 0.12 |
| Entorhinal NFT score- median (range) | frequent (none-frequent) | frequent (mild-frequent) | 0.37 |
| Temporal Lobe NFT score- median (range) | sparse (none-frequent) | sparse (none-frequent) | 0.69 |
| Hippocampus NFT score- median (range) | moderate (none-frequent) | moderate (sparse-frequent) | 0.13 |
| CERAD plaque score- median (range) | sparse (none- frequent) | sparse (none-frequent) | 0.80 |
| Entorhinal plaque score- median (range) | none (none-frequent) | sparse (none-frequent) | 0.63 |
| Temporal Lobe plaque score- median (range) | sparse (none-frequent) | sparse (none-frequent) | 0.71 |
| Hippocampus NFT score- median (range) | none (none-frequent) | none (none-frequent) | 0.96 |
| ARG positive cases, n (%) | 13 (19%) | 16 (40%) | 0.03 |
| Average ARG Density | sparse | moderate | 0.02 |
| WMR positive cases, n (%) | 34 (49%) | 16 (40%) | 0.50 |
| Incidental LB cases, n (%) | 13 (19%) | 10 (25%) | 0.58 |
| PMI | 3.0 ± 1.3 | 3.0 ± 1.3 | 0.92 |
Abbreviations: M = Male, F = Female, NFT = Neurofibrillary tangles, CERAD = Consortium to Establish a Registry for Alzheimer’s disease, ARG = Argyrophilic grains, WMR = White matter rarefaction, LB = Lewy bodies, MMSE = Mini-mental state exam, PMI = Post mortem interval; Stdev = standard deviation.
When the cohort is broken down by decade of death, TDP-43 positivity was documented in 4/13 (30.8%) of the 70–79 year old cohort, 17/60 (28.3%) of the 80–89 year old cohort and 17/39 (43.6%) of the 90–99 year old cohort. There was one subject over the age of 100 years at the time of death that did not have pathological TDP-43 protein deposition.
Discussion
No study to date has examined a large series of documented, prospectively followed non-demented elderly subjects to determine both the prevalence of pathological TDP-43 staining as well as its concurrence with other neuropathological features commonly found in the elderly. We found phosphorylated TDP-43 deposits in the hippocampus and/or amygdala in 36.4% of 110 non-demented aged individuals. Additionally, there was a significant association of pathological TDP-43 with argyrophilic grains (ARG). Other common normal aging neuropathological findings, including senile plaques, neurofibrillary tangles, cerebral white matter rarefaction and incidental Lewy bodies, showed no association, although further and more detailed studies would be necessary to confirm these negative findings.
In one of the first reports of TDP-43 pathology in normal aging, Nakashima-Yasuda and colleagues reported a 3% frequency (n = 1) in a series of 33 individuals aged 63–87 [28], while in a small study of two control cases, ages 48 and 63, Hasegawa did not observe TDP-43 positivity [17]. Another study including 63 neurologically normal individuals found 2/63 or 3% of cases showed abnormal TDP-43 deposition [40]. There has been only one prior study that incorporated standardized clinical assessment with immunohistochemical demonstration of TDP-43 in a large series; in this study, 10/60 (17%) were positive for TDP-43 [14], and when only looking at subjects over the age of 65, the percentage increased to 29%, with no positive cases under the age of 65. [14].
Characterized by spindle-shaped lesions in neuronal processes predominantly within mesial temporal lobe limbic regions, often accompanied by abnormal, phosphorylated tau-positive glia, ARG are of uncertain clinical significance [21,36,33,20]. They are found in subjects with dementia but are also common in cognitively unimpaired older people as well as together with other neuropathological conditions [21,23,35]. ARG are commonly found in association with temporal lobe neurofibrillary tangles, particularly Braak stage IV, leading to the suggestion that they may represent distal dendrites and/or axons of neurons undergoing active neurofibrillary degeneration [33]. Interestingly, a recent study on patients with ALS revealed concomitant ARG pathology in 38% of their subjects [35]. This is not the first report of ARG in ALS. Yokota reported a case study of coexisting ALS and ARG disease in a non-demented patient in 2007 [42]. At present, it is unclear whether ARG and TDP-43 are benign incidental findings in the elderly or may eventually lead to clinically manifest disease had the subjects lived long enough.
Although the relationship between the deposition of tau positive ARG and pathologic TDP-43 deposition remains unclear, it is tempting to speculate there is a synergistic or common pathological mechanism resulting in the accumulation of these abnormal protein forms. Fujishiro and colleagues found in a small series of 15 subjects with ARG as well as dementia or schizophrenia, 60% (or 9 of 15) of cases exhibited concomitant TDP-43 pathology [13]; furthermore, those cases with concomitant TDP-43 pathology were statistically more likely to be in a higher severity stage of ARG; we confirmed this likelihood to association with a more severe stage of ARG. Fujishiro and colleagues also reported that, in some of the cases with ARG and TDP-43 pathology, co-localization of phospho-tau and phospho-TDP-43 within individual grains as well as within individual neuronal perikarya was observed in the entorhinal cortex and amygdala, although in most such structures, co-localization was more often not present [13]. As most ‘primary’ TDP-43 proteinopathies are of younger onset, the pathological mechanisms might also be different from that seen in our “normal aging” group here. The relationship between tau and TDP-43 pathology therefore seems to be inconsistent and at present it is still unclear why subjects with ARG are more likely to possess TDP-43 pathology [37].
Acknowledgments
The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Adler CH, Connor DJ, Hentz JG, Sabbagh MN, Caviness JN, Shill HA, Noble B, Beach TG. Incidental Lewy body disease: clinical comparison to a control cohort. Movement disorders: official journal of the Movement Disorder Society. 2010;25(5):642–646. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica. 2009;117(6):613–634. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell and tissue banking. 2008;9(3):229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH. Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: a quantitative evaluation of a one-year autopsy population from a geriatric hospital. Cereb Cortex. 1994;4(2):138–150. doi: 10.1093/cercor/4.2.138. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Braak E. Demonstration of amyloid deposits and neurofibrillary changes in whole brain sections. Brain Pathol. 1991;1(3):213–216. doi: 10.1111/j.1750-3639.1991.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiology of aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 8.Caselli RJ, Walker D, Sue L, Sabbagh M, Beach T. Amyloid load in nondemented brains correlates with APOE e4. Neuroscience letters. 2010;473(3):168–171. doi: 10.1016/j.neulet.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crystal HA, Dickson DW, Sliwinski MJ, Lipton RB, Grober E, Marks-Nelson H, Antis P. Pathological markers associated with normal aging and dementia in the elderly. Annals of neurology. 1993;34(4):566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 10.Davies L, Wolska B, Hilbich C, Multhaup G, Martins R, Simms G, Beyreuther K, Masters CL. A4 amyloid protein deposition and the diagnosis of Alzheimer’s disease: prevalence in aged brains determined by immunocytochemistry compared with conventional neuropathologic techniques. Neurology. 1988;38(11):1688–1693. doi: 10.1212/wnl.38.11.1688. [DOI] [PubMed] [Google Scholar]
- 11.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiology of aging. 1992;13(1):179–189. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 13.Fujishiro H, Uchikado H, Arai T, Hasegawa M, Akiyama H, Yokota O, Tsuchiya K, Togo T, Iseki E, Hirayasu Y. Accumulation of phosphorylated TDP-43 in brains of patients with argyrophilic grain disease. Acta neuropathologica. 2009;117(2):151–158. doi: 10.1007/s00401-008-0463-2. [DOI] [PubMed] [Google Scholar]
- 14.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM, Arnold SE, Trojanowski JQ. Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Archives of neurology. 2010;67(10):1238–1250. doi: 10.1001/archneurol.2010.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibb WR. Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathology and applied neurobiology. 1986;12(3):223–234. doi: 10.1111/j.1365-2990.1986.tb00136.x. [DOI] [PubMed] [Google Scholar]
- 16.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain: a journal of neurology. 2007;130(Pt 5):1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- 18.Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Annals of neurology. 2008;64(1):60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. Journal of neuropathology and experimental neurology. 1997;56(10):1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Jicha GA, Petersen RC, Knopman DS, Boeve BF, Smith GE, Geda YE, Johnson KA, Cha R, Delucia MW, Braak H, Dickson DW, Parisi JE. Argyrophilic grain disease in demented subjects presenting initially with amnestic mild cognitive impairment. Journal of neuropathology and experimental neurology. 2006;65(6):602–609. doi: 10.1097/01.jnen.0000225312.11858.57. [DOI] [PubMed] [Google Scholar]
- 21.Josephs KA, Whitwell JL, Parisi JE, Knopman DS, Boeve BF, Geda YE, Jack CR, Jr, Petersen RC, Dickson DW. Argyrophilic grains: a distinct disease or an additive pathology? Neurobiology of aging. 2008;29(4):566–573. doi: 10.1016/j.neurobiolaging.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66(7):1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 23.Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC. Neuropathology of cognitively normal elderly. Journal of neuropathology and experimental neurology. 2003;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Meyer JS, Kawamura J, Terayama Y. White matter lesions in the elderly. Journal of the neurological sciences. 1992;110(1–2):1–7. doi: 10.1016/0022-510x(92)90002-3. [DOI] [PubMed] [Google Scholar]
- 26.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41(4):479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 27.Murray ME, Senjem ML, Petersen RC, Hollman JH, Preboske GM, Weigand SD, Knopman DS, Ferman TJ, Dickson DW, Jack CR., Jr Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Archives of neurology. 2010;67(11):1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta neuropathologica. 2007;114(3):221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Kwong LK, Truax AC, Vanmassenhove B, Kretzschmar HA, Van Deerlin VM, Clark CM, Grossman M, Miller BL, Trojanowski JQ, Lee VM. TDP-43-positive white matter pathology in frontotemporal lobar degeneration with ubiquitin-positive inclusions. Journal of neuropathology and experimental neurology. 2007;66(3):177–183. doi: 10.1097/01.jnen.0000248554.45456.58. [DOI] [PubMed] [Google Scholar]
- 30.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Experimental neurology. 2008;210(2):409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiology of aging. 2009;30(7):1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, et al. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250–260. doi: 10.1212/wnl.43.2.250. [DOI] [PubMed] [Google Scholar]
- 33.Sabbagh MN, Sandhu SS, Farlow MR, Vedders L, Shill HA, Caviness JN, Connor DJ, Sue L, Adler CH, Beach TG. Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer disease and associated disorders. 2009;23(3):229–233. doi: 10.1097/WAD.0b013e318199d833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000;55(3):370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 35.Soma K, Fu YJ, Wakabayashi K, Onodera O, Kakita A, Takahashi H. Co-occurrence of argyrophilic grain disease in sporadic amyotrophic lateral sclerosis. Neuropathology and applied neurobiology. 2012;38(1):54–60. doi: 10.1111/j.1365-2990.2011.01175.x. [DOI] [PubMed] [Google Scholar]
- 36.Thal DR, Schultz C, Botez G, Del Tredici K, Mrak RE, Griffin WS, Wiestler OD, Braak H, Ghebremedhin E. The impact of argyrophilic grain disease on the development of dementia and its relationship to concurrent Alzheimer’s disease-related pathology. Neuropathology and applied neurobiology. 2005;31(3):270–279. doi: 10.1111/j.1365-2990.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 37.Uryu K, Nakashima-Yasuda H, Forman MS, Kwong LK, Clark CM, Grossman M, Miller BL, Kretzschmar HA, Lee VM, Trojanowski JQ, Neumann M. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. Journal of neuropathology and experimental neurology. 2008;67(6):555–564. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O’Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia. 2008;46(6):1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG. Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Experimental neurology. 2012;240C:190–204. doi: 10.1016/j.expneurol.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease – a review. International journal of clinical and experimental pathology. 2011;4(2):147–155. [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer disease and associated disorders. 1999;13(4):226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Yokota O, Tsuchiya K, Noguchi Y, Akabane H, Ishizu H, Saito Y, Akiyama H. Coexistence of amyotrophic lateral sclerosis and argyrophilic grain disease: a non-demented autopsy case showing circumscribed temporal atrophy and involvement of the amygdala. Neuropathology: official journal of the Japanese Society of Neuropathology. 2007;27(6):539–550. doi: 10.1111/j.1440-1789.2007.00805.x. [DOI] [PubMed] [Google Scholar]


