Abstract
Neural mechanisms underlying the capacity of memory to be rich with sensory detail are largely unknown. A candidate mechanism is learning-induced plasticity that remodels adult sensory cortex. Here, expansion in the primary auditory cortical (A1) tonotopic map of rats was induced by pairing a 3.66 kHz tone with activation of the nucleus basalis, mimicking the effects of natural associative learning. Remodeling of A1 produced de novo specific behavioral memory, but neither memory nor plasticity were consistently at the frequency of the paired tone, which typically decreased in A1 representation. Rather, there was a specific match between individual subjects’ area of expansion and the tone that was strongest in each animal’s memory, as determined by post-training frequency generalization gradients. These findings provide the first demonstration of a match between the artificial induction of specific neural representational plasticity and artificial induction of behavioral memory. As such, together with prior and present findings for detection, correlation and mimicry of plasticity with the acquisition of memory, they satisfy a key criterion for neural substrates of memory. This demonstrates that directly remodeling sensory cortical maps is sufficient for the specificity of memory formation.
Keywords: acetylcholine, auditory cortex, brain stimulation, neurophysiology, nucleus basalis
INTRODUCTION
The neural basis of memory remains a central problem in neuroscience. A prevalent approach has been to determine the brain regions that are necessary for memory formation. For example, the medial temporal lobe has been identified as requisite for the establishment of recent declarative memory, although not for long-term storage which has been attributed largely to the cerebral cortex (Squire et al., 2004). A less frequent but complementary research focus is on how the actual contents of memory become stored. Memory content includes the particular sensory information about experienced events that is initially provided by transient neural activity across the several sensory modalities. Sensory cortical areas are particularly well suited to represent details of experiences because they include neurons with highly specific receptive fields. Moreover, primary sensory fields are convenient targets for the study of the representations of remembered experiences because they contain organized dimensions of content-specific representations (Gilbert and Wiesel, 1992; Weinberger, 1995), which facilitated the discovery of learning and memory related plasticity in primary auditory (A1) (Weinberger, 1995), somatosensory (S1) (Diamond et al., 1999) and visual (V1) (Super, 2002) fields.
Regardless of the brain region or type of learning-related plasticity under consideration as a memory substrate, certain commonly accepted criteria need to be satisfied. First, behavioral evidence of memory formation should be accompanied by (a) detection of a neural change. Further, the neural plasticity detected should be (b) correlated with the behavioral parameters of learning. Additionally, (c) mimicry should be demonstrable, i.e., artificial production of the neural change should produce behavioral signs of memory that mimic its natural induction (Martin et al., 2000). Satisfaction of these criteria is required to conclude that a candidate neural change is sufficient for memory.
However, neural plasticity that can account for memory contents also requires an additional criterion, that of (d) specificity. Thus, it is not adequate to simply find neural plasticity (i.e., detection) that is related to with behavioral expression of memory by their co-emergence (i.e., correlation), because such plasticity might be involved in, e.g., processes that enable the acquisition and storage of a particular experience, rather than encoding a sensory aspect (or content) of the experience itself. Consequently, neural candidates for memory contents have also to match the specific details of those contents. Therefore, in order to conclude sufficiency, both natural and also (c) mimicked plasticity ought to match the specific contents of the behavioral memory, e.g., that of a specific sound. Furthermore, specificity at the group-level, while important, is inadequate to fully meet the criterion of content-specificity. Individuals seldom form identical memory, even within a group that has undergone the same training. Therefore a neural change in individual subjects needs to match the content of the individual subject’s memory in order to be a valid candidate substrate of those contents.
The primary auditory cortex has been studied more extensively than any other primary sensory field for neural substrates of learning and memory. As such, neural plasticity in A1 has met cardinal criteria outlined above. The (a) detection of associative plasticity (e.g., increased evoked potentials and unit discharges to a signal tone) has been established across tasks and species for more than fifty years (reviewed in Scheich et al., 2011; Weinberger, 2011). More recently, combined behavioral studies of memory and auditory neurophysiology have revealed (b) correlation, in that associative learning can produce signal-specific persistent associative shifts in receptive field tuning to favor representation of a tone-frequency that signals reinforcement (Bakin and Weinberger, 1990; Weinberger, 2004). Furthermore, representational tuning shifts across A1 expand the signal-frequency area in the tonotopic map (Recanzone et al., 1993) where they appear to encode the level of acquired stimulus importance (Rutkowski and Weinberger, 2005) and serve as a substrate for strengthening specific memory (Polley et al., 2006; Bieszczad and Weinberger, 2010a, 2011). Moreover, representational plasticity correlates of learning have the same attributes as behavioral memory (e.g., associativity, specificity, consolidation and long-term retention) (Weinberger, 2007) and are ubiquitous, developing in humans and animals in a wide variety of tasks (Ohl and Scheich, 2005).
Brain stimulation techniques have been used to satisfy the third criterion of (c) mimicry for auditory learning and A1 plasticity. For example, stimulation of the cholinergic nucleus basalis (NBstm) paired with the presentation of an auditory tone induces representational plasticity in A1 that has the same major features as plasticity induced during natural learning, i.e., associativity, specificity, consolidation and long-term retention (Weinberger, 2007). Moreover it is dependent on muscarinic acetylcholine receptors (Miasnikov et al., 2001; Zhang et al., 2006; Chen and Yan, 2007; Zhang and Yan, 2008). Perhaps most importantly, this procedure also implants de novo behavioral memory that also has the main characteristics of natural behavioral memory (McLin et al., 2002; Miasnikov et al., 2011). Thus, NBstm procedure successfully mimics natural memory formation by artificially inducing A1 representational plasticity and also implanted auditory memory.
However, there has not yet been any established link between mimicry of A1 representational plasticity and mimicry of behavioral memory. For example, whether or not the induction of plasticity in A1 by stimulation of the nucleus basalis is sufficient to implant of behavioral memory with this procedure is unknown. This issue could be resolved by addressing the fourth criterion for sufficiency, that of (d) specificity, to determine whether there are matches in NB-induced plasticity and implanted memory at the level of the individual. Studies of natural auditory learning and memory in rodents have shown at the group level that neural plasticity in A1 is significantly related to the training frequency (Bakin and Weinberger, 1990; Edeline and Weinberger, 1993; Gonzalez-Lima and Scheich, 1986; Kisley and Gerstein, 2001; Fritz et al., 2003; Recanzone et al., 1993; Reed et al., 2011). Recent experiments have focused on the fact that identical training can induce different representational changes in A1 in individual subjects, and discovered that these individual differences in natural learning correlate with the strength of the specific memory an animal had acquired during training (Ohl et al., 2001; Polley et al., 2006; Bieszczad and Weinberger 2010a, 2010b, 2012). Similarly, a recent study of artificially implanted memory has shown that the NBstm procedure is susceptible to a “peak shift” learning phenomenon in which the training can implant a specific auditory memory that is unique in individual animals and different from the actual frequency of the paired tone — despite their identical training (Miasnikov and Weinberger, 2012). Therefore, the ultimate specificity of mimicked and natural behavioral memory may depend not on the parameters of training, but on the specificity of the reorganization of frequency-representation in A1 within an individual subject.
One paper has studied a link between mimicry of A1 representational plasticity and mimicry of behavioral memory by exploring the degree of match in specificity between NB-induced A1 plasticity and memory. A significant relationship was found between the magnitude of increase in evoked potential amplitude for frequencies at or adjacent to the conditioned stimulus frequency (CS) and the magnitude of change in behavioral heart rate or respiration measures of memory (Miasnikov et al., 2006). However, this study did not demonstrate specificity of A1 plasticity and behavior for the same unique frequency. The conclusion that cortical plasticity is actually highly specific to the memory requires an individual analysis of the match in frequency-specificity between NB-induced plasticity in A1 and NB-induced memory. The purpose of the present study was to investigate this open issue of individual matches in specificity to determine the role of A1 reorganization for the induction of auditory memory.
Because the area of representational gain is known to encode both stimulus importance (Rutkowski and Weinberger, 2005) and strength of frequency-specific memory (Bieszczad and Weinberger, 2010) in natural learning, we focused on the relationship between frequency representational A1 area and its specificity to memory.
EXPERIMENTAL PROCEDURES
The general methods were identical to those previously reported (Miasnikov et al., 2011) and thus are only briefly reported here. All procedures were performed in accordance with the University of California, Irvine, Animal Research Committee and the NIH Animal Welfare guidelines.
Subjects and surgery
The subjects were 6 adult male Sprague–Dawley rats (~400 g) that, under pentobarbital general anesthesia, had received a concentric bipolar stimulating electrode in the right caudal nucleus basalis (NB), and an epidural screw electrode over the ipsilateral primary auditory cortex. Two threaded standoffs were affixed to the skull to mount a thermistor assembly for recording respiration. After recovery the effective NB locus was confirmed by repeatedly obtaining several seconds of auditory cortical EEG activation to NB-stimulation (NBstm; 0.2 s train) while subjects were in a state of slow wave sleep.
Stimuli, experimental design and analysis of respiratory behavior
Training and testing took place while subjects were in an acoustically damped box with a double-walled acoustic chamber. Acoustic stimuli were nine 2.0 s pure tones separated by ~0.58 octaves (1.00–27.64 kHz; cosine 10 ms rise/fall time [10–90%]; 70 dB SPL) delivered via calibrated overhead speakers. Stimulation parameters of the nucleus basalis consisted of a 0.2 s train of 100 Hz (pairs of 0.2 ms opposite polarity) pulses at a current level of 100 μA. These parameters of NB stimulation were insufficient to disrupt or initiate motor movements.
On Days 1, 2 and 6, animals were presented with the nine test tones (~20 repetitions of each, randomly delivered; inter-tone intervals ~1.0 min) while respiratory responses to each were recorded. These yielded behavioral frequency generalization gradients (FGG). On Days 3, 4 and 5, animals were trained with a single conditioned stimulus (CS) frequency. Training consisted of 200 trials/day of the CS tone (3.66 kHz, 2.0 s), which co-terminated with a 0.2 s train of NBstm (inter-trial intervals = ~1 min). The sensitive behavioral measure of conditioned disruption of ongoing respiration discovered by Sherrington (1900) was obtained in response to the test tones (Days 1, 2, 6) and during training (Days 3, 4, 5). Respiration was monitored during single-tone training to determine learning (Fig. 1A & B). Implantation of the frequency-specificity of the auditory memory was assessed by subtracting the pretraining FGG (Day 2) from the posttraining FGG (Day 6) to yield a difference FGG (ΔFGG). A non-associative control group (e.g., NBstm with unpaired presentation of tones) was not included because all previous studies of NB-induced memory implantation have shown the effects of pairing tone with NBstm are associative (e.g., Bakin and Weinberger, 1996b; McLin et al., 2002, 2003; Miasnikov et al., 2006).
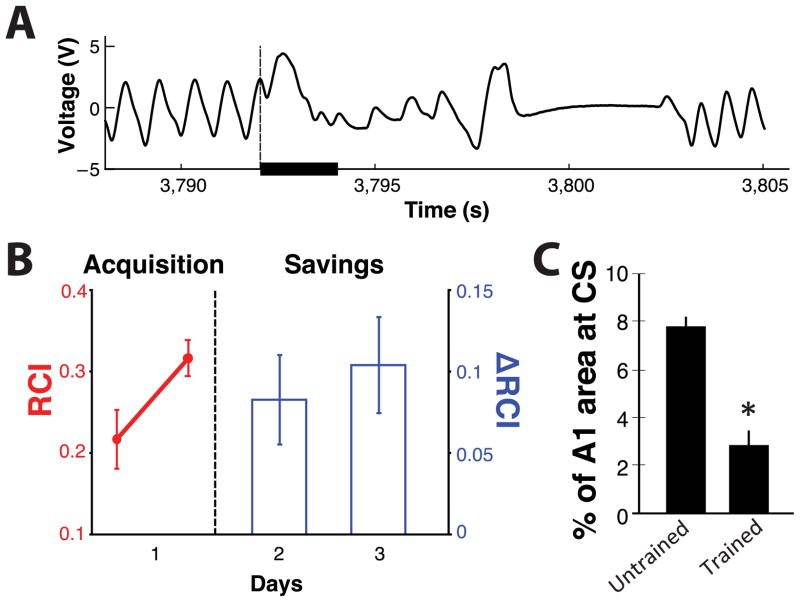
Fig. 1.
Animals learn without CS-specific tonotopic expansion. (a) Conditioned disruption of ongoing respiration by the CS tone (solid bar). (b) Day 1, group learning response during last 100 trials of training with the 3.66 kHz tone (the two last 50 trial blocks are shown); Days 2 & 3, savings (increased response) from last trial block of preceding day demonstrates consolidation of conditioned responding. Note that this conditioned disruption of respiration is determined during training with only a single tone. (c) CS/NBstm trained rats (n = 6) lack CS-specific expansion compared to a group of untrained naïve rats (n = 9). Instead, trained animals appear to have a significant decrease in CS-area in A1, which suggests that an expansion had occurred elsewhere in the frequency map. Bar graph: Untrained (n = 9) and Trained (n = 6) group means ± s.e.m.
Respiration was detected as breathing-related thermal fluctuations by a glass-encapsulated thermistor attached to a lightweight pedestal-mounted assembly that was pre-adjusted with a sensor positioned in front of a naris. The amplified output signal was fed to an ADC module, and the autocorrelation function (AC) was calculated on-line. The AC was used to present tones only when the subject was in a quiescent behavioral state, defined as four (4) consecutive seconds of 0.700 ≤ AC ≤ 0.975. Prior studies had found that animals were, e.g., exploring, grooming, or in paradoxical sleep, when the AC was < 0.700 and in slow wave sleep (SWS) when AC was > 0.975 (Weinberger et al., 2006). This state control prevented ceiling and floor effects during stimulus presentation, as when very high levels of acetylcholine are intrinsically released in the cortex during exploration or paradoxical sleep, and when levels are very low, as during SWS, respectively (Jasper and Tessier, 1971).
FGGs were determined by calculating the amount to which test tones (Days 2 and 6) disrupted ongoing respiration, during the 13 sec following the onset of each tone compared to the 4 s immediately preceding that tone. This yielded a trial-by-trial “Respiration Change Index”: RCIi = (|Posti − Pre|)/(Posti + Pre) where Post and Pre were the values of the power of the respiration signal upon the presentation of any one test tone-frequency. RCI is sensitive to increases and decreases of both frequency and amplitude of the respiration response. An RCI value of zero would indicate no change and a value of 1.0 would indicate complete cessation of respiration. RCI values for each of the test tones together comprised the FGG. The same RCI method was used to determine respiratory behavior during three consecutive training trials with the CS frequency alone (Days 3, 4, 5). Analysis of respiration was limited to < 1.8 s after the tone onset to avoid any direct effects on respiration due to NB stimulation itself. This analysis is sufficient to detect the development and maintenance of respiratory conditioned responding. Learning and savings over the three days of training (Fig. 1B) were revealed by increases in RCI to the tone between the first and second 50-trial block of the last 100 trials of the first training session (Fig. 1B, Acquisition), and change in RCI between the first and second, as well as first and third session (days) of training (Fig. 1B, Savings).
Mapping of the primary auditory cortex
The tonotopic map in A1 was determined following CS/NBstm training by the cortical distribution of characteristic frequency (CF) in a terminal session 24–48 h after the final behavioral test. An additional group of untrained naïve animals (n = 9) had been mapped as a comparison group. Electrophysiological mapping methods have been reported previously (Bieszczad and Weinberger, 2012) and were identical and the results standard in the untrained, naïve group, for direct comparison with the trained group. Briefly, mapping to determine characteristic frequency (CF) and the borders of A1 was conducted while rats were anesthetized with sodium pentobarbital to minimize pain and discomfort during electrophysiological recording (50 mg/kg, i.p.). Stimuli used to construct frequency-evoked responses at each recording site were pure-tone bursts delivered to the ear contralateral to the recording hemisphere (50 ms, cosine-squared gate with rise/fall time [10–90%] of 7 ms, 0.5–54.0 kHz, 0–70 dB SPL). Frequency response areas (FRAs) were constructed offline for evoked spike-timing data using custom software built in Matlab R2011a.
The characteristic frequency (CF) of a responsive site was defined as the stimulus frequency having the lowest threshold (CF threshold) for an evoked response (i.e., highest neural sensitivity). CF was determined from each FRA. Recording sites were identified by Cartesian coordinates across the surface of the cortex. Each coordinate point (i.e., that references each recording site) was labeled with the determined CF and entered into a Voronoi tessellation algorithm (Matlab R2011a toolbox) to construct areal polygons around each point. Sites that were determined to be within the borders of A1 had evoked responses that were short-latency (within 6–40 ms of tone onsets), a single CF that could be determined from the tuning shape of the FRA, and with a CF that followed an organized progression from low- to high-CF (posterior to anterior) across the surface of the cortex. The cortical areal distribution of frequency-representation was expressed in 0.58 octave-wide bands of CF (CF-band). Each CF-band area was expressed relative to the total size of A1 across the audible frequency range for the rat (i.e., % of A1 area) The sizes of polygons were summed within CF bands (< 0.9, 0.9–1.3, 1.3–2.0, 2.0–3.0, 3.0–4.5 (CS frequency CF-band), 4.5–6.7, 6.7–10.1, 10.1–15.1, 15.1–22.6, 22.6–33.8 and 33.8–50.7 kHz) to represent the areal distribution of frequency. A1 areas were expressed in relative terms as percentages with respect to the total area (100%) of A1 to account for natural variability in the absolute size and orientation of topographic cortical maps.
Supra-threshold analysis was identical to that for threshold (i.e., at CF). FRAs of sites that were determined from CF analysis to be within A1were subjected to best frequency (BF) analysis. An absolute measure of best frequency, “BF60”, was determined as the frequency that evoked the maximal response at 60 dB SPL. This level was chosen for analysis because it was nearest the sound level of the trained signal (i.e., 70 dB SPL) that was contained well within the stimulus set used to determine the FRA for each A1 site (i.e., 0–70 dB SPL) and for which BF could be accurately determined.
Analysis of the distribution of A1 area
The amounts of A1 area occupied by each CF-band (i.e., for threshold analyses) or BF-bands (i.e., for supra-threshold analyses) in individual animals were averaged (± s.e.m.) to determine the areal distribution of frequency in A1 for the group. Group means were generated in two ways: (1) with respect to absolute frequency such that the same frequency-band in all subjects was averaged (i.e., CS-frequency alignment); or (2) with respect to the frequency at the unique peak of ΔFGG for each subject such that different CF or BF frequency-bands from each subject were averaged (i.e., peak-ΔFGG alignment). The peak was defined as one of seven frequencies (1.5, 2.4, 3.7, 5.5, 8.2, 12.3, or 18.4 kHz) that was at the highest point of the ΔFGG (had the greatest positive difference) within a systematic curve about that peak.
Statistics
All statistics used functions built-in to Matlab R2011a software for appropriate parametric comparisons, corrected when necessary, as indicated. α = 0.05.
RESULTS
We used electrical stimulation of the nucleus basalis (NB), the primary source of cortical acetylcholine, to induce areal expansion in A1 (Bakin and Weinberger, 1996a; Kilgard and Merzenich, 1998) and bridge the gap between cortical plasticity and behaviorally specific auditory memory. In particular, we assessed whether pairing tone with NB-activation results in memory implantation, i.e., as evident in the development of corresponding tone-specific auditory behavior, that matches the tone’s specific representational expansion in A1. To place this somewhat novel approach within the broader context, we begin with a standard group analysis based on the CS frequency.
Learning for an auditory tone after pairing with nucleus basalis stimulation
As noted above, rats received 200 daily presentations of a pure-tone conditioned signal-frequency (CS) (3.66 kHz; 2.0 s, 70 dB SPL) that co-terminated with nucleus-basalis stimulation (NBstm; 100 μA, 0.2 s) for three consecutive days. An increase in the disruption of respiration to the conditioned tone (Fig. 1A) revealed that all animals developed an auditory memory (effect of acquisition: Wilcoxon signed rank test, P < 0.05) that persisted over days (savings from initial acquisition on Day 2: Wilcoxon signed rank test, P < 0.05; and on Day 3: Wilcoxon signed rank test, P < 0.05) (Fig. 1B). Note that training was with a single frequency. Thus, the specificity of the auditory memory was determined after training, with a test that measured behavioral responses to many frequencies in addition to the 3.66 kHz trained signal (see Section 3.3).
Primary auditory cortical reorganization after pairing tone with NB stimulation
Prior studies have reported expansions in A1 for the representation of tone-frequencies that have ibeen paired with NBstm. However, no significant increase in A1 map area for the CS-frequency (± 0.29 octaves) was found (one-tailed test for CS-gain: t(12) = 2.99, P > 0.05) (Fig. 1C). This negative finding initially appears to be inconsistent with previous reports. However while tone paired with NBstm does produce cortical reorganization for a representational bias toward the CS frequency, the only similar studies actually did not obtain highly specific effects. For example, maximal enhancements in cortical activation were for frequencies more than half an octave (see Fig. 8A in Puckett et al., 2007; Fig. 3C), even over an octave in spectral distance from the CS frequency (see Fig. 3C in Kilgard and Merzenich, 1998), or for a range of frequencies near that of the CS (see Fig. 5C in Miasnikov et al., 2008). Thus, the apparent failure to observe a significant increase in area for the CS frequency of 3.66 kHz actually is compatible with prior reports. Indeed, there was an actual decrease in A1 representational area for the CS-frequency (± 0.29 octaves) (one-tailed test for CS-loss: t(12) = 2.99, P < 0.01) (Fig. 1C), which suggests that there was a complementary increase in area elsewhere in A1 to increase cortical representation of a frequency that was not the CS.
Fig. 3.
Tonotopic expansion occurs for tone-frequency with the greatest acquired importance. (A) It is possible that an expansion in A1 had occurred for a frequency an individual had actually remembered (and not for the frequency of the CS) because the animal had remembered that frequency to be more important than the CS. Thus, more area should exist for the frequency with greater importance, i.e., the greater behavioral ΔFGG values at the peak-frequency, than for the amount of A1 area and behavioral ΔFGG values for the CS-frequency. This relationship was confirmed by a comparison of individual ΔFGG amplitudes for the CS-frequency and those for the frequency at the peak of the ΔFGG (x-axis) to the amount of representational area for those corresponding frequencies in A1 (y-axis). Frequencies that evoked greater behavioral responses (peaks of the ΔFGG) positively correlate with larger A1 area. Therefore, specific A1 expansions had occurred not for the CS frequency, but rather had enlarged the representation of the frequency at the ΔFGG peak. Crosses show group means for A1 area and amplitude of the ΔFGG, which were both significantly different between CS and ΔFGG-peak analyses (indicated by asterisk). (B) Examples of A1 maps after CS/NBstm training show expansion not at the CS-frequency, but for the frequency at the peak of the behavioral ΔFGG. An example of an untrained animal’s A1 map (i) shows the relative amount of area for the frequency that was targeted for expansion with CS/NBstm training (outlined in white: 3.66 kHz ± 0.29 octaves). Examples of maps from trained animals show an enlarged area below (ii), at (iii) or above (iv) the target CS-frequency. Maps from animals indicated by (ii) and (iv) lacked any detectable area of representation of the CS frequency (± 0.29 octaves). Scale bars = 1.0 mm. (C) The area in A1 with expanded representation can be predicted by the peaks of individual ΔFGGs. More A1 area represents frequencies near ΔFGG-peaks (red) than the CS-frequency (blue). The right panel shows the subtraction of curves in (c) illustrating the specificity of the effect; the center CF-band of alignment (“0” at the CS vs. the ΔFGG-peak) is significantly different and without effect on A1 areas of frequency-representation neighboring the frequency at the ΔFGG-peak. Shaded area shows mean ± s.e.m. *P < 0.05.
However, an alternative possibility is that plasticity for the frequency of the trained 3.66 kHz signal might have developed instead near the sound level of the signal, rather than at threshold. Thus, the A1 representation of best frequency (BF) at 60 dB SPL was determined to complement the analysis of representation of characteristic frequency (determined at the sound level threshold for a tone-evoked response). However there was no change in representational area at this sound level (two-tailed test: t(12) = 0.01, P > 0.05). Therefore, no group analyses revealed an expansion for the 3.66 kHz signal.
Frequency-specificity of memory after pairing tone with NB stimulation
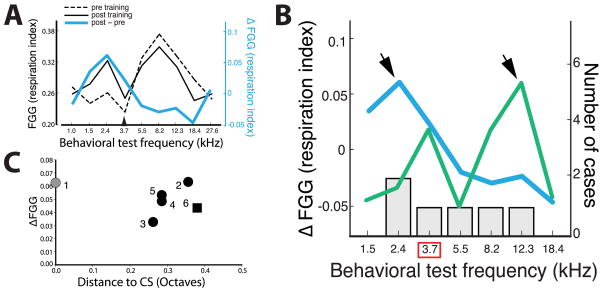
An implicit expectation about the specificity of NBstm-induced effects is that the training frequency is necessarily the frequency that becomes most salient, which assumes that the contents of auditory memory (natural or artificially-induced) are dominantly for the training frequency. This assumption permits group analyses that rely on the specificity of learning to be predicted by experimental parameters alone (e.g., the frequency of the paired signal tone). However, learning is often not precise due to individual differences in prior experiences and predispositions (Miasnikov and Weinberger, 2012) (see Discussion section 4.2). Thus, the group’s lack of significant increase in area at the CS frequency may simply reflect the development of dominant memory contents for various other frequencies across individual animals. To investigate this possibility it is necessary to test individuals for behavioral effects using many frequencies, yielding behavioral frequency generalization gradients (FGG) that reveal the actual frequency-specificity of memory (Mostofsky, 1965). The peak of the FGG reveals specificity of memory for the CS or for a non-CS frequency. Subtraction of pre-training from post-training FGGs yielded behavioral difference gradients (ΔFGG) (Fig. 2A). These revealed that frequency-specific stimulus salience after NBstm/tone training actually differed across animals.
Fig. 2.
Individuals acquire a different tone-frequency as the most important following identical CS/NBstm training. (a) Determining the difference frequency generalization gradient (ΔFGG). An example of FGGs obtained using conditioned disruption of ongoing respiration show the computation of a ΔFGG (blue line, right axis) by subtraction of the post-training FGG (solid line, left axis) from the pre-training FGG (dashed line, left axis). (b) Arrows indicate the identified peaks of ΔFGGs in individual rats (as per the left axis). Examples show one animal with a ΔFGG-peak at a frequency below (left arrow), or above (right arrow) the trained CS-frequency (3.66 kHz, indicated by the red box). Histogram indicates a count of animals with peaks at each of the possible frequencies (as per the right axis). There was a range of frequencies that had been learned as the most important, despite identical training. (c) The peak of the group difference frequency generalization gradients (ΔFGGs) in the five prior studies of NB-induced memory implantation (1–5) and the current study (6). Note that only the first study (Experiment 1: McLin et al., 2002) found that the peak behavioral response was at the CS frequency. All subsequent studies employed pre-training tonal presentation (see Supplemental Procedures). In all of these studies, the peak of the group ΔFGG was in the range of 0.26–0.36 octaves from the CS. Experiment 2: Miasnikov et al., 2006, CS = 8.00 kHz, Peak = 6.25 kHz; Experiment 3: Miasnikov et al., 2008b, CS = 8.00 kHz, Peak = 9.75 kHz; Experiment 4: Miasnikov et al., 2008a, CS= 8.00 kHz; Peak (reanalyzed data from mean of 3 frequencies centered on CS = 9.75 kHz; Experiment 5: Miasnikov et al., 2011, CS = 8.00 kHz, Peak (24 h post) = 9.75 kHz. The current study (Experiment 6) found the peak of the ΔFGG to be shifted −0.38 octaves (CS = 3.66 kHz, Peak = 2.81 kHz). Most importantly, this study revealed the frequency of the cortical expansions matched the peaks of the ΔFGG on an individual animal basis.
All ΔFGGs contained a peak (i.e., were not flat), which indicated that each animal had acquired a frequency-specific auditory memory. The peak of each ΔFGG identifies the frequency with the greatest acquired salience. The ΔFGGs in 5/6 animals peaked at a non-CS frequency, as far as 1.5 octaves away from the CS tone (Fig. 2B). This finding appears to differ from reports of CS-specific implantation of memory by pairing tone with NB stimulation (McLin et al., 2002). However, such precision was actually obtained only in one study, in which animals did not hear any tones prior to training (McLin et al., 2002; point #1 in Fig. 2C). Subsequent experiments had exposed animals to various tones before training, to obtain within-subject baselines (Miasnikov et al., 2006, 2008a, 2008b, 2011), resulting in ΔFGGs that were not at the CS frequency (points #2–6 in Fig. 2C). We recently discovered that the presence of pre-training tones produce this deviation from the CS frequency (Miasnikov and Weinberger, 2012) (see Discussion section 4.2).
Match in the frequency-specificity of memory with the specificity of A1 reorganization
Given the variety of frequencies remembered as most important, the lack of CS-specific A1 expansion does not demonstrate the absence of underlying cortical area gains for the actual frequency-specificity of auditory memory. A1 expansion may have developed instead for the specific frequency an animal had best remembered, i.e., the frequency at the peak of its behavioral ΔFGG. Indeed, behavioral amplitudes of the ΔFGG at the peak were significantly greater than that for the CS (CS vs. ΔFGG-peak behavioral amplitude: paired t-test t(6) = 2.18, P < 0.05). Therefore, we compared within-animal representational area in A1 to their peak frequencies. This analysis revealed that maximally expanded cortical area was correlated with the best remembered frequency (r(12) = 0.60, P < 0.05) (Fig. 3A), rather than the CS training frequency.
To determine if the specificity of cortical remodeling predicts the specificity of memory we re-aligned the distribution of A1 frequency-representation to center on the tone-frequency of individual ΔFGG peaks (vs. prior centering on CS-frequency). This analysis revealed an enlarged cortical representation for the frequency at the ΔFGG-peak (CS vs. ΔFGG-peak A1 area: paired t-test t(6) = 3.64, P < 0.05). The specificity of the expansion in A1 was further characterized by an analysis of the distribution of all frequency cortical representation. Fig. 3B shows individual A1 maps that illustrate the distribution of frequency representation and the relative representation of area at the CS vs. at the peak of the ΔFGG. Cortical area was significantly larger only for the frequency-band containing the frequency at the ΔFGG peak (alignment × A1 frequency-band interaction: F(5,60) = 3.18, P < 0.05; posthoc CS-frequency vs. ΔFGG-peak alignment for A1 frequency-band at center: t(10) = 3.02, P < 0.05). There were no significant differences for any other frequency-band (posthoc for all other A1 bands: t(10) < |1.40|, P > 0.05). Thus the frequency-specificity of sensory cortical expansion predicted the specificity of auditory memory induced by training (Fig. 3C).
To determine if behavioral specificity could also be predicted by frequency-specific reorganization above threshold, we subjected BF data to the same re-alignment analysis at 60 dB SPL. There was a significant expansion at the ΔFGG-peak (alignment × A1 frequency-band interaction: F(5,60) = 2.49, P < 0.05; posthoc CS-frequency vs. ΔFGG-peak alignment for A1 frequency-band at center: t(10) = 3.11, P < 0.05), without any significant differences for any other frequency-band (posthoc for all other A1 bands: t(10) < |2.03|, P > 0.05). Therefore, the representation of frequency at 60 dB SPL also predicted the frequency-specificity of auditory memory induced by training.
DISCUSSION
Resume and validity of findings
Analysis of the relationship between auditory cortical expansion and the specificity of behavioral memory, both of which were induced by pairing a tone with stimulation of the nucleus basalis, revealed a specific match between the greatest area gain in A1 and the tone frequency that was strongest in memory. Uncovering this relationship required individual rather than group approaches. This is consistent with the reality that training with identical protocols often results in variability in learning and memory. The current findings provide the first demonstration that direct remodeling of primary sensory cortex is sufficient for the induction of specific behavioral memory.
An alternative is that the cortical expansions are not responsible for the specificity of memory because stimulation of the nucleus basalis might have critically affected the subcortical auditory system or perhaps other brain structures. This explanation is quite possible in the case of previous research that has not been based on the additional criterion of a specific match between plasticity and memory content. However, only the ventral medial geniculate nucleus (MGv) has a sufficiently frequency specific organization that might account for the frequency-specificity of both A1 plasticity and memory. Although the MGv does develop representative plasticity, consisting of associative tuning shifts directed toward the CS frequency, the effect is transient, dissipating within an hour (Edeline and Weinberger, 1991; Froemke et al., 2013) while plasticity in A1 can last at least for two months (Weinberger et al., 1993). It was at a more remote time point (i.e., at least 24 h after training) that the frequency-specificity of behavior and that of A1 re-organization was determined. Therefore, it is extremely unlikely that transient MGv frequency tuning plasticity could be responsible for the current findings.
Further support for a cortical locus of the specificity of memory is that cholinergic blockade via muscarinic antagonists blocks the implantation of NB-stimulation mediated frequency-specific auditory cortical plasticity (Edeline et al., 1994; Miasnikov et al., 2001; Ji and Suga, 2003; Yan and Zhang, 2005; Froemke et al., 2013) and frequency-specific behavior (Miasnikov et al., 2008a; Froemke et al., 2013). Furthermore, the level of acetylcholine release within primary auditory cortex controls the degree of induced specificity of associative behavior both between modalities (visual vs. auditory: Butt et al., 2009) and within modality in the dimension of auditory frequency (Weinberger et al., 2006). Overall, this evidence indicates that remodeling the representation of frequency in A1 is sufficient for the formation of specific auditory memory.
The current findings constitute the first report that direct remodeling of the functional organization of cortex produces de novo specific and behaviorally validated memory. They bridge the gap between mimicry of A1 representational plasticity and the artificial implantation of specific behavioral memory. In so doing, they satisfy an important criterion for identifying a neural basis for learning and memory, i.e., that an established neural correlate of memory (cortical representational expansion), which can be induced artificially (by NB stimulation), is itself sufficient to implant specific memory (Martin et al., 2000).
The role of adult cortical reorganization for the induction of memory
Future studies to determine if remodeling sensory cortex is necessary for specific memory will have to satisfy the additional criteria of (i) blocking memory by blocking remodeling of A1 (or other primary sensory fields) and (ii) reversing memory by reversing cortical plasticity (Martin et al., 2000). There is already evidence to support the latter. Extinction following training eliminates behavioral memory for animals in which the gain of plasticity in A1 is also eliminated, while lesser reversals of plasticity produce lesser losses of memory (Bieszczad and Weinberger, 2012).
There has been some confusion about the necessity of cortical plasticity for memory because the former can dissipate while auditory perceptual learning can remain (Reed et al, 2011). However, a careful distinction must be made between initial acquisition of memory and the effects of extensive over-training — that is continued training well after behavioral asymptote has been reached. Reed and colleagues conducted overtraining for a period of weeks before A1 plasticity disappeared. Such over-training is known to shift behavior from cognitive memory to habit (James, 1890) and from cortically- to subcortically-dependent (Mishkin and Petrie, 1984). So plasticity might be necessary for the acquisition of specific and recent memory. Furthermore, in the absence of overtraining, or reversal (e.g., with extinction training), representational plasticity in A1 shows retention for at least two months (Weinberger et al., 1993). The current findings of the direct induction of cortical remodeling and specific memory pertain to acquisition. How long cortical remodeling is maintained with overtraining remains to be determined.
Also, there has been some uncertainty about the various uses of the term “memory”. Within the broader context, memory is seen as multidimensional. Thus, virtually all experiences include representations of stimuli in the several sensory systems engaged (particularly those signals predictive of rewarding or aversive events), contextual stimuli, interoceptive signals about bodily state, emotions induced, and even stored representations of similar experiences. All of these changes produce a widely distributed network of neurons that essentially encode the entire memory. The present findings have shown that the specific acoustic frequency within memory is tightly linked to the increase in representational area within A1. As such, it is likely that A1 plasticity is part of the distributed network involved in natural learning situations.
Relation to previous studies
In contemporary neuroscience, the requirement that only one locus/mechanism be at hand continues to be a criterion for necessity rather than for sufficiency (Martin et al., 2000). Thus, sufficiency requires only that instantiation of a process will produce the outcome in question. Multiple processes or other factors may produce similar outcomes of A1 plasticity for memory, e.g., other neuromodulators (Kilgard, 2005; Shepard et al., 2012) in a variety of natural learning paradigms (Weinberger, 2004; Ohl and Scheich, 2005). As noted above, the present findings pertain to the frequency-specific aspect of auditory memory and pertain to its acquisition rather than indefinite storage in cases of overtraining or changes in associative contingencies such as extinction.
Beyond the current findings, other levels of representation within A1 itself may also serve different functional outcomes for behaviors related to auditory memory. For example, the current findings come from studies of the continuous tonotopy of A1 determined largely at neural response threshold and in a narrow time window after tone onset determined here (e.g., within 35 ms of tone onset). Thus, these findings may reflect a different computational organization than sparse representations that have been reported with calcium imaging techniques determined above-threshold (e.g., at 40–60 dB SPL; (Bandyopadhyay et al., 2010) and at longer time scales of resolution (e.g., within 200 ms of tone onset). Also, the precision of frequency-tuning may decrease with longer time integration windows and at higher sound levels for which neurons respond to a broader range of frequencies, which may permit the integration of multiple inputs. Nonetheless, the current findings show that the match in frequency-specific A1 expansion with frequency-specific behavior is maintained for BFs determined above threshold (near the sound level of the trained tone), which is compatible with recent findings of specific reorganizational plasticity for frequency and intensity near that of the conditioned stimulus (Polley et al., 2006; Froemke et al., 2013). The effect for the specificity of memory in longer latency A1 responses remains to be investigated.
Similarly, differences may arise when comparing A1 plasticity in different layers of the cortical microcolumn that can reflect changes in various sources of cortical input (intracortical or thalamocortical synapses) onto A1 neurons (Guo et al., 2012). Recordings to determine cortical frequency organization in the current study were made in layer IV/V, which exhibits the strongest and most frequency-specific tuning receptive fields as can be recorded in A1. It is at this most sensitive (i.e., sound level at response threshold) and specific (i.e., in layers IV/V) level of frequency representation in which continuous tonotopy along the posterior to anterior dimension of the cortex has been determined in A1 (Recanzone et al., 1993; Kilgard & Merzenich 1998; Rutkowski & Weinberger, 2005; Polley et al., 2006, 2007; Berlau & Weinberger, 2008; Hui et al., 2009; Bieszczad & Weinberger, 2010a, 2010b, 2010c, 2012; Takahashi et al., 2011; Reed et al., 2011). Other cortical layers have different computational properties and thus also varying susceptibilities to functional plasticity. For example, changes in intracortical inputs to A1 appear to be best correlated to the development of learned behavior (Guo et al., 2013).
The current findings of specific reorganization in A1 to induce de novo memory (i.e., without prior training) is compatible with a similar study by Froemke et al., (2013) that shows NBstm-pairing with tones can further enhance (or impede) the effects of prior natural learning on auditory detection and discrimination. While Froemke and colleagues focus their report of specific plasticity to only the combined frequency-intensity identity of the paired tone signal (the CS), their data also appear to show remarkable changes near threshold. For example, in their Fig. 1C, the receptive field shows the expected increase in activity at the CS frequency/intensity (circled cell), however the excitatory (Exc) receptive field also exhibits a change in shape at the tuning tip in near-threshold responses. There, the A1 receptive field changed its characteristic frequency (CF)-tuning from between 8 and 32 kHz in “Exc (before)” to between 2 and 8 kHz in “Exc (after)”. Sound level tuning also changes from an excitation threshold of about 30 dB SPL, to an excitation threshold of about 20 dB SPL. This suggests that analyses of CF (i.e., best response at threshold) could have also reported changes in A1 tonotopy that would reflect frequency-specific expansions, independent of intensity-specific changes. Another example from their data that supports putative CF changes can be seen in the lower panel of Fig. 5A. There, the “Before” tuning curve shows an evoked response at 20 and 30 dB SPL, but in the “After” tuning curve, these responses have disappeared. Therefore, the threshold of this A1 site has changed, and likely along with it, the CF at the new threshold.
Collectively, these findings add to existing knowledge by providing evidence to support that the actual specificity of memory (e.g., the particular frequency that will be strongest in memory) is predicted by the frequency-specificity of plasticity that develops in the tonotopic map of A1. They support that A1 re-organization is sufficient first for the acquisition of specific memory (current findings), and also for the initial maintenance of specificity in recent auditory memory (Froemke et al., 2013).
Specificity of memory in individual subjects
It is noteworthy that detection of the match between cortical remodeling and behavioral memory required accounting for individual differences in learning (Fig. 3). While inexplicable individual differences in cortical specificity have been reported with similar NBstm treatment (e.g., Bao et al., 2003), they might be better understood given the additional determination of specifically what the animals had learned.
We presented test tones prior to training in order to best reveal the specificity of memory after training. Animals received nine tones (1.00–27.64 kHz), one of which was the CS frequency (3.66 kHz), ~20 times each, randomly intermixed (mean interval = 60 s), on the two days preceding the three days of training. This procedure better approximates the natural situation in which human and animal subjects have had relevant auditory experiences prior to a bout of training, rather than hearing pure tones as completely novel stimuli during training. Specificity of memory can be revealed by obtaining frequency generalization gradients (FGGs) before and after training, thereby yielding a difference generalization gradient (ΔFGG). The peak of the ΔFGG denotes the strongest frequency memory.
Prior reports emphasized that the effects of pairing tone and NBstm to implant memory were “specific” to the frequency of the conditioned stimulus. However, only in the first study of memory implantation (McLin et al., 2002) was the peak of the generalization gradient actually at the CS frequency. Unfortunately, this initial experiment lacked pre-training tones, so within-subjects ΔFGGs could not be obtained. Subsequent experiments used the same protocol as the current study, yielding more accurate determinations of tonal memory. Although they found that implanted memory was biased toward the CS tone, it was never at this frequency, but displaced ~0.25–0.35 octaves (Fig. 2C). Such “peak shift” recently was shown to be due to an interaction between prior auditory experience (pre-training tones) and the effects of learning (tone–NBstm pairing) (Miasnikov and Weinberger, 2012). This is compatible with the findings of Froemke et al. (2013) who also show evidence of behavioral variability, despite identical training and NB stimulation treatment, which appears to be dependent on an animal’s intervening experience with pure tones (see Froemke et al., 2013 Figs. 7H and 7I). In conjunction with the Miasnikov et al. (2013) study, the present findings that show a direct correlation between the specificity of memory and the specificity of map expansion actually posits a mechanism for variability induced by exposure to tonal stimuli outside of training per se. Thus, while training-induced bias to frequencies near the CS were sufficient in earlier studies to establish a degree of specificity both for NB implanted memory (e.g., Miasnikov et al., 2006), and areal expansion in A1 (e.g., Kilgard and Merzenich, 1998), a more precise match between cortical expansion and memory is necessary for the former to potentially explain the latter. The ΔFGG provides a level of behavioral specificity for implanted memory that satisfies this criterion. Thus, ascertaining the attributes of memory that are likely to be acquired by treatments that directly remodel cortical representations can both explicate resultant neural plasticity and reveal its links to memory. The current findings underscore the advantages of including analyses based on individual differences in learning and supplement standard approaches limited to the training stimulus.
Specificity of sensory cortical plasticity for memory
Traditional conceptions of primary sensory cortical fields have assumed their function to be purely sensory-perceptual. A growing body of research has enlarged their functions to learning, memory, attention and other cognitive processes (recently reviewed in Scheich et al., 2011; Weinberger 2011). Prior reports of adult primary sensory cortical plasticity have focused largely either on induction by experience/learning or by activation of brain systems, such as the NB. Within those contexts, and given the then surprising nature of sensory cortical malleability, it was sufficient to emphasize the specificity of effect with reference to the paired sensory training stimuli (e.g., CS). This was critical in establishing the candidacy of primary sensory fields as potential substrates of cognitive processes. That the degree of specificity was not always as great as assumed received less emphasis. However, as a further stage of inquiry concerns the relationship between representational plasticity and the actual contents of memory, the criteria for specificity must be more stringent, because non-matching sensory plasticity could not be a basis of a specific behavioral memory. Therefore, it would be helpful if future studies include an estimate of the actual degree of specificity between induced neural plasticity and resultant behavioral assessment of memory. Likewise, individual, as well as group analyses may prove revealing.
Conclusions
The current findings open a novel line of inquiry into the mechanisms by which an apparent increase in the number of neurons that become “tuned” to a sensory stimulus induces the initial formation of a corresponding behavioral memory. Insofar as increased representational area has been linked both to the acquired importance of a stimulus (Rutkowski and Weinberger, 2005) and the strength of the memory for that stimulus (Bieszczad and Weinberger, 2010c), perhaps cortical networks that command greater resources facilitate network synchrony (Goard and Dan, 2009) and thereby dominate in competition for behavioral control (Headley and Weinberger, 2011; 2013; Kilgard, 2012). Future circuit analyses, including determination of temporal dynamics, are needed to reveal mechanisms of synaptic plasticity for induction and maintenance of memory based on sensory cortex (Froemke et al., 2007). Likewise, synergistic engagement of multiple neuromodulatory mechanisms or of other factors in addition to the cortical cholinergic influence may contribute to the accuracy of sensory cortical plasticity (and resultant behavioral expression of memory) for the actual training signal experienced. Indeed, CS-specificity is possible and frequently observed in natural learning conditions during which various available mechanisms can become together engaged to dictate the sensory cortical plasticity (e.g., Shepard et al., 2012). The generality of cortically-based memory implantation is applicable to any sensory modality, e.g., by directly reorganizing representations in the visual and somatosensory cortices, also awaits investigation. Memory induction by remodeling of multi-modal cortical association fields also may occur.
Furthermore, the ability to induce specific memory by directed reorganization of auditory cortical representations has clinical implications. Stimulation-based treatments have already shown promise for cortical remodeling to alter perceptual functions (Reed et al., 2011), including the potential to reverse maladaptive effects of tinnitus (Engineer et al., 2011; 2012). However, individual differences indicate the need to adjust training regimens in behavior-based therapies for the success of effective and enduring effects. Reduction of potential harmful, e.g., phantom-limb like, effects of pain or aggravation that may result if map expansion develops “off-target” (i.e., for an unintended stimulus) (Lozano, 2011) may be achieved by taking advantage of individualized approaches that promote intended therapeutic functional benefits.
HIGHLIGHTS.
Pairing tone with nucleus basalis stimulation expands A1 frequency representation.
Pairing induces specific memory as detected in behavioral frequency generalization.
Individual cortical expansions predict individual specific frequency memory.
Directly remodeling sensory representations is sufficient to induce specific memory.
Acknowledgments
We thank Jemmy C. Chen for help with data analysis and figures, and also Jacquie D. Weinberger and Gabriel K. Hui for laboratory administrative assistance. This study was funded by (NIDCD/NIH) DC-010013 to NMW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Vol. 13. Nature Publishing Group; 2010. pp. 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. J Neurosci. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlau KM, Weinberger NM. Learning strategy determines auditory cortical plasticity. Neurobiol Learn Mem. 2008;89:153–219. doi: 10.1016/j.nlm.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Remodeling the cortex in memory: Increased use of a learning strategy increases the representational area of relevant acoustic cues. Neurobiol Learn Mem. 2010a;94:127–171. doi: 10.1016/j.nlm.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Learning strategy trumps motivational level in determining learning-induced auditory cortical plasticity. Neurobiol Learn Mem. 2010b;93:229–268. doi: 10.1016/j.nlm.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Representational gain in cortical area underlies increase of memory strength. Proc Natl Acad Sci USA. 2010c;107:3793–3798. doi: 10.1073/pnas.1000159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieszczad KM, Weinberger NM. Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci. 2012;35:598–613. doi: 10.1111/j.1460-9568.2011.07974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AE, Chavez CM, Flesher MM, Kinney-Hurd BL, Araujo GC, Miasnikov AA, Weinberger NM. Association learning-dependent increases in acetylcholine release in the rat auditory cortex during auditory classical conditioning. Neurobiol Learn Mem. 2009;92:400–409. doi: 10.1016/j.nlm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Chen G, Yan J. Cholinergic modulation incorporated with a tone presentation induces frequency-specific threshold decreases in the auditory cortex of the mouse. Eur J Neurosci. 2007;25:1793–2596. doi: 10.1111/j.1460-9568.2007.05432.x. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Petersen RS, Harris JA. Learning through maps: functional significance of topographic organization in primary sensory cortex. J Neurobiol. 1999;41:64–68. [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Thalamic short-term plasticity in the auditory system: Associative retuning of receptive fields in the ventral medial geniculate body. Behav Neurosci. 1991;105:618–639. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Edeline JM, Weinberger NM. Non-awaking basal forebrain stimulation enhances auditory cortex responsiveness during slow-wave-sleep. Brain Res. 1994;636:333–337. doi: 10.1016/0006-8993(94)91033-2. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Møller AR, Kilgard MP. Directing neural plasticity to understand and treat tinnitus. Hear Res. 2012:1–9. doi: 10.1016/j.heares.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins ARO, Zaika N, Bernstein H, Wachs M, Levis PA, Polley DB, Merzenich MM, Schreiner CE. Long-term modification of cortical synapses improves sensory perception. Nat Neurosci. 2013;16(1):79–88. doi: 10.1038/nn.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Goard M, Dan Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat Neurosci. 2009;12:1444–1449. doi: 10.1038/nn.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose. II. Auditory cortex plasticity. Behav Brain Res. 1986;20:281–374. doi: 10.1016/0166-4328(86)90228-7. [DOI] [PubMed] [Google Scholar]
- Guo W, Chambers AR, Darrow KN, Hancock KE, Shinn-Cunningham BG, Polley DB. Robustness of cortical topography across fields, laminae, anesthetic states, and neurophysiological signal types. J Neurosci. 2012;32:9159–9172. doi: 10.1523/JNEUROSCI.0065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Intskirveli I, Blake DT, Metherate R. Tone-detection training enhances spectral integration mediated by intracortical pathways in primary auditory cortex. Neurobiol Learn Mem. 2013;101:75–84. doi: 10.1016/j.nlm.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Gamma-band activation predicts both associative memory and cortical plasticity. J Neurosci. 2011;31:12748–12758. doi: 10.1523/JNEUROSCI.2528-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Fear conditioning enhances gamma oscillations and their entrainment of neurons representing the conditioned stimulus. J Neurosci. 2013;33:5705–5717. doi: 10.1523/JNEUROSCI.4915-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui GK, Wong KL, Chavez CM, Leon MI, Robin KM, Weinberger NM. Conditioned tone control of brain reward behavior produces highly specific representational gain in the primary auditory cortex. Neurobiol Learn Mem. 2009;92:27–34. doi: 10.1016/j.nlm.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. Habit. New York: H. Holt & Co; 1890. [Google Scholar]
- Jasper HH, Tessier J. Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science. 1971;172:601–602. doi: 10.1126/science.172.3983.601. [DOI] [PubMed] [Google Scholar]
- Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: Effect of atropine. J Neurophysiol. 2003;90:1904–1909. doi: 10.1152/jn.00363.2003. [DOI] [PubMed] [Google Scholar]
- Kilgard MP. Cortical map reorganization without cholinergic modulation. Neuron. 2005;48:529–530. doi: 10.1016/j.neuron.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Kilgard MP. Harnessing plasticity to understand learning and treat disease. TINS. 2012;35(12):715–722. doi: 10.1016/j.tins.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kisley M, Gerstein G. Daily variation and appetitive conditioning-induced plasticity of auditory cortex receptive fields. Eur J Neurosci. 2001;13:1993–3996. doi: 10.1046/j.0953-816x.2001.01568.x. [DOI] [PubMed] [Google Scholar]
- Lozano AM. Harnessing plasticity to reset dysfunctional neurons. N Engl J Med. 2011;364:1367–1368. doi: 10.1056/NEJMcibr1100496. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. Induction of behavioral associative memory by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 2002;99:4002–4007. doi: 10.1073/pnas.062057099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin DE, III, Miasnikov AA, Weinberger NM. CS-specific gamma, theta, and alpha EEG activity detected in stimulus generalization following induction of behavioral memory by stimulation of the nucleus basalis. Neurobiol Learn Mem. 2003;79:152–176. doi: 10.1016/s1074-7427(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, McLin DE, III, Weinberger NM. Muscarinic dependence of nucleus basalis induced conditioned receptive field plasticity. NeuroReport. 2001;12:1537–1542. doi: 10.1097/00001756-200105250-00047. [DOI] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008a;90:443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Gross N, Poytress BS, Weinberger NM. Motivationally neutral stimulation of the nucleus basalis induces specific behavioral memory. Neurobiol Learn Mem. 2008a;90:125–137. doi: 10.1016/j.nlm.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Rapid induction of specific associative behavioral memory by stimulation of the nucleus basalis in the rat. Neurobiol Learn Mem. 2006;86:47–65. doi: 10.1016/j.nlm.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Specific auditory memory induced by nucleus basalis stimulation depends on intrinsic acetylcholine. Neurobiol Learn Mem. 2008b;90:443–454. doi: 10.1016/j.nlm.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Chen JC, Weinberger NM. Consolidation and long-term retention of an implanted behavioral memory. Neurobiol Learn Mem. 2011;95:286–295. doi: 10.1016/j.nlm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miasnikov AA, Weinberger NM. Detection of an inhibitory cortical gradient underlying peak shift in learning: A neural basis for a false memory. Neurobiol Learn Mem. 2012;98:368–379. doi: 10.1016/j.nlm.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Petrie HL. Neuropsychology of Memory. New York, NY: Guilford; 1984. Memories and Habits: Some implications for the analysis of learning and retention; pp. 287–296. [Google Scholar]
- Mostofsky DI. Stimulus Generalization. Boston University; 1965. [Google Scholar]
- Ohl F, Scheich H. Learning-induced plasticity in animal and human auditory cortex. Curr Opin Neurobiol. 2005;15:470–477. doi: 10.1016/j.conb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ohl F, Scheich H, Freeman W. Change in pattern of ongoing cortical activity with auditory category learning. Nature. 2001;412:733–739. doi: 10.1038/35089076. [DOI] [PubMed] [Google Scholar]
- Polley D, Steinberg E, Merzenich M. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–5052. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett A, Pandya P, Moucha R, Dai W, Kilgard M. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98:253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheich H, Brechmann A, Brosch M, Budinger E, Ohl F, Selezneva E, Stark H, Tischmeyer W, Wetzel W. Behavioral semantics of learning and crossmodal processing in auditory cortex: the semantic processor concept. Hear Res. 2011;271:3–18. doi: 10.1016/j.heares.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Shepard KN, Kilgard MP, Liu RC. Springer Handbook of Auditory Research. New York, NY: Springer New York; 2012. Experience-Dependent Plasticity and Auditory Cortex; pp. 309–315. [Google Scholar]
- Sherrington CS. Experiments on the Value of Vascular and Visceral Factors for the Genesis of Emotion. Proceedings of the Royal Society of London. 1900;66:390–403. [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Super H. Cognitive processes in the primary visual cortex: from perception to memory. Rev Neurosci. 2002;13:287–298. doi: 10.1515/revneuro.2002.13.4.287. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Funamizu A, Mitsumori Y, Kose H, Kanzaki R. Progressive plasticity of auditory cortex during appetitive operant conditioning. Bio Systems. 2010;101:37–41. doi: 10.1016/j.biosystems.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Ann Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 1993;90:2394–2398. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM, Miasnikov AA, Chen J. The level of cholinergic nucleus basalis activation controls the specificity of auditory associative memory. Neurobiol Learn Mem. 2006;86:270–285. doi: 10.1016/j.nlm.2006.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Associative representational plasticity in the auditory cortex: A synthesis of two disciplines. Learn Mem. 2007;14:1–16. doi: 10.1101/lm.421807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Reconceptualizing the primary auditory cortex: Learning, memory and specific plasticity. In: Winer J, Schreiner CE, editors. The Auditory Cortex. New York: Springer; 2011. pp. 465–91. [Google Scholar]
- Yan J, Zhang Y. Sound-guided shaping of the receptive field in the mouse auditory cortex by basal forebrain stimulation. Eur J Neurosci. 2005;21:563–576. doi: 10.1111/j.1460-9568.2005.03878.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hamilton SE, Nathanson NM, Yan J. Decreased Input-Specific Plasticity of the Auditory Cortex in Mice Lacking M1 Muscarinic Acetylcholine Receptors. Cerebral Cortex. 2006;16:1258–1265. doi: 10.1093/cercor/bhj067. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yan J. Corticothalamic feedback for sound-specific plasticity of auditory thalamic neurons elicited by tones paired with basal forebrain stimulation. Cereb Cortex. 2008;18:1521–1528. doi: 10.1093/cercor/bhm188. [DOI] [PubMed] [Google Scholar]