Abstract
It is well established that social recognition memory is mediated, in part, by arginine vasopressin (AVP). AVP cells within the bed nucleus of the stria terminalis (BST) and medial amygdala (MeA) send AVP-ergic projections to the lateral septum (LS). We have demonstrated that progesterone treatment decreases AVP immunoreactivity within the BST, the MeA and the LS, and that progesterone treatment impairs social recognition. These data suggested that progesterone may impair social recognition memory by decreasing AVP. In the present experiment, we hypothesized that infusions of AVP into the LS would rescue the progesterone induced impairment in social recognition within adult male rats. One week after adult male rats underwent cannula surgery, they were given systemic injections of either a physiological dose of progesterone or oil control for three days. Four hours after the last injection, we tested social recognition memory using the social discrimination paradigm, a two-trial test that is based on the natural propensity for rats to be highly motivated to investigate novel conspecifics. Immediately after the first exposure to a juvenile, each animal received bilateral infusions of either AVP or artificial CSF (aCSF) into the LS. Our results show that, as expected, control animals exhibited normal social discrimination. In corroboration with our previous results, animals given progesterone have impaired social discrimination. Interestingly, animals treated with progesterone and AVP exhibited normal social discrimination, suggesting that AVP treatment rescued the impairment in social recognition caused by progesterone. These data also further support a role for progesterone in modulating vasopressin dependent behavior within the male brain.
Keywords: vasopressin, progesterone, social recognition behavior, social memory, lateral septum
Introduction
Social recognition allows animals to distinguish friend from foe and family member from stranger. Short-term social recognition is a way to differentiate between previously encountered conspecific individuals (Ferguson et al., 2002). The role of arginine vasopressin (AVP) in the regulation of this behavior has been studied for decades, and these studies indicate that AVP is important for normal social recognition in intact, adult male rats (Ferguson et al., 2002). In male rats, the social memory of a familiar conspecific seems to be maintained for at least 30–60 minutes (Thor and Holloway, 1982) after an initial encounter. Infusion of an AVP agonist into the lateral septum (LS) enhances while antagonist impairs this memory (Dantzer et al., 1988).
Dantzer et al. (1987) found that non-treated control rats do not maintain a social memory 120 minutes after an initial encounter. However, rats treated with AVP do demonstrate social recognition memory of a previously encountered juvenile even after this lengthened intertrial interval (Dantzer et al., 1987; Le Moal et al., 1987). These data suggest that increasing AVP availability in the brain can maintain social olfactory based recognition(Le Moal et al., 1987). Conversely, administration of a V1a receptor antagonist impairs social recognition memory for a familiar juvenile within 30 minutes after initial exposure. Engelmann and Landgraf (1994) found that Brattleboro rats demonstrated impaired social recognition, even 30 minutes after an initial encounter with a juvenile rat (Engelmann and Landgraf, 1994). Brattleboro rats, an inbred strain that spontaneously arose from Long-Evans rats, do not synthesize biologically active AVP in the brain (Engelmann and Landgraf, 1994). Importantly, the impairment in social recognition in Brattleboro rats was rescued with site specific microdialysis treatment with synthetic AVP into the LS (Engelmann and Landgraf, 1994). Additionally, recent data indicate that in intact animals AVP is released in the LS during the acquisition of a social recognition memory (Lukas et al., 2011). These data not only provide additional evidence for the importance of AVP in social recognition, but also the importance of AVP within the LS in social recognition.
The LS, which contains AVP receptors (Tribollet et al., 1988), receives AVPergic projections from neurons located in the bed nucleus of the stria terminalis (BST) and the medial amygdala (MeA). We have shown that the AVP cells in the BST and MeA are virtually 100% co-localized with progestin receptors (Auger and De Vries, 2002). Indeed, treatment with progesterone functions to reduce AVP protein levels in male rats within the BST and MeA as well as AVP fiber density in the LS (Auger and Vanzo, 2006). As progesterone decreased AVP levels, we previously examined the impact of progesterone on regulating social recognition memory. We found that progesterone treatment impaired social recognition memory in a social discrimination task (Bychowski and Auger, 2012). Here we hypothesize that the mechanism by which progesterone impairs social recognition is through reduction in AVP levels. Indeed, we tested if the impairment in social discrimination seen in progesterone treated rats could be rescued by infusion of AVP into the LS. We replicated our previous findings that progesterone impairs social recognition memory and we extended these findings by demonstrating that infusions of AVP into the LS can rescue the impairments in social recognition induced by progesterone treatment.
Experimental Procedures
Animals
Twenty, three-month-old male Sprague-Dawley rats were bred in our animal facility from breeding stock obtained from Charles River (Charles River Laboratories, Inc., Wilmington, MA). Juvenile male stimulus animals (between 20–30 days old) were purchased directly from Charles River. All animals were group housed in our animal facility on a reversed 12:12 light/dark cycle, with lights off at 11:00 am, and had free access to food and water. This research was approved by the University of Wisconsin Animal Care and Use Committee.
Treatment paradigm and drugs
Twenty male rats were separated into 2 groups of 10 animals each, 1 group was pretreated with progesterone and the other was pretreated with oil. Progesterone pretreated animals were then infused with either AVP or aCSF, and control oil pretreated animals were infused with either AVP or aCSF and tested for social recognition. Three weeks later, in a counter balanced design, animals were reassigned to different infusion groups and re-tested. In this way we were able to maintain 9–10 animals in each treatment group (oil-aCSF, oil-AVP, progesterone-aCSF, progesterone-AVP; see experimental procedure for detail of timing of infusions during behavior testing). Animals were pretreated treated with progesterone (Steraloids, Newport R.I.; 1 mg), dissolved in 0.1 mL of sesame oil, or 0.1 mL of sesame oil. The dose of 1mg progesterone was used because we have empirically shown the levels produced by this dose are similar to those physiological levels of progesterone that are observed following stress in male rats(Andersen et al., 2004; Auger et al., 2006). Following trial one of the social discrimination task, animals were infused with an AVP agonist (Deamino-Cys1,D-Arg8)-Vasopressin (0.1 ng/0.5 µL; Bachem, Torrance, CA) or aCSF (0.5 µL) into the lateral septum (as described below). The dose of AVP used was based on previous studies that showed an enhancement of social recognition in animals infused with AVP (Dantzer et al., 1988; Le Moal et al., 1987). All infusions occurred during the "lights off" portion of the light cycle.
Cannula surgery
Rats were anesthetized with Isofluorane gas and secured in a Kopf stereotaxic frame. Before surgery commenced, rats were given subcutaneous injections of ampicillin (30 mg/kg; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO) and ketoprofin (200 µg/0.1 mL; MP Biomedicals, Inc. Aurora, OH). The toothbar was set at −4.0 mm below the interaural line for all surgeries. Bilateral stainless steel cannulae (10 mm long, 23 gauge) were implanted according to standard stereotaxic procedures. To target the lateral septum, cannulae were placed at an 8 degree angle to avoid the lateral ventricle. Coordinates of the injection site were AP: −0.2 mm from bregma, ML: ±1.32 mm from midline, DV: −5 mm from skull surface. Self-made cannulae were fixed to the skull 2 mm above the target site with cranial plastic (Esschem, Linwood, PA) and anchoring screws (McMaster-Carr, Chicago, IL). Wire stylets (10 mm long, 30 gauge) were placed in the cannulae to maintain patency. Rats were returned to their home cages upon awakening and given a recovery period of no less than five days (with daily health checks) before behavioral testing began. Sepsis did not occur and no changes in behavior were observed. Upon sacrifice of the animals and examination of the brains, at the completion of behavioral testing, there appeared to be to no tissue reaction or damage around the cannula site.
Microinfusion procedure
Intracerebral microinfusions occurred immediately after the test animal completed trial 1 of the social discrimination paradigm (further described below in procedure). Stylets were removed from the guide cannulae and stainless steel injectors, connected via polyethylene tubing (PE-10, Becton Dickinson, Sparks, MD) to 10 µL capacity Hamilton syringes (Hamilton, Reno, NV) on a Harvard microdrive pump, were lowered through the cannulae to the site of infusion. The flow rate for infusions was 0.32 uL/min. The total infusate volume for the bilateral infusions was 0.5 µL/side. After infusions, injectors were left in place for an additional minute to allow for diffusion of the injectate into the tissue. Injectors were then removed, and wire stylets replaced. The entire infusion procedure took approximately 6 minutes per animal.
Animals were handled every day between surgery and behavioral testing. On the first day of progesterone or oil injection, wire stylets in cannulae were removed and replaced. On the second day of injections, animals received infusions of saline into the LS. This procedure functioned to familiarize the rats with the minimal restraint associated with the microinfusion procedure as well as minimize any adverse effects of infusion procedure on tissue damage that could influence behavioral outcome.
Verification of cannula placement
At the end the experiment, rats were deeply anesthetized with Isofluorane and perfused transcardially with a 4% formaldehyde solution. Brains were collected and stored in 4% formaldehyde. Coronal sections (60 µm) were cut through the infusion site on a cryostat microtome, collected on slides, stained with cresyl violet, and subsequently reviewed to verify correct placement of the injections. Images of representative sections were captured using Scion Image software on a computer interfaced with a microscope-mounted Hitachi HV-C20 CCD camera. All animals were found to have the tip of the injector positioned correctly within the lateral septum, as determined with the aid of the rat brain atlas of Paxinos and Watson (Paxinos and Watson, 1986).
Behavioral testing and statistical analyses
Testing took place during the “lights off” portion of the light cycle under dim red light in our behavior room. All behavior was digitally recorded and then analyzed by a trained researcher blind to all treatments using The Observer (Noldus Information Technologies, Leesburg, VA) behavioral observation software. Sigma Stat 3.5 was used to conduct all statistical analyses. Paired t-tests were used to statistically analyze amount of time spent investigating novel and familiar juveniles in trial 2. Also, ratio of investigation duration (RID) scores of the familiar/novel juvenile were analyzed using a one-way ANOVA. A One Way ANOVA was also used to analyze total investigation time in trial 2 (i.e., investigation of novel + familiar juvenile). Outliers were determined using Grubbs outlier test. One outlier was removed from the RID analysis. This animal was in the progesterone-AVP group.
Procedure: Social discrimination, progesterone and AVP infusion
There are several widely accepted behavioral paradigms for studying social recognition. The paradigm used in this experiment is a two-trial social discrimination paradigm based on the social recognition test described previously (Engelmann et al., 1995; Thor and Holloway, 1981; Thor and Holloway, 1982).
On day 1 of treatment, rats were separated from their cage mates and singly housed, also on this day; animals were pretreated with progesterone or oil for 3 consecutive days. On the third day of progesterone or oil pretreatment, animals underwent social discrimination testing 4 hours after the last round of pretreatment injections. On trial 1 of this test, a male juvenile stimulus rat (between 20–30 days old) was placed in the home cage of the adult rat and the adult was allowed to freely investigate both the juvenile and the cage for 5 minutes. After the 5 minute investigation period, the juvenile was removed from the cage and the adult began a 30 minute intertrial interval. As soon as the intertrial interval began, the adult was removed from the cage to undergo the microinfusion procedure, as described above. After the 30 minute intertrial interval elapsed, the juvenile from trial 1 plus a novel juvenile were placed in the adult’s cage, and the adult was again free to investigate both individuals as well as the cage for 5 minutes. Discrimination of the novel versus familiar juveniles is indicated by a significant decrease in the amount of time the adult rat spends investigating the familiar juvenile and an increase in the amount time investigating the novel juvenile. The juvenile rats were distinguishable to the researcher scoring the video by unique tail marks drawn with low odor permanent marker no less than 30 minutes prior to trial 1 of social recognition testing. No residual odor from the marker was detected by the experimenter at the beginning of the experiment. Both the novel and familiar juveniles were marked with tail marks (either one or two rings around the tail), and these marks were counterbalanced between the novel and familiar juvenile across groups. Adult investigation of the juvenile(s) was scored to include direct contact between the nose of the adult and the body of the juvenile and close following behavior.
Results
Social discrimination, progesterone and AVP
We have previously shown that progesterone treatment impairs social discrimination, and we hypothesize that this effect of progesterone on behavior is due to its suppression of AVP levels, as we have seen previously. Therefore, it was our goal to determine if administration of AVP into the lateral septum could rescue the impairment in social recognition. Using the typical social discrimination paradigm, control animals (oil + aCSF and oil + AVP) discriminated normally. That is, adult males spent significantly more time investigating the novel juvenile than familiar juvenile in trial 2 (p = 0.048, p = 0.003, respectively; Fig. 1a and 1b). Similar to our previous data (Bychowski and Auger, 2012), animals in the progesterone + aCSF group failed to discriminate normally (p = 0.639, Fig. 1c), as progesterone appeared to impair social discrimination. Animals in the progesterone + AVP group demonstrated normal social discrimination (p = 0.045, Fig. 1d), indicating that AVP administration successfully rescued the deficit in social recognition memory seen in progesterone treated rats. Figure 2 is a representative photomicrograph showing the cannula tract aimed at the LS (Fig. 2). Figure 3 shows a schematic diagram summarizing injector tip placement in the LS (Fig 3). A ratio of investigation (RID) score (investigation of familiar juvenile/investigation of novel juvenile) was also calculated to directly compare the treatment groups. The RID scores of animals pretreated with progesterone and later infused with aCSF were significantly higher than the RID scores in all the other groups (F (3,31)=4.278, p=0.012). Higher RID values, or those at or above 1.0 are indicative of there being little difference between the amount of time the adult experimental animal spent investigating the familiar juvenile compared to the novel one (Fig. 4).
Figure 1.
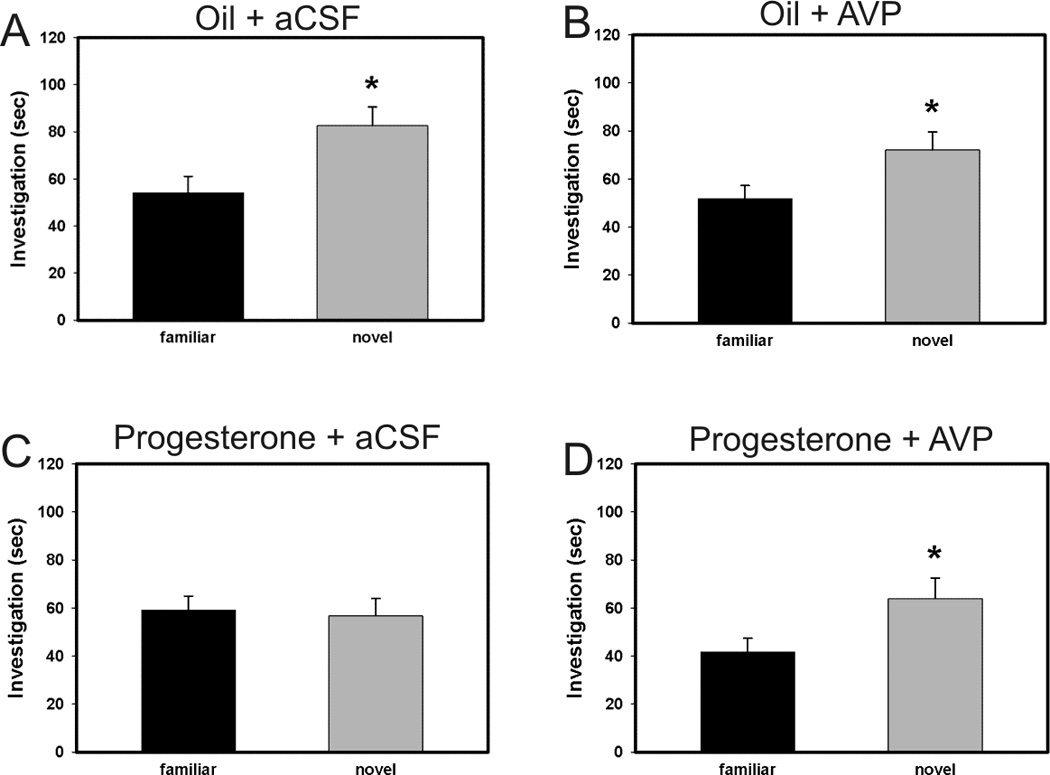
Effect of treatment with progesterone or oil and infusion of AVP or aCSF into LS on social recognition in the social discrimination paradigm. Investigation of juvenile rats in trial 2. (A) Animals in the oil + aCSF group discriminated normally (significantly more investigation of familiar juvenile than novel juvenile, (* p = 0.048). (B) Animals in the oil + AVP group discriminated normally (* p = 0.003). (C) Animals in the progesterone + aCSF group failed to discriminate normally (p = 0.639). (D) Animals in the progesterone group discriminated normally (* p = 0.045), indicating that AVP administration successfully rescued the deficit in social recognition memory seen in progesterone treated rats. Bars represent mean and SEM.
Figure 2.
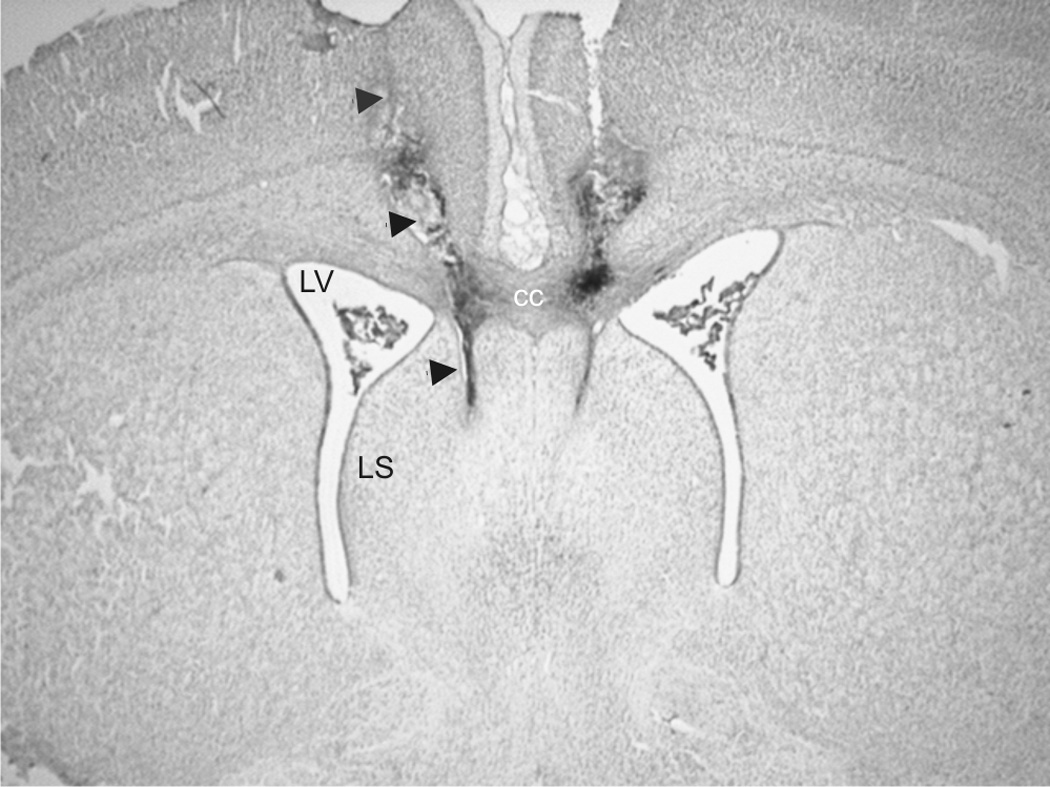
Representative photomicrograph of infusion placements into the male rat brain that target the LS. Abbreviations: cc- corpus callosum, LV- lateral ventricle, LS- lateral septum.
Figure 3.
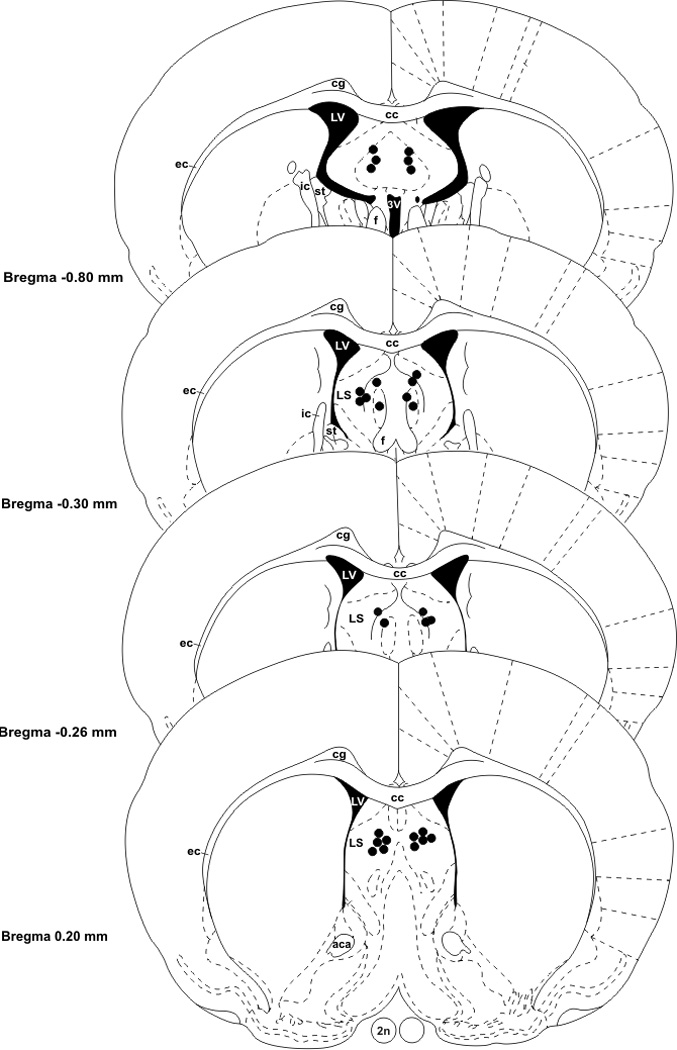
Schematic diagram indicating infusions sites in the LS. Abbreviations: 2n-optic nerve, 3V- 3rd ventricle, aca- anterior commissure, anterior part, cc- corpus callosum, cg- cingulum, ec- external capsule, f- fornix, ic- internal capsule, LV- lateral ventricle, LS- lateral septum, st- stria terminalis.
Figure 4.
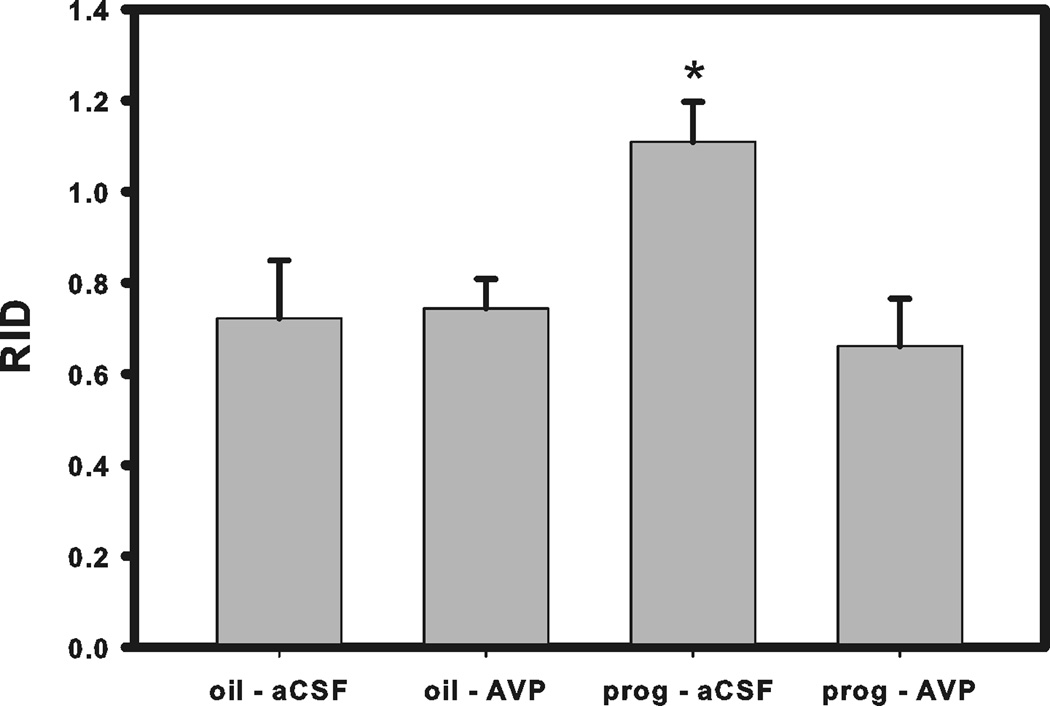
The effect of AVP receptor agonist on social recognition. Ratio of investigation (RID) scores; higher ratios indicate impaired social discrimination performance. Bars represent mean and SEM.
In order to examine if our agonist treatment, given immediately after trial 1, influenced any subsequent behavior in trial 2, we analyzed total investigation by the adult experimental animal of both the novel and familiar juveniles. This analysis revealed that there was no difference in the amount total investigation time in trial 2 as a result of any of the infusion treatments (F (3,35) = 1.376, p = 0.268; data not shown).
Discussion
Our data provide strong evidence that progesterone, by acting on the AVP system, can influence social behavior in male rats. We have shown in a previous study (Bychowski and Auger, 2012), that progesterone impairs social recognition. We confirm and extend these results by demonstrating that AVP infusion into the LS rescued this progesterone-induced impairment in social discrimination. As we have shown that progesterone reduces AVP in the BST and MeA, and that progesterone impairs social recognition behavior in adult male rats, these data provide a strong link between progesterone’s actions and their potential functional significance for AVP-linked social behavior in males (Auger and Vanzo, 2006) (Bychowski and Auger, 2012).
The role that AVP plays in the regulation of social recognition behavior has been investigated for over 30 years. A subcutaneous injection of AVP enhances social recognition when the period between the first exposure to a juvenile and the second exposure is lengthened to 2 hours(Dantzer et al., 1987). Also, social recognition behavior is impaired by infusions of AVP antagonists into the LS, one of the projection sites of the AVP cells in the BST and MeA (Dantzer et al., 1988). It has also been shown that castration, which depletes AVP in the BST and MeA and in the projection sites of these cells, blocks social recognition (Bluthe et al., 1990). These studies illustrate the basic importance of AVP in social recognition. Interestingly, AVP does not seem to be important in all forms of recognition.
Studies have demonstrated that AVP is not involved in non-social investigatory behavior. A study by Everts and Koolhaas (1997) examined a simple two-trial recognition test that used two objects instead of two juveniles. In this study, they found that control rats recognized a familiar object upon second exposure after a 30 minute intertrial interval (as evidenced by decreased investigation of the familiar object in trial 2). Unlike social recognition, animals infused with an AVP antagonist showed no impairment in object recognition (Everts and Koolhaas, 1997). These findings are similar to previous data from our laboratory demonstrating that systemic progesterone treatment, which has been demonstrated to suppress AVP levels, did not affect object recognition, although the same treatment impaired social recognition memory (Bychowski and Auger, 2012). It is likely that progesterone is influencing social recognition by directly acting on the AVP system, as progesterone treatment suppresses AVP levels in the BST and MeA as well as the projection sites of these cells (Auger and Vanzo, 2006). These data also further support the concept that progesterone serves an important regulatory function within the male brain.
Although the mechanism by which progesterone acts on AVP is uncertain, the notion that progesterone levels are impacted in male rats under a number of circumstances is clear. Progesterone levels can be sensitive to changes in social and environmental conditions, and this suggests a physiological mechanism by which progesterone can influence social memory. For example, stress inducing situations, like foot shock, cause a significant increase in progesterone levels in male rats (Andersen et al., 2004). Also, animals sleep deprived for more than 24 hours had significantly elevated serum levels of progesterone compared to control animals (Andersen et al., 2005). These authors suggested that the stress of sleep deprivation induces changes in progesterone. Similarly, Persengiev, Kanchev and Vezenkova (1991) not only found that sleep deprivation significantly elevated progesterone levels, but constant light conditions also impacted these levels in male rats (Persengiev et al., 1991). These data suggest that environmental changes, some very common, can induce changes in levels of progesterone. These changes in progesterone levels could potentially alter AVP levels, resulting in altered social functioning. While these data suggest that stressful situations can elevate progesterone levels in the blood, it is important to note that inevitable biological processes unrelated to stress can also influence progesterone levels.
Normal non-stress progesterone levels in young, male rats can vary over the course of the day (Simpkins et al., 1981). In fact, repeated blood sampling showed that plasma progesterone levels increased 2.5-fold from lowest to highest levels over a 24 hour period in 3–4 months old rats (Simpkins et al., 1981). It has also been shown that levels of progesterone can change as animals age. Plasma progesterone levels reached highs and lows at the same times of day as seen in young rats but at a greater than 4-fold increase in 19–20 month old rats (Simpkins et al., 1981). In another study, serum progesterone levels were significantly increased in intact 24 month old rats compared to 3 or 12 month old rats (Gruenewald et al., 1992). These authors suggest that the increases in progesterone are due to age-related changes in endocrine physiology. As these elevated levels of progesterone in aged animals may influence socially-relevant AVP levels in the brain, it would be interesting to see if these changes in hormonal profiles play a role in the age-related decline in recognition behavior.
Compared to young rats, control aged rats are unable to successfully discriminate between familiar and unfamiliar chambers in a simple olfactory discrimination task, and more importantly, between novel and familiar animals in a social recognition task (Prediger et al., 2005; Prediger and Takahashi, 2005). Other studies have also shown a decline in recognition memory with age that do not seem to be linked to a reduced olfactory ability (Terranova et al., 1994). The decline in social recognition ability seen in aged animals may be dependent on the strain of rats used, as not all strains show this deficit. Nevertheless these data indicate that normal social and olfactory recognition abilities can decline with age. Whether or not this decline in ability is associated with impaired aging in rats is unclear. However, these data do point toward a potential physiological mechanism by which changes in progesterone levels can act on the brain to impact specific behaviors.
In summary, we have shown that a progesterone-induced impairment in social recognition behavior in male rats can be rescued by infusing an AVP agonist into the LS. These data provide a strong link between progesterone action on AVP in the BST and MeA and its influence on behavior within the male brain
Progesterone treatment impairs social recognition by acting on AVP.
We examined if AVP treatment would rescue progesterone impairment of social recognition.
AVP treatment rescued the impairment in social recognition caused by progesterone.
The data support a role for progesterone in modulating vasopressin linked behavior in male brain.
Acknowledgements
The authors would like to thank Brooke E. Schmeichel and Craig W. Berridge for training on stereotaxic technique. This work was supported by National Institute of Mental Health – National Research Service Award (NRSA) T32 Gm007507, a National Science Foundation (NSF) Grant IOS-1122074 to CJA as well as the Psychology Department, the Graduate School and the College of Letters and Sciences at the University of Wisconsin-Madison.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andersen ML, Bignotto M, Machado RB, Tufik S. Different stress modalities result in distinct steroid hormone responses by male rats. Braz J Med Biol Res. 2004;37:791–797. doi: 10.1590/s0100-879x2004000600003. [DOI] [PubMed] [Google Scholar]
- Andersen ML, Martins PJ, D'Almeida V, Bignotto M, Tufik S. Endocrinological and catecholaminergic alterations during sleep deprivation and recovery in male rats. J Sleep Res. 2005;14:83–90. doi: 10.1111/j.1365-2869.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Auger CJ, De Vries GJ. Progestin receptor immunoreactivity within steroid-responsive vasopressin-immunoreactive cells in the male and female rat brain. J Neuroendocrinol. 2002;14:561–567. doi: 10.1046/j.1365-2826.2002.00809.x. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Jessen HM, Auger AP. Microarray profiling of gene expression patterns in adult male rat brain following acute progesterone treatment. Brain Res. 2006;1067:58–66. doi: 10.1016/j.brainres.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Auger CJ, Vanzo RJ. Progesterone treatment of adult male rats suppresses arginine vasopressin expression in the bed nucleus of the stria terminalis and the centromedial amygdala. J Neuroendocrinol. 2006;18:187–194. doi: 10.1111/j.1365-2826.2005.01400.x. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–157. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Bychowski ME, Auger CJ. Progesterone impairs social recognition in male rats. Horm Behav. 2012;61:598–604. doi: 10.1016/j.yhbeh.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe RM, Koob GF, Le MM. Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology (Berl) 1987;91:363–368. doi: 10.1007/BF00518192. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthe R-M, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in brattleboro rats. Phsy Beh. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Lateral septal vasopressin in rats: role in social and object recognition? Brain Res. 1997;760:1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Hess DL, Wilkinson CW, Matsumoto AM. Excessive testicular progesterone secretion in aged male Fischer 344 rats: a potential cause of age-related gonadotropin suppression and confounding variable in aging studies. J Gerontol. 1992;47:B164–B170. doi: 10.1093/geronj/47.5.b164. [DOI] [PubMed] [Google Scholar]
- Le Moal M, Dantzer R, Michaud B, Koob GF. Centrally injected arginine vasopressin (AVP) facilitates social memory in rats. Neurosci Lett. 1987;77:353–359. doi: 10.1016/0304-3940(87)90527-1. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendo. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Orlando: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Persengiev S, Kanchev L, Vezenkova G. Circadian patterns of melatonin, corticosterone, and progesterone in male rats subjected to chronic stress: effect of constant illumination. J Pineal Res. 1991;11:57–62. doi: 10.1111/j.1600-079x.1991.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging. 2005;26:957–964. doi: 10.1016/j.neurobiolaging.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Takahashi RN. Modulation of short-term social memory in rats by adenosine A1 and A(2A) receptors. Neurosci Lett. 2005;376:160–165. doi: 10.1016/j.neulet.2004.11.049. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Kalra PS, Kalra SP. Alterations in daily rhythms of testosterone and progesterone in old male rats. Exp Aging Res. 1981;7:25–32. doi: 10.1080/03610738108259783. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Perio A, Worms P, le FG, Soubrie P. Social olfactory recognition in rodents: deterioration with age, cerebral ischaemia and septal lesion. Behav Pharmacol. 1994;5:90–98. [PubMed] [Google Scholar]
- Thor DH, Holloway WR. Persistence of social investigatory behavior in the male rat: Evidence for long-term memory of initial copulatory experience. Animal Learning and Behavior. 1981;9:561–565. [Google Scholar]
- Thor DH, Holloway WR. Social memory of the male laboratory rat. J of Comp Phys Psych. 1982;96:1000–1006. [Google Scholar]
- Tribollet E, Barberis C, Jard S, Dubois-Dauphin M, Dreifuss JJ. Localization and pharmacological characterization of high affinity binding sites for vasopressin and oxytocin in the rat brain by light microscopic autoradiography. Brain Res. 1988;442:105–118. doi: 10.1016/0006-8993(88)91437-0. [DOI] [PubMed] [Google Scholar]


