Abstract
NK cells play an important role in innate immune control of the infection with vaccinia virus (VV). However, it remains incompletely defined how the activation of NK cells in response to VV is regulated. In this study, we showed that STAT1 was critical for NK cell activation upon VV infection and the subsequent clearance of VV infection in vivo. We further demonstrated that STAT1 signaling in both NK and accessory cells such as DCs was required for efficient NK cell activation upon VV infection. Mechanistically, STAT1 signaling in DCs promoted the expression of NKG2D ligands, which is required for NK cell activation via the NKG2D pathway. Taken together, our data suggest that STAT1 mediates anti-VV effect by promoting NK cell activation through both NK-intrinsic and extrinsic mechanisms and may provide insights into the design of effective NK cell-based therapies for viral infections.
Introduction
NK cells represent an important component of the innate immune system and play a critical role in antiviral responses (1, 2). NK cells are essential for the control of poxviruses. Upon poxviral infection, NK cells are activated and accumulate at the site of infection, leading to effective viral control (3–6). Vaccinia virus (VV) is the most studied member of the poxvirus family and is the live vaccine responsible for the successful elimination of smallpox in the late 1970s (7). Recent studies have shown that effective activation of NK cells and subsequent control of VV infection in vivo are dependent on multiple pathways including both the type I IFN and NKG2D pathways (5, 6, 8). However, how these different pathways are coordinated to achieve efficient NK cell activation in response to VV infection remains incompletely defined.
STAT1 belongs to the STAT family that consists of seven members (9). Among the STAT family members, STAT1 is the best characterized transcription factor that regulates many of the biological effects mediated by type I IFNs and other cytokines in response to viral infections in vivo (10). Indeed, studies have shown that STAT1-deficient (STAT1−/−) mice are highly susceptible to viral infections (11, 12). This is due, in part, to lack of direct antiviral defense mediated by type I IFNs. In addition, STAT1 signaling is also required for the activation of NK cells (13, 14). However, it remains to be defined the precise role of STAT1 in mediating NK cell activation in response to viral infection in vivo.
In this study, we showed that NK cell activation and function were severely compromised in STAT1−/− mice, leading to impaired viral clearance, and lethality upon live VV infection. We further demonstrated that STAT1 signaling in both NK cells and accessory cells such as dendritic cells (DCs), was critical for efficient NK cell activation in vitro. Similarly, STAT1 signaling in NK and non-NK cells was required for NK cell activation in vivo. Furthermore, STAT1−/− DCs failed to upregulate NKG2D ligand expression, which is required for efficient NK cell activation upon VV infection via the NKG2D pathway. Collectively, the data presented here suggest a critical role for both NK cell-intrinsic and –extrinsic STAT1 signaling in NK cell response to VV infection.
Materials and Methods
Mice
Wild-type (WT) 129/Sv mice were obtained from Charles River Breeding Laboratories. STAT1−/− mice on the 129/Sv background were purchased from Taconic. All mice used in this study were between 6 and 8 wk of age. Experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Duke University (Durham, NC).
Antibodies and flow cytometry
PE-conjugated anti-CD49b (clone DX5), PE-Cy5-conjugated anti-CD3e (clone 145 -2C11), APC-conjugated anti-IFN-γ, APC-conjugated anti-CD3e (clone 145-2C11), biotin-conjugated anti-Ly49C/I (clone 5E6), PE-Cy5-conjugated Streptavidin, and FITC-conjugated anti-CD11c (clone HL3) were purchased from BD Biosciences (San Diego, CA). PE -Cy5-conjugated anti-B220 (clone RA3-6B2), PE-conjugated anti-Rae-1 (clone CX1), FITC-conjugated anti-CD49b (clone DX5), FITC-conjugated anti-Granzyme B (GRB, clone NGZB), biotin -conjugated anti-NKG2D (clone CX5), and PE-Cy7-conjugated anti-GRB (clone NGZB) were purchased from eBioscience (San Diego, CA). To assess the production of IFN-γ and Granzyme B intracellularly, splenocytes were incubated with live VV at a MOI of 0.1 for 48 hr at 37°C. 5 μg/ml Brefeldin A was added during the last 6 h r of incubation, and intracellular staining was performed as previously described (15).
NK cell proliferation was assessed by BrdU incorporation. Briefly, 2 mg BrdU was injected intraperitoneally in each mouse 1 hr before sacrifice and incorporated BrdU was detected by a FITC-conjugated anti -BrdU antibody after DNA digestion, according to the manufacturer’s instruction (FITC BrdU Flow Kit, BD Biosciences, San Diego, CA). FACS Canto (BD Biosciences, San Diego, CA) was used for flow cytometry event collection, which was analyzed using FACS DiVA software (BD Biosciences, San Diego, CA).
Vaccinia virus
The Western Reserve (WR) strain of VV was purchased from American Type Culture Collection (ATCC, Manassas, VA). VV was grown in TK-143B cells (ATCC) and purified by a 35% sucrose cushion as described (16). The titer was determined by plaque assay on TK-143B cells and VV was stored at −80°C until use. For in vivo studies, 2 × 106 pfu, or as indicated, of live VV in 0.05 ml of 1 mM Tris pH 9.0 was injected intraperitoneally.
Ovarian VV titer assay
Viral load in the ovaries was measured by plaque-forming assay as described (5). In brief, female mice were sacrificed 36 h after infection, and ovaries were harvested and stored at −80°C. In some experiments, mice were depleted of NK cells by two intravenous injections of anti-Asialo GM1 antibody (Wako Chemicals USA, Richmond, VA) before infection. Ovaries from individual mice were homogenized and freeze-thawed three times. Serial dilutions were performed and plated on confluent TK-143B cells. And, viral titers were determined 2 days later by crystal violet staining.
NK cell cytotoxicity assay
Splenocytes were enriched for CD49b+ NK cells by positive selection with PE -conjugated anti- CD49b and anti-PE microbeads (Miltenyi Biotec, Auburn, CA). CD49b+ splenocytes were then incubated with 51Cr-labeled NK sensitive targets, YAC-1 cells (ATCC) at different effector: target ratios for 4 hr at 37°C (5). The supernatants obtained after the incubation were subjected to gamma counting. The maximum or spontaneous release was defined as counts from samples incubated with 1% SDS or medium alone, respectively. The cytolytic activity was calculated with the following formula: percentage specific lysis = (experimentalcpm − spontaneouscpm) release × 100/(maximumcpm − spontaneouscpm) release.
NK-DC in vitro co-culture system
DCs were generated from the bone marrow of WT or STAT1−/− mice in the presence of GM -CSF and IL-4 as described (5). In brief, bone marrow cells were harvested from femurs and tibiae of mice and cultured in the presence of mouse GM-CSF (1,000 U/ml) and IL-4 (500 U/ml) (R&D Systems, Minneapolis, MN) for 5 days. GM -CSF and IL-4 were replenished on days 2 and 4. On day 5, CD11c+ DCs were harvested for NK cell stimulation. In another series of experiments, 2 × 105CD11c+ DCs from WT or STAT1−/− mice were incubated 24 h with live VV at a MOI of 0.1 or with 100 ng/ml IFN-α in a final volume of 200 μl.
NK-DC co-culture was performed as described (17) with some modification s. In brief, purified CD49b+CD3− NK cells (2 × 105) were co-cultured with CD11c+ DCs (1 × 105) at an NK:DC ratio of 2:1. The co-culture was subsequently infected with VV with MOI of 1, relative to DCs, for 24 hr at 37°C. To assess the intracellular production of IFN-γ and Granzyme B, 5 μg/ml Brefeldin A was added during the last 6 hr of incubation, and intracellular staining was performed as previously described (18).
In vivo transfer experiments
CD49b+CD3− NK cells were purified from naïve WT or STAT1−/− mice, and labeled with 3.3 mM CFSE. Thereafter, 2.5 × 105 WT or STAT1 −/− NK cells were administered intravenously to WT or STAT1−/− recipients as indicated. The recipients were immediately infected by intraperitoneal injection of 2 × 106 pfu of live VV and splenocytes were harvested 36 hr later for analysis.
Quantitative real-time PCR
Total RNA was extracted from CD11c+ DCs with TRIzol solution (Invitrogen). Reverse transcription was carried out using the ImProm-II reverse-transcription system (Promega) and random primers. Oligonucleotide primers were as followed: IL-15 (FWD: 5′-TAACAGCTCAGAGAGGTCAGGA-3′; REV: 5′-GCTGACATGGGTTTCTGTGTTC-3′); IL-12 (FWD: 5′-GAGTGGGATGTGTCCTCAGAAG -3′; REV: 5′-GCCTATGACTCCATGTCTCTGG-3′); Rae1 (FWD: 5′-CTATGGATACACCAACGGGCT- 3′; REV: 5′-ACTTCGCTTCATACCAGAGAGG-3′); Mult1 (FWD: 5′-TTCAAAGGTCTGCTGCTTCAC-3′; REV: 5′-GGCTAACAATGGCGTATCCTG-3′); Actin (FWD: 5′-AGCCATGTACGTAGCCATCC-3′; REV: 5′-CTCTCAGCTGTGGTGGTGAA- 3′). Quantitative PCR was performed with the MyiQ iCycler system (Bio-Rad) and the iQ SYBR Green PCR mastermix (Bio-Rad). All experiments were done three separate times in duplicate. Amounts of mRNA were normalized to β-actin mRNA levels within each sample.
Statistical analysis
A two-sided, unpaired student t-test with 95% confidence bounds and Welch’s correction was used for all statistical analysis, except for survival curve where Log-rank (Mantel-Cox) test analysis was used. All statistical analyses were performed using GraphPad Prism Version 5.0f for Mac OS X (GraphPad Software, San Diego, CA).
Results
Vaccinia viral infection is fatal in STAT1−/− mice
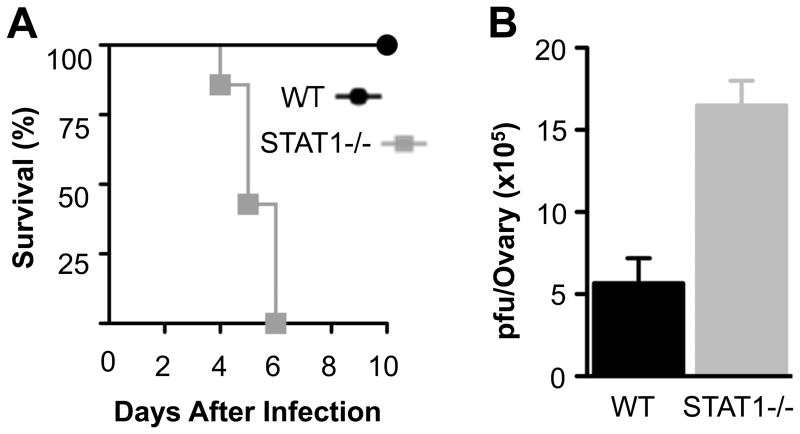
Since it is unknown whether STAT1 is required for the clearance of VV in vivo, we performed a series of experiment to gain insight into this. We first evaluated the effect of STAT1 deletion on the survival of infected mice (Fig. 1A). We found that VV-infected STAT1−/− mice had a dramatically reduced survival compared to WT mice (p < 0.01). Indeed, all of the STAT1−/− mice were deceased by day 6 after infection, whereas all WT mice survived with the same dose of VV. We further observed that the morbidity in STAT1−/− mice was associated with a significantly (p < 0.01) higher viral load. Specifically, we found that the ovarian viral titers were increased by threefold in infected STAT1−/− mice compared to WT mice (Fig. 1B). Thus, STAT1 is crucial to the innate immune control of VV infection in vivo.
FIGURE 1.
Vaccinia virus infection is fatal in STAT1−/− mice. WT and STAT1 −/− mice were infected with 2 × 106 pfu of VV intraperitoneally on day 0. (A) Survival was monitored over 10 days and the Kaplan-Meier plot of mouse survival is shown (n = 8). (B) 36 hr after infection, ovaries from infected mice were harvested and assayed for viral load. Data represents viral titer ± SD as pfu per ovary (n = 6).
STAT1−/− mice have impaired NK cell proliferation and activation in response to VV infection
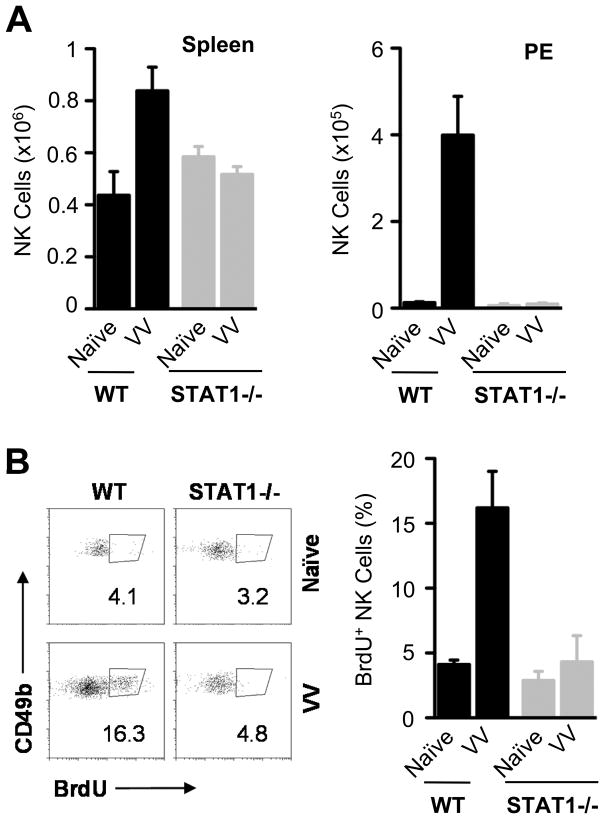
How does STAT1 confer innate immune defense against VV infection? One possibility is that STAT1 could mediate direct antiviral defense by type I IFN(10). However, we have previously shown that type I IFNs exert anti -VV infection by promoting NK cell activation and function, rather than protecting cells from VV infection directly (5). To determine whether STAT1 is critical for NK cell activation and function upon VV infection in vivo, we first examined the total NK cell numbers and assessed their proliferation during VV infection in WT and STAT1−/− mice. WT and STAT1 −/− mice were infected with VV intraperitoneally. 36 hr after infection, splenic NK cells in WT mice expanded about twice as much compared to the naïve mice, whereas no significant (p> 0.05) splenic NK cell expansion was observed in STAT1−/− mice upon VV infection (Fig. 2A). Similarly, significant (p < 0.001) expansion of NK cells was observed in the peritoneal cavity of WT mice, but not in that of STAT1−/− mice (Fig. 2A), suggesting that STAT1 is critical for NK cell expansion and accumulation at the site of infection. We next examined whether defective accumulation of NK cells in the peritoneal cavity was due to impaired NK cell proliferation by pulse BrdU labeling. Indeed, we found that the percentage of BrdU-positive NK cells was significantly (p <0.05) increased in WT, but not in STAT1−/− mice upon VV infection (Fig. 2B). These results indicate that STAT1 is important for NK cell proliferation and suggest that lack of NK cell accumulation in the peritoneal cavity of STAT1−/− mice is at least, in part, due to defective NK proliferation upon VV infection.
FIGURE 2.
STAT1 −/− mice have decreased NK cell numbers. WT and STAT1 −/− mice were infected with 2 × 106 pfu of VV intraperitoneally or left uninfected (Naïve). Mice were sacrificed 36 hr after infection and NK cells were analyzed in the spleen and in peritoneal cavity exudates (PE). Also, NK cell proliferation in PE was assessed by BrdU incorporation. (A) The mean cell numbers ± SD of CD49b+CD3− NK cells are shown (n = 6). (B) FACS plots showing the percentage of NK cell proliferation by BrdU staining among CD49b+CD3− NK cells (left panel); the mean percentage of NK cell proliferation ± SD among CD49b+CD3− NK cells is indicated (right panel)(n = 4). Data shown is representative of two independent experiments.
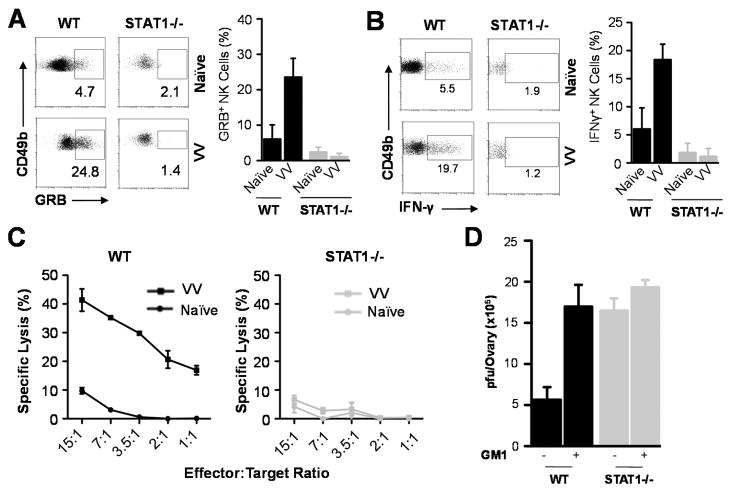
We next investigated whether STAT1 was also essential for NK cell activation. WT or STAT1−/− mice were infected with VV intraperitoneally and splenic NK cells were analyzed 36 h r later. WT NK cells produced significant (p < 0.01) amounts of Granzyme B (Fig. 3A) and IFN -γ (Fig. 3B), as well as lytic activity on NK cell target, YAC-1 cells (Fig. 3C), compared to the naïve control, whereas STAT1−/− NK cells failed to produce significant amounts of Granzyme B (Fig. 3A) and IFN-γ (Fig. 3B) or lytic activity (Fig. 3C)(p > 0.05), indicating that STAT1 is critical for NK cell activation in response to VV infection. To further support this observation, we examined the effect of NK cell depletion on viral clearance in WT and STAT1−/− mice. Consistent with our previous observation (5), depletion of NK cells led to a significant (p < 0.01) increase in viral titers in WT mice (Fig. 3D). However, NK depletion had no significant effect on viral titers in STAT1−/− mice (Fig. 3D). These results further confirm that the essential role of STAT1 in VV control in vivo is mediated through activation of NK cells.
FIGURE 3.
Impaired NK cell function in STAT1−/− mice. WT and STAT1−/− mice were infected with 2×106 pfu of VV intraperitoneally or left uninfected (Naïve). Mice were sacrificed 36 h r after infection and splenic NK cells were assayed for intracellular IFN-γ and Granzyme B (GRB) production and lytic activity. (A) FACS plots of intracellular Granzyme B production by NK cells are shown with the percentage of Granzyme B positive cells among CD49b+CD3− NK cells indicated. The mean percentage ± SD of Granzyme B positive cells among CD49b+CD3− NK cells is provided (n = 6). (B) FACS plots of intracellular IFN-γ production by NK cells are shown with the percentage of IFN-γ positive cells among CD49b+CD3− NK cells indicated. The mean percentage ± SD of IFN-γ positive cells among CD49b+CD3− NK cells is provided (n = 6). (C) NK cell lytic activity was assayed on YAC-1 cells at different effector:target ratios. Naïve splenocytes from WT and STAT1−/− mice were used as the respective controls. The mean percentage ± SD of specific lysis is indicated (n = 4). (D) Some mice were depleted of NK cells with anti-Asialo GM1 antibody (+GM1), or without depletion (−GM1), and the ovaries were harvested for measurement of viral load. Data represents viral titer ± SD as pfu per ovary (n = 4). Data shown is representative of three independent experiments.
STAT1 in both NK and accessory cells is required for NK cell activation in vitro
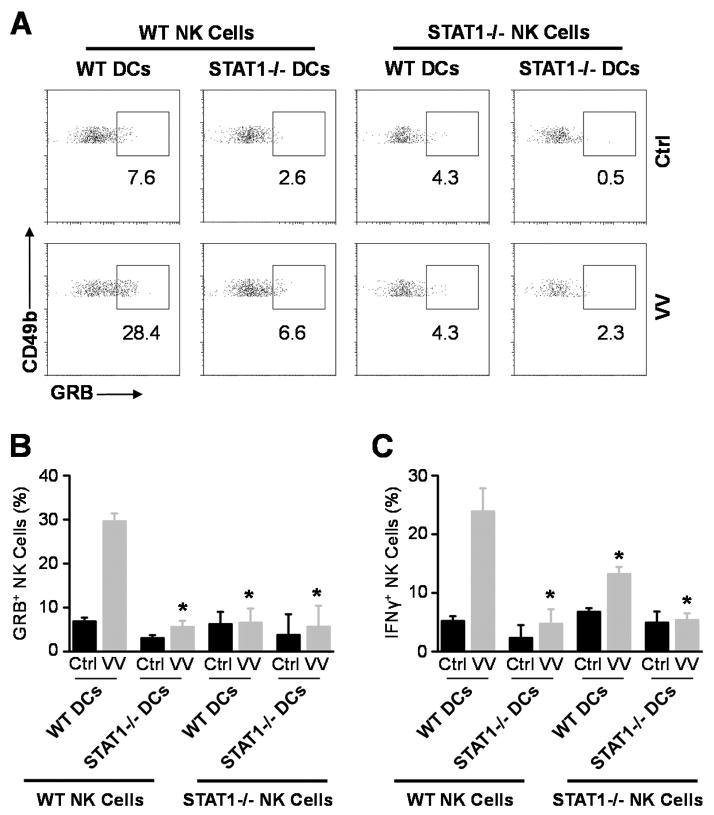
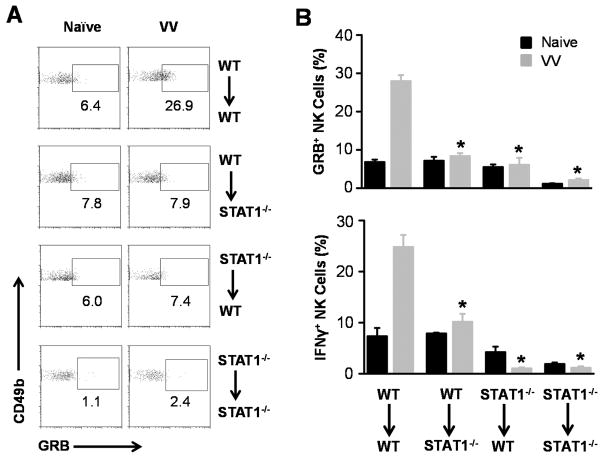
How does STAT1 regulate NK cell activation upon VV infection? Since direct action of type I IFNs is critical for NK cell activation in response to VV infection (5), STAT1 could mediate the type I IFN effect on NK cells. Alternatively, accessory cells such as CD11c+ conventional DCs have been shown to play an important role in NK cell activation (19). Thus, STAT1 signaling in DCs could also be important for NK cell activation. To address this question, we utilized an in vitro DC-NK cell co-culture system as described (5). Purified WT or STAT1−/− NK cells were co-cultured with WT or STAT1−/− CD11c+ DCs, followed by infection with VV. 24 hr after infection, NK cells were analyzed for the production of IFN-γ and Granzyme B. Our results showed that in the absence of STAT1 in either NK cells or DCs, the production of IFN -γ and Granzyme B by NK cells in response to VV is significantly (p < 0.05) reduced (Fig. 4). These results indicate that STAT1 signaling on both NK and accessory cells is required for the activation of NK cells by VV in vitro.
FIGURE 4.
STAT1 in both NK and accessory cells is required for NK cell activation in vitro. WT or STAT1−/− CD49b+CD3e− NK cells were co -cultured with WT or STAT1−/− CD11c+DCs and VV or left uninfected (Ctrl). 24 hr after infection, NK cells were assayed for IFN-γ and Granzyme B (GRB) production. (A) FACS plots of intracellular Granzyme B production by NK cells are shown with the percentage of Granzyme B positive cells among CD49b+CD3− NK cells indicated. Data shown is representative of three independent experiments. (B &C) The mean percentage ± SD of Granzyme B (B) or IFN-γ (C) positive cells among CD49b+CD3− NK cells are provided. * = p <0.05 compared to WT VV.
STAT1 in both NK and non-NK cells is essential for NK cell responses to VV infection in vivo
To validate the physiological relevance of this in vitro observation, CFSE-labeled NK cells from WT or STAT1−/− donor mice were adoptively transferred into WT or STAT1−/− recipient mice, followed by VV infection intraperitoneally. 36 hr after infection, the production of IFN-γ and Granzyme B was assessed on the transferred NK cells. WT NK cells transferred into WT recipients were able to mount an adequate response after VV infection (Fig. 5A, B). However, the production of IFN-γ or Granzyme B was significantly (p < 0.05) reduced when STAT1−/− NK cells were transferred into WT recipients (Fig. 5A, B), suggesting that STAT1 signaling in NK cells is required for NK cell activation. This is consistent with our previous observation that type I IFN signaling on NK cells is critical for NK cell activation upon VV infection (5). We also observed that the production of IFN-γ or G ranzyme B was also significantly (p < 0.05) reduced when WT NK cells were transferred into STAT1−/− recipients (Fig. 5A, B), suggesting that STAT1 signaling in non -NK cells is also required for NK cell activation in vivo. Taken together, our results demonstrate that STAT1 in both NK and non-NK cells is required for NK cell responses during VV infection in vivo.
FIGURE 5.
STAT1 signaling in both NK and non-NK cells is critical for NK cell activation during VV infection in vivo. CD49b+CD3− NK cells were purified from the spleen of WT and STAT1−/− mice and labeled with CFSE. These cells were then adoptively transferred into either WT or STAT1−/− mice, as indicated next to each panel. The mice were then infected by injecting 2×106 pfu of VV in traperitoneally. Some mice were left uninfected (Naïve). 36 hr after infection, transferred NK cells (CFSE-positive) were assayed for intracellular Granzyme B (GRB) and IFN-γ production. (A) FACS plots of intracellular Granzyme B production by NK cells are shown with the percentages of Granzyme B positive cells among CFSE +CD49b+CD3− NK cells indicated. Data shown is representative of two independent experiments. (B) The mean percentage ± SD of Granzyme B or IFN-γ positive cells among CFSE+CD49b+CD3− NK cells are provided (n = 6). * = p <0.05 compared to WT VV.
STAT1 signaling in DCs is required for upregulation of NKG2D ligands
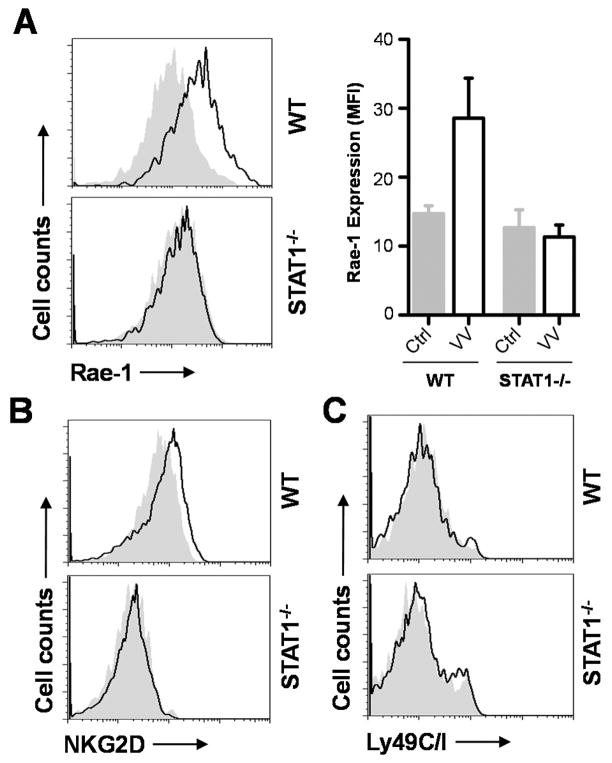
The requirement of STAT1 in DCs for NK cell activation prompted us to investigate the underlying mechanism(s). Since the interaction between NKG2D ligand expressed on DCs and NKG2D on NK cells is critical for NK cell activation to VV infection (6), we hypothesized that upregulation ofNKG2D ligands upon VV infection is dependent on STAT 1 signaling in DCs. To test this, we examined the expression of the NKG2D ligand, Rae-1, on splenic CD11c+ DCs in WT andSTAT1 −/− mice 24 h rafter VV infection. We found a significant (p < 0.01) upregulation of Rae-1 expression in WT DCs compared to the uninfected naïve control, whereas no significant (p > 0.05) upregulation of Rae-1 was observed in DCs of STAT1−/− mice (Fig. 6A). We also observed some upregulation of NKG2D on WT, but not STAT1−/− NK cells in response to VV infection (Fig. 6B). However, no changes in the expression of NK inhibitory receptor, Ly49C/I were detected in WT or STAT1 −/− mice (Fig. 6C). Taken together, our results suggest that STAT1 signaling in DCs is required for upregulation of NKG2D ligand expression, which in turn promotes NK cell activation via the NKG2D pathway.
FIGURE 6.
Upregulation of the NKG2D ligand, Rae-1 is dependent on STAT1 signaling in DCs. WT and STAT1 −/− mice were infected with 2×106 pfu of VV intraperitoneally or left uninfected (Naïve). (A) 24 hr after infection, splenic CD11c+B220− DCs were assayed for Rae -1 expression. Solid lines represent Rae-1 expression in infected mice whereas gray areas indicate Rae-1 expression in naïve mice. The median fluorescence intensities (MFI) ± SD of Rae-1 expression for WT and STAT1−/− DCs are provided (n = 6). (B–C) NK cells were analyzed for NKG2D (B) and Ly49C/I (C) expression. Solid lines represent expression in infected mice whereas gray areas indicate expression in naïve mice. Data shown is representative of three independent experiments.
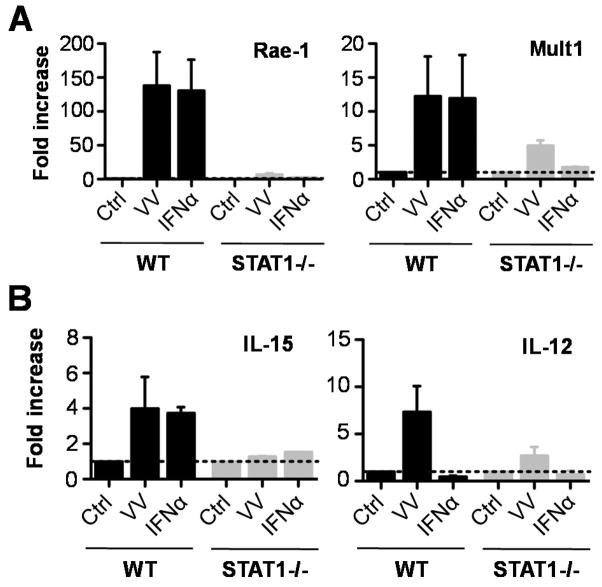
To investigate whether type I IFNs play a role in the STAT1-dependent upregulation of NKG2D ligand in DCs upon VV infection, WT or STAT1−/− DCs were stimulated with IFN-α and measured for the expression of Rae-1 and Mult1, another NKG2D ligand by real-time quantitative PCR. Indeed, similar to VV, stimulation with IFN-α significantly (p < 0.001) promoted the expression of both Rae-1 and Mult1 by WT DCs compared to the unstimulated controls (Fig. 7A). However, the upregulation of NKG2D ligands by STAT1−/− DCs in response to VV or IFN-α was severely compromised (Fig. 7A), suggesting type I IFNs contribute to STAT1-dependent upregulation of NKG2D ligand in DCs. However, this result did not exclude the potential involvement of other cytokines such as IL-21 in response to VV infection. We further showed that IFN-α or VV also promoted the production of IL-15 and IL-12 in a STAT1-dependent manner (Fig. 7B), which could also contribute to NK cell activation. This is consistent with the previous report in a model of hepatitis C infection (20).
FIGURE 7.
Upregulation of NKG2D ligand and cytokine expression is dependent on STAT1 signaling in DCs. CD11c+ DCs generated from the bone marrow of WT and STAT1−/− mice were stimulated for 24 h with live VV, IFN-α, or left untreated (Ctrl). Thereafter, RNA was purified and the expression of NKG2D ligands, Rae-1 and Mult1 (A), and IL-15 and IL-12 (B) was assayed by quantitative real-time PCR. The mean fold increase ± SD of expression over control (Ctrl) is provided (n = 3). mRNA abundance was normalized to β-actin.
Discussion
In this study, we showed that STAT1 was critical for NK cell activation upon VV infection and the subsequent clearance of VV infection in vivo. We further demonstrated that STAT1 signaling in both NK cells and accessory cells such as DCs was required for efficient NK cell activation upon VV infection in vitro and in vivo. Mechanistically, STAT1 signaling in DCs promoted the expression of the NKG2D ligand, Rae-1, which is required for NK cell activation via the NKG2D pathway.
Although STAT1 has been shown to play an important role in the control of various viral infections (11, 14, 21), this is the first report showing that STAT1 is essential for the clearance of VV, a member of the poxviridae family. The observed lethality with VV infection is consistent with that VSV (11) and MCMV (14) infection in STAT1 −/− mice. However, STAT1−/− mice had a higher survival rate after infection with dengue virus (21). The reason for this discrepancy is not entirely clear, but may be related to a role of STAT1-independent pathways in the control of primary dengue virus infection (21).
Our observation that STAT1 is essential for NK cell activation upon VV infection is in line with the previous reports showing a critical role of STAT1 in NK cell activation in response to MCMV infection and tumor cells (13, 14). We further demonstrated here that STAT1 signaling in both NK and accessary cells are important for NK cell activation. The NK cell-intrinsic role of STAT1 is consistent with our previous observation that direct action of type I IFN is critical for NK cell activation in response to VV infection (5). However, the NK cell-extrinsic role of STAT1 in NK activation upon a viral infection represents a novel finding and the underlying mechanism(s) responsible for this role remains to be defined.
Studies have shown that NK cell activation in response to viral infection is regulated by engagement of NK activating receptors such as Ly49H, NKp46 and NKG2D (22–24). In the setting of VV infection, we have previously shown that the interaction between NKG2D ligands on accessory cells such as DCs, and NKG2D on NK cells is critical for NK cell activation and function (6). However, it remains unclear how the upregulation of NKG2D ligands on accessary cells in response to VV infection is regulated. In this study, we provided the first evidence that STAT1 signaling on DCs is critical for upregulating the NKG2D ligand expression, leading to the enhanced NK cell activation upon VV infection. Although IFN-α could promote the upregulation of NKG2D ligands by DCs, what triggers the activation of STAT1 in accessory cells upon VV infection remains to be further defined. This is because STAT1 regulates many of the biological effects mediated by type I and type II IFNs, as well as other cytokines such as IL-21 in response to viral infections in vivo (10, 18). Thus, future studies are needed to define mechanisms responsible for STAT1 activation. Similarly, how STAT1 activation leads to NKG2D ligand upregulation on accessory cells requires further investigation.
In conclusion, we have shown that STAT1 is required for the innate immune control of VV in vivo. This is mediated by promoting the activation and effector function of NK cells. This STAT1-dependent NK cell activation is dependent on STAT1 signaling on both NK and accessory cells in response to VV. Furthermore, STAT1 signaling in accessory cells upregulates the expression of the NKG2D ligand, which is required for NK cell activation via the NKG2D pathway. Taken together, our data suggest that STAT1 mediates anti-VV effect by promoting NK cell activation through both NK-intrinsic and extrinsic mechanisms and may provide insight into the design of effective NK cell-based therapies for viruses.
Acknowledgments
This work was supported by the National Institutes of Health grants AI083000, CA136934, and CA047741 (to Y.Y.). C.F. is supported by a post-doctoral fellowship award from the FRSQ (Fonds de recherche du Québec -Santé).
References
- 1.Lee SH, Miyagi T, Biron CA. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 4.Natuk RJ, Welsh RM. Accumulation and chemotaxis of natural killer/large granular lymphocytes at sites of virus replication. J Immunol. 1987;138:877–883. [PubMed] [Google Scholar]
- 5.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 6.Martinez J, Huang X, Yang Y. Direct TLR2 signaling is critical for NK cell activation and function in response to vaccinia viral infection. PLoS Pathog. 2010;6:e1000811. doi: 10.1371/journal.ppat.1000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenner FHD, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 8.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-{beta} Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Sastre A, Biron CA. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- 11.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, Kaplan DH, Riley JK, Greenlund AC, Campbell D, Carver-Moore K, DuBois RN, Clark R, Aguet M, Schreiber RD. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 12.Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK, Rao DT, Gertner R, Gimeno R, Frey AB, Levy DE. Distinct requirements for IFNs and STAT1 in NK cell function. J Immunol. 2000;165:3571–3577. doi: 10.4049/jimmunol.165.7.3571. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 15.Fortin C, Huang X, Yang Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J Immunol. 2012;189:1843–1849. doi: 10.4049/jimmunol.1200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Huang CT, Huang X, Pardoll DM. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat Immunol. 2004;5:508–515. doi: 10.1038/ni1059. [DOI] [PubMed] [Google Scholar]
- 17.Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 18.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 signaling is critical for CD8 T cell survival and memory formation in response to vaccinia viral infection. J Immunol. 2011;186:2729–2738. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newman KC, Riley EM. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol. 2007;7:279–291. doi: 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 20.Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Suzuki T, Miyagi T, Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- 21.Shresta S, Sharar KL, Prigozhin DM, Snider HM, Beatty PR, Harris E. Critical roles for both STAT1-dependent and STAT1-independent pathways in the control of primary dengue virus infection in mice. J Immunol. 2005;175:3946–3954. doi: 10.4049/jimmunol.175.6.3946. [DOI] [PubMed] [Google Scholar]
- 22.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 23.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 24.Guma M, Angulo A, Lopez-Botet M. NK cell receptors involved in the response to human cytomegalovirus infection. Curr Top Microbiol Immunol. 2006;298:207–223. doi: 10.1007/3-540-27743-9_11. [DOI] [PubMed] [Google Scholar]