Abstract
Background
Gastroparesis affects predominantly females; however, the biological basis for this gender bias is completely unknown. Several lines of evidence suggest that nitrergic dependent stomach motility function is reduced in diabetic gastroparesis and that nNOS is estrogen-regulated.
Aims
The purpose of this study was to investigate whether reduced levels of estradiol-17β (E2) down-regulates tetrahydrobiopterin (BH4, a cofactor for nNOS dimerization and enzyme activity) biosynthesis and therefore nNOS mediated gastric motility would be impaired in a mouse model of chronic estrogen deficiency, follicle stimulating hormone receptor knock-out female mice (FORKO).
Methods
In-bred 12-week-old female FORKO mice were obtained from our FORKO breeding colony. Gastric emptying was measured in overnight fasting mice. Nitrergic relaxation (AUC/mg tissue) was measured at 2 Hz through electric field stimulation using gastric antrum strips prepared from WT and FORKO mice. Protein expression for nNOSα, BH4 biosynthesis enzymes (GCH-1, DHFR) and estrogen receptors (α, β) were measured in gastric antrum by western blotting. Levels of BH4 and oxidized BH2, B biopterin levels were determined by HPLC.
Results
In FORKO, compared to wild type (WT) stomachs we indentified (1) reduced (%) gastric emptying (64 ± 2.5 vs. 77.6 ± 0.88), (2) greater reduction in nitregic relaxation (−0.13 ± 0.012 vs. −0.28 ± 0.012), (3) increased nNOS dimerization (0.48 ± 0.02 vs. 0.34 ± 0.05), (4) decreased NO release whether measured at 24 h (0.6 ± 0.04 vs. 1.7 ± 0.22, p < 0.05) or at 48 h (3.4 ± 0.26 vs. 5.0 ± 0.15, p < 0.05) of incubation, (5) decreased GCH-1 (1.9 ± 0.06 vs. 2.3 ± 0.04), DHFR (1.8 ± 0.14 vs. 2.4 ± 0.07) and ERα (2.7 ± 0.4 vs. 3.9 ± 0.4) and (6) increased oxidized biopterin levels and decreased ratio of BH4 versus BH2 + B.
Conclusion
We conclude that chronic estrogen deficiency negatively modifies the function of both BH4 and nNOS thereby contributing to the development of gastroparesis in a FORKO mouse model.
Keywords: Follicle stimulating hormone, Estrogen, Nitric oxide, nNOS dimerization, Biopterins, Gastroparesis
Introduction
Gastroparesis (delayed gastric emptying) is a clinical condition that primarily affects young women and is associated with abnormal gastric motility. The most common forms include idiopathic, diabetic (types 1 and 2) or post-surgical complications [1]. Although gastroparesis is a significant health problem, its pathogenesis and the apparent gender bias are not well understood. Gastric motility is regulated in large part by the enteric nervous system primarily consisting of excitatory acetylcholine, and inhibitory NO and vasoactive intestinal peptide effectors [2, 3]. The importance of NO for gastric function, produced in this case by neuronal nitric oxide synthase (nNOS), has been established by findings of pyloric hypertrophy and gastric dilation in nNOS (−/−) mice [4, 5]. The catalytic activity of NOS depends on a dimerization step, facilitated by (6R)-tetrahydrobiopterin (BH4). BH4 is an endogenously synthesized molecule and is a cofactor for all NOS enzymes (nNOS, eNOS, and iNOS) [6]. Our laboratory previously demonstrated that gastric antral nitrergic relaxation and nNOS alpha (α) dimerization are higher in healthy female than male rats. Diabetes induction by streptozotocin significantly impaired these parameters and led to delayed gastric emptying in female rats [3]. We also recently showed that gastric nitrergic neuron dysfunction due to altered levels of BH4 biosynthesis significantly contributes to the pathogenesis of diabetic-induced gastroparesis [7].
Thus female rats are more dependent on nitrergic regulation of gastric function than males and this may have clinical implications in patients. Thus it is possible that females will be more vulnerable to gastroparesis because of changes in the BH4-nNOS pathway secondary to changes in female hormones. Although conflicting reports exist, the preponderance of evidence suggests that elevated levels of circulatory estradiol-17β (E2) but not progesterone regulate gastric emptying in healthy females [8, 9]. These studies further indicate that E2 elevates NO and regulates gastric motility function. However, conclusive evidence demonstrating that a deficiency in E2 levels contributes to the pathogenesis of diabetic gastroparesis is lacking [9].
FORKO mice lacking follicle stimulating hormone receptor (FSH-R) exhibit profound changes in ovarian structure and secondary sex organs. FSH-R gene disruption causes complete loss of ovarian estrogen production (>95 %). However circulatory progesterone levels were decreased by 70 % in FSH-R null mice. The chronic deficiency of circulating estrogen in these mice also causes important metabolic alterations that can induce obesity with aging, a well known detrimental factor for the development of type 2 diabetes in humans [10]. Estrogen replacement therapy promptly induced uterine growth and reversed the accumulation of adipose tissue suggesting the functional role of estrogen in these mice [10]. Therefore, we hypothesized that reduced levels of E2 would down-regulate BH4 biosynthesis and therefore impair nNOS mediated gastric motility in the FORKO mouse model of chronic estrogen deficiency compared to WT SVE129 female mice.
Materials and Methods
Experimental Mice
Adult female inbred FORKO and WT mice (12 weeks old) were maintained in our institutional animal care facility under controlled temperature, humidity and a light–dark cycle (12:12-h), with free access to normal rodent chow and water. The protocols used for these mice were approved by the Institutional Animal Care and Use Committees at the Meharry Medical College, Nashville, Tennessee, in accordance with the recommendations of National Institutes of Health, Guide for the Care and Use of Laboratory Animals. Animals were sacrificed by cervical dislocation to collect gastric tissue for future analysis. Tissue samples (antrum, pylorus or fundus, as indicated) collected from animals were snap frozen in liquid nitrogen and stored at −80 °C until analyzed.
Measurement of Glucose
A drop of blood was collected from the tail vein of fasted (12 h) FORKO and WT mice without anesthesia for determination of plasma glucose using an Alphatrack™ Blood monitoring pack (Abbott laboratories, Abbott park, IL, USA).
Solid Gastric Emptying Studies
Solid gastric emptying studies were performed in both WT and FORKO female mice as reported earlier [3, 7, 11]. Briefly, after fasting overnight, a known amount of normal diet (along with water) was fed to the animals for 3 h. At the end of 3 h, the amount of food intake over the 3 h period was determined. Animals were then fasted again for another 2 h without food or water. At the end of this fast, animals were sacrificed and the weight of the whole stomach was measured before and after opening and emptying the stomach of its contents. The rate of gastric emptying was calculated according to the following equation: gastric emptying (% in 2 h) = (1 − gastric content food intake-1) × 100.
Organ Bath Studies
Electric field stimulation (EFS)-induced NANC (nonadrenergic, non-cholinergic) relaxation was studied in circular gastric antrum strips [12]. Strips tied with a silk thread at both ends were mounted in 10-ml water-jacketed organ baths containing Krebs buffer at 37 °C and continuously bubbled with 95 % O2–5 % CO2 (Radnoti Glass Technology, Monrovia, CA, USA). Tension for each muscle strip was monitored with an isometric force transducer and analyzed by a digital recording system (Biopac Systems, Goleta, CA, USA). A passive tension equal to 2 g was applied to each strip in the 1 h equilibration period through incremental increases (0.5 g, four times, at 15 min intervals). Strips were exposed to atropine, phentolamine and propranolol (10 μM each) in the bath solution for 1 h to block cholinergic and adrenergic responses. 5-Hydroxy-tryptamine (100 μM) pre-contracted strips were exposed to EFS (90 V, 2 Hz, 1-ms pulse for duration of 1 min) to elicit NANC relaxation. Relaxation response elicited by low frequency (2 Hz) stimulus under NANC conditions, as used in this study, was demonstrated as predominantly nitrergic in origin. To investigate EFS induced NANC relaxation in WT and FORKO mice, gastric pyloric strips from WT and FORKO mice were collected and NANC relaxation measured through EFS induction. The NO dependence of nitrergic relaxations was confirmed by preincubation with NG-nitro-L-arginine-methyl ester (L-NAME, 100 μM). At the end of each experiment, muscle strips were blotted dry with filter paper and weighed. Comparisons between groups were performed by measuring the areas under the curve (AUC/mg tissue) for the EFS-induced relaxation (AUCR) for 1 min versus the baseline for 1 min (AUCB) according to the formula (AUCR − AUCB)/weight of tissue (mg) = AUC/mg of tissue.
nNOSα Dimerization in Mice Gastric Antrum
Levels of nNOSα monomer and dimer were quantified by western blotting via low temperature (LT)-PAGE in WT and FORKO gastric antrum homogenates as described [3, 7, 13]. LT-SDS-PAGE was performed on ice; 30 μg of protein in standard Laemmli buffer was incubated at 0 °C for 30 min and then separated using a 6 % separating gel. All gels and buffers were pre-equilibrated to 4 °C prior to electrophoresis; the buffer tank was also placed in an ice-bath during electrophoresis to maintain the gel temperature below 15 °C. A polyclonal antibody specific to nNOSα (1:1,000 dilution) (Zymed Laboratories, Grand Island, NY, USA) and anti-rabbit IgG conjugated with horseradish peroxidase (1:5,000) (Sigma Chemical, St. Louis, MO, USA) were used as the primary and secondary antibodies, respectively.
Western Blot Analysis
nNOSα protein was quantified in gastric antrum homogenates from the two groups of mice, using standard western blot analysis, as described in our previous study [3, 7, 14]. Proteins were measured by Bio-Rad protein assay (Bio-Rad laboratories, Hercules, CA, USA) and 30 μg of protein were separated by 6 % SDS polyacrylamide gel electrophoresis (SDS-PAGE). The membrane was immunoblotted with polyclonal nNOSα primary antibody (Zymed Laboratories Inc.) and anti-rabbit IgG conjugated with a horseradish peroxidase (Sigma Chemical) secondary antibody.
GCH-1 (1:250), DHFR (1:250) and ERα (1:500) proteins were quantified in gastric antrum homogenates using standard western blot analysis. Proteins were measured by Bio-Rad protein assay (Bio-Rad, Hercules, CA), and 30 μg of protein were separated by 15 % SDS-PAGE. The membrane was immunoblotted with polyclonal primary antibodies (Santacruz Biotechnology, Santa Cruz, CA, USA) and their respective secondary antibodies [anti-mouse IgG (1:2 500) for GCH-1, DHFR and anti-rabbit IgG (1:5 000) for ERα].
Binding of antibodies to the blots was detected with an enhanced chemiluminescence system ECL (Amersham Pharmacia Biotech, Piscataway, NJ, USA) following the manufacturer’s instructions. Stripped blots were re-probed with γ-tubulin specific polyclonal antibodies (Sigma Chemical) to enable normalization of signals between samples. Band intensities were analyzed using Bio-Rad Gel Doc (Bio-Rad, USA).
In Vitro NO Release
In vitro NO release experiments were performed as described previously [9]. Briefly, animals from both groups were sacrificed by CO2 asphyxiation and the whole dissected stomach was transferred in chilled oxygenated krebs bicarbonate solution. Gastric antrum muscular tissue was harvested and cut into mucosa-free strips and individual portions cultured for either 24 or 48 h in 500 μl of phenol red-free DMEM supplemented with NB27 (2 %) and antibiotics (1 %). Following the timed incubations, the DMEM portions were collected and stored at −80°C for analysis of NO released into the medium during the incubation period. NO released into the medium was analyzed as total nitrite (metabolic by-product of NO) according to the manufacturer’s protocol that was supplied with a commercially available kit (EMD Chemicals, Gibbstown, NJ, USA).
Measurement of Biopterin Levels
Biopterin levels were determined in antrum homogenates by HPLC followed by electrochemical and fluorescent detection, as described previously [11, 15]. Briefly, samples were homogenized in 50 mM phosphate-buffered saline, pH 7.4, containing 1 mM dithioerythritol and 100 μM EDTA. Following centrifugation (15 min at 13,000 rpm and 4 °C), the samples were transferred to new, cooled micro tubes and precipitated with cold 1 M phosphoric acid, 2 M trichloroacetic acid and 1 mM dithioerythritol. The samples were vigorously mixed and then centrifuged for 15 min at 13,000 rpm and 4 °C. The samples were injected onto an isocratic HPLC system and quantified using sequential electrochemical (Coulochem III, ESA Inc., Sunnyvale, CA, USA.) and fluorescence (Jasco, Easton, MD, USA) detection. HPLC separation was performed using a 250 mm, ACE C-18 column (Hichrom, Berkshire, UK) and mobile phase comprising of 50 mM sodium acetate, 5 mM citric acid, 48 μM EDTA, and 160 μM dithioerythritol, pH 5.2, (all ultrapure electrochemical HPLC grade) at a flow rate of 1.3 ml/min. Background currents of +500 and −50 μA were used for the detection of BH4 on electrochemical cells E1 and E2, respectively; 7,8-BH2 and biopterin were measured using a Jasco FP2020 fluorescence detector. Quantification of BH4, BH2, and biopterin was done by comparison with authentic external standards and normalized to sample protein content.
Statistics
Data were presented as mean ± standard error (SE). Statistical comparisons between groups were determined by Student’s t test or the Tukey test after one-way analysis of variance (ANOVA). A p value of less than 0.05 was considered statistically significant.
Results
Body Weights and Glucose Levels in FORKO Mice
As shown in Table 1, there was no significant difference in body weights in FORKO compared to WT mice. FORKO mice on average, had a slightly higher plasma glucose level following an overnight fast, but the increase was not statistically different from similarly fasted WT female mice.
Table 1.
Blood glucose levels, body weights and % gastric emptying (2 h) in the wild type (WT) and FORKO female mice
| Measure | WT | FORKO |
|---|---|---|
| Body weight (g) | 18.5 ± 1.0 | 19.4 ± 0.9 |
| Blood glucose (mg/dl 12 h) | 97.4 ± 3.7 | 108.0 ± 4.4 |
| Gastric emptying (% 2 h) | 77.6 ± 0.88 | 64.0 ± 2.5* |
Results are expressed as mean ± SEM (N = 4)
WT wild type, KO knock out, FORKO follicle stimulating hormone receptor knock-out female mice
p < 0.05 compared to WT
Delayed Gastric Emptying and Reduced Nitrergic Relaxation in FORKO Mice
There was, however, a significantly slower solid gastric emptying (% in 2 h) which was observed in FORKO compared to WT (64 % ± 2.5 vs. 77.6 % ± 0.88; p < 0.05) mice (Table 1). Next we examined if an impaired nitrergic relaxation was associated with the delay in gastric emptying in these mice. As shown in Fig. 1a, gastric nitrergic relaxation was significantly reduced in female FORKO mice compared to WT mice (−0.13 ± 0.012 vs. −0.28 ± 0.012; p < 0.05). The above data suggest that estrogen deficiency led to an impairment of nitrergic (functional activity of nNOS) mediated gastric motility and thus, a delay in gastric emptying.
Fig. 1.
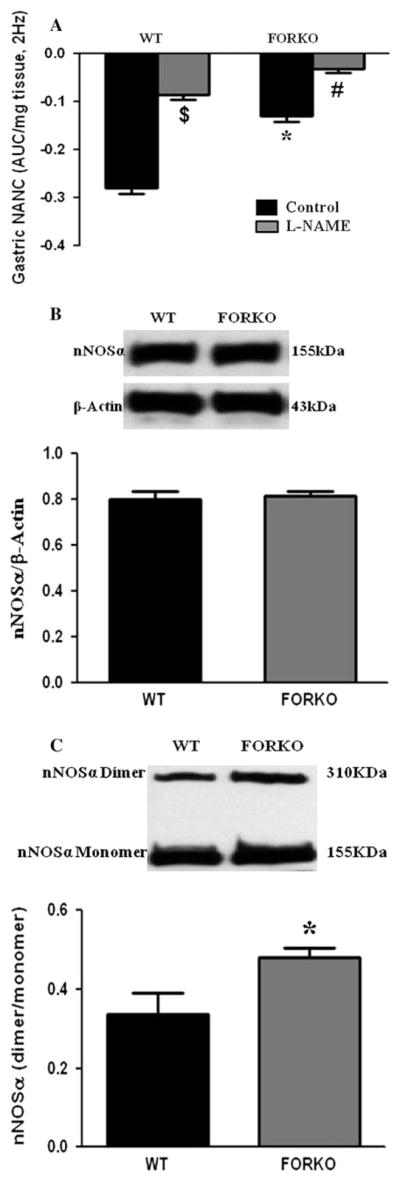
Nitrergic relaxation in female wild type (WT) and follicle stimulating hormone receptor knock-out female mice (FORKO) gastric muscular tissues. a Nitrergic relaxation was measured in WT and FORKO gastric muscular tissue. Representative immunoblots and densitometric analysis data for nNOSα protein (b) and nNOSα dimer (c) in female mice gastric muscular tissue. Values are mean ± SE (n = 4 mice per group). Statistical significance was determined by Tukey test after one-way ANOVA. *p < 0.05 FORKO control group compared with WT control group; #p < 0.05 FORKO + L-NAME compared with FORKO control group; and $p < 0.05 WT + L-NAME compared with WT control group
Gastric nNOSα Protein Expression, nNOSα Dimers/Monomers in FORKO Mice
There was no change in the protein level of nNOSα, the only functional isoform of nNOS in gastric muscular tissue (Fig. 1b). We then checked the nNOSα dimer/monomer ratio. There is a significant increase in nNOSα dimer/monomer ratio in FORKO compared to WT mice (0.48 ± 0.02 vs. 0.34 ± 0.05, p < 0.05) (Fig. 1c).
Reduced NO Levels in WT and FORKO Mice Gastric Muscular Tissue
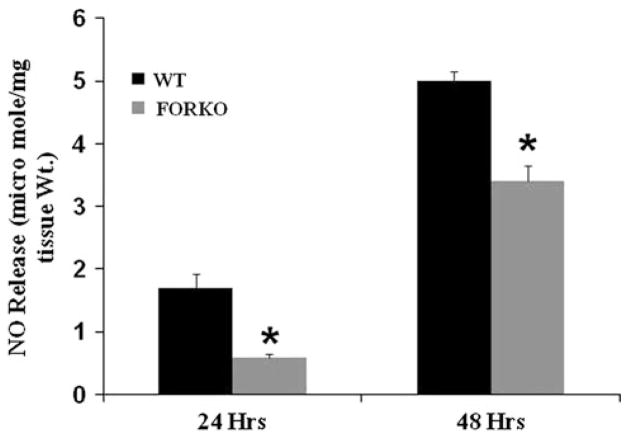
Even though nNOS dimerization was increased, we found that NO release (μmole/mg tissue wt) was reduced in FORKO mice gastric muscular tissue strips when compared to WT at both 24-h (0.6 ± 0.04 vs. 1.7 ± 0.22, p < 0.05) and 48-h (3.4 ± 0.26 vs. 5.0 ± 0.15, p < 0.05) time points (Fig. 2). Collectively, chronic estrogen deficiency led to reduced NO levels, a nitrergic mediated gastric dysmotility and delayed gastric emptying in FORKO female mice.
Fig. 2.
In vitro NO release in female wild type (WT) and follicle stimulating hormone receptor knock-out female mice (FORKO) gastric muscular tissue. In vitro NO levels were measured in WT and FORKO gastric muscular tissue at both 24- and 48-h time points. Values are mean ± SE (n = 4 mice per group). Statistical significance was determined by Tukey test after one-way ANOVA. * p < 0.05 compared with control group
Reduced Expression of Gastric GCH-1 in FORKO Mice
GCH-1 (GTP cyclohydrolase 1) is a rate limiting enzyme responsible for BH4 biosynthesis via the de novo pathway [16]. We investigated if the protein expression of GCH-1 was altered in gastric muscular tissues obtained from FORKO mice. As shown in Fig. 3a, there was a significant decrease in GCH-1 protein expression in female FORKO mice gastric muscular tissue compared to WT mice (1.9 ± 0.06 vs. 2.3 ± 0.04, p < 0.05).
Fig. 3.
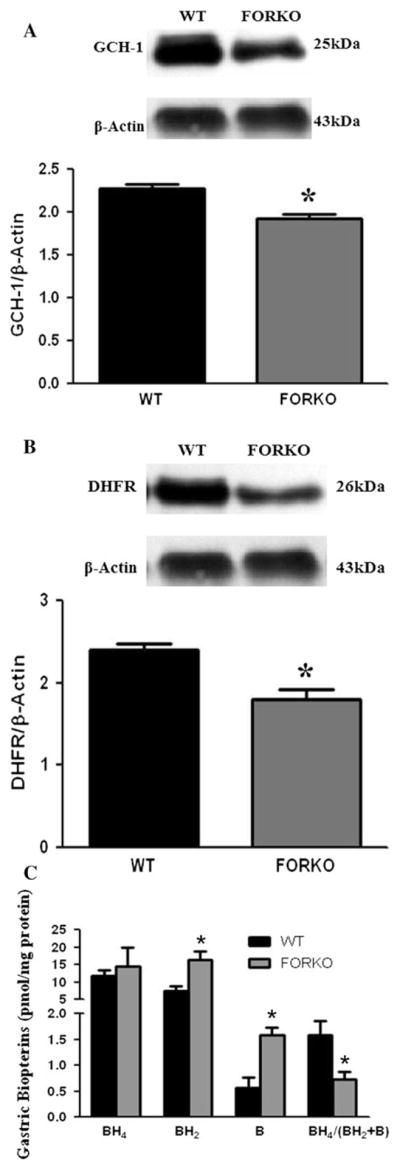
Protein expression of GCH-1, DHFR and biopterin in female wild type (WT) and follicle stimulating hormone receptor knock-out female mice (FORKO) mouse gastric muscular tissue. Representative immunoblots and densitometric analysis data for GCH-1 (a) and DHFR (b) proteins in female mice gastric muscular tissue. c The levels of biopterins (BH4, BH2 and B) in WT and FORKO mice. Values are mean ± SE (n = 4 mice per group). Statistical significance was determined by Tukey test after one-way ANOVA. * p < 0.05 compared with control group
Reduced Expression of Gastric DHFR in FORKO Mice
We measured protein levels of DHFR (dihydrofolate reducase), an enzyme responsible for the conversion of oxidized BH2 into reduced BH4 via the salvage pathway [16]. Figure 3b shows there was a significant decrease in DHFR protein expression in female FORKO mice gastric muscular tissue compared to WT mice (1.8 ± 0.14 vs. 2.4 ± 0.07, p < 0.05). The above data suggest that both de novo (Fig. 3a) and salvage (Fig. 3b) pathway enzymes responsible for BH4 biosynthesis were altered by chronic estrogen deficiency.
Elevated Gastric Oxidized Biopterin Levels in FORKO Mice
Figure 3c shows the levels (pmol/mg protein) of total biopterins (BH4, BH2 and B) in gastric tissue from WT and FORKO mice. No significant change was observed in the absolute BH4 (reduced form) level as measured by HPLC, whereas there was a significant increase in oxidized BH2 as well as B levels in FORKO mice compared to WT mice. The ratio of BH4 and total biopterin was therefore significantly (p < 0.05) reduced in FORKO compared to WT mice suggesting a lack of BH4 bioavailability. These data suggest that estrogen deficiency is leading to increased oxidized biopterins (reduced BH4 availability), a reduced nNOS function and delayed gastric emptying.
Reduced Expression of Gastric ERα but Not ERβ Protein in FORKO Mice
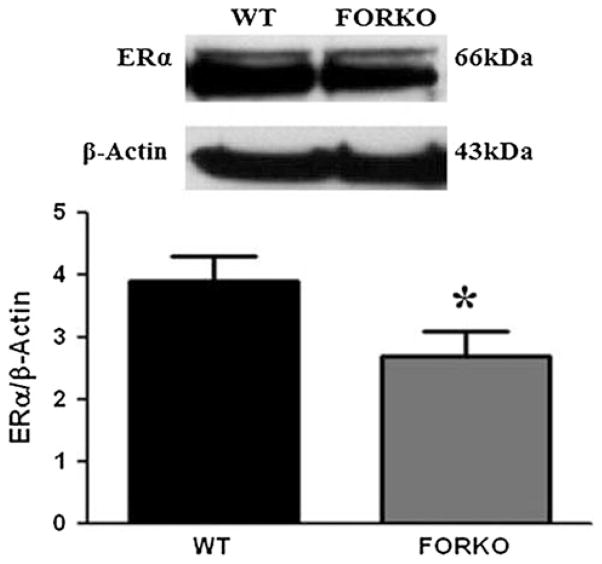
Estrogen binds to its receptors and relaxes smooth muscle. Thus we investigated if estrogen receptors alpha (ERα) and/or beta (ERβ) were altered in gastric muscular tissue obtained from FORKO mice compared to controls. Figure 4 demonstrates that gastric ERα was significantly reduced in FORKO (2.7 ± 0.4 vs. 3.9 ± 0.4, p < 0.05) compared to WT mice. However, no change in ERβ protein expression was observed (2.9 ± 0.3 vs. 3.0 ± 0.4).
Fig. 4.
Expression of ERα in wild type (WT) and follicle stimulating hormone receptor knock-out female mice (FORKO) female mice gastric muscular tissue. Representative immunoblots and densitometric analysis data for ERα protein in female mice gastric muscular tissue. Values are mean ± SE (n = 4 mice per group). Statistical significance was determined by Tukey test after one-way ANOVA. *p < 0.05 compared with WT control group
Discussion
Many studies using the FORKO model in recent years indicated that estrogen deficiency links to several health complications in these mice that are similar to those observed in humans. As an example, in FORKO mice blood pressure is elevated and production of reactive oxygen species is increased leading to vascular damage, as in postmenopausal women [17]. Further, in the context of low estrogen levels, a high fat intake in these mice leads to NO mediated vascular damage [18]. Lack of E2 due to the loss of FSH-R signaling in these mice causes important metabolic alterations that induce obesity over time, a well-known detrimental factor for developing type 2 diabetes mellitus in humans [10]. Several lines of evidence in both rodents and humans suggest that circulating E2 are reduced in diabetes due to a hypothalamopitutary ovarian axis dysfunction [9, 19]. Therefore, FORKO mice have been used as a chronic E2 deficiency model. Here we used them to investigate gastric motility abnormalities often observed at the onset of diabetes, and more often in women.
The importance of estrogens to BH4 synthesis comes from the known fact that E2 treatment elevated both the expression of GCH-1 and BH4 in rat brain neurons via a estrogen receptor-mediated event [20]. A previous report using in vitro hyperglycemic conditions suggested that E2 supplementation restored the impairment of both BH4 biosynthesis and NO generation via estrogen receptor α in bovine aortic endothelial cell cultures [21]. Estrogen supplementation also attenuated the progression of diabetic nephropathy in diabetic rats [22]. Most importantly, Shah et al. [12] found that nNOS protein expression and nitrergic relaxation in gastric fundus was increased in estrogen but not progesterone treated ovariectomized mice. Together with our earlier reports [3, 9, 12] these data collectively indicate that the elevated nitrergic function observed in healthy females compared to age-matched males, is regulated by increased levels of endogenous circulating estrogens. Thus a reduction in E2 levels can lead to reduced nitrergic relaxation and delayed gastric emptying (gastroparesis). The exact mechanism(s), however, of E2 effects on gastric BH4 biosynthesis and nNOS function remain unknown.
This study is the first comprehensive report of gastric dysmotility in FORKO mice, an animal model of chronic estrogen deficiency. We have shown that estrogen deficient FORKO mice have a slower gastric emptying and reduced nitrergic relaxation similar to that of diabetic rodents [3, 7] (Table 1, Fig. 1a). Several cofactors have been shown to be important for nNOS activity, including BH4. The level of BH4 is tightly regulated by both de novo and salvage pathways. GCH-1 is a rate limiting enzyme and regulates BH4 levels via the de novo pathway, while DHFR reduces oxidized (inactive) BH2 and B to active BH4 via the salvage pathway [17]. As shown in Fig. 3a, b, E2 deficiency reduced expression of GCH-1 and DHFR levels in female FORKO gastric muscular tissue. We next quantified the levels of BH4 and its oxidized metabolites BH2 and B in this tissue. Similar to our previous findings using diabetic, hyperlipidemia and moderate oxidative stress models [7, 11], we saw increased levels of oxidized biopterins (BH2 and B) and decreased ratios of BH4:BH2 + B, without a change in absolute BH4 levels (Fig. 3c). Cai et al. [23] demonstrated that BH4 levels are reduced at the onset of diabetes as well as in endothelial cells exposed to hyperglycemia conditions in vitro. Thus increased levels of oxidized biopterins may compete with L-arginine for nNOS, resulting in a further impairment in nNOS bioactivity and the production of reactive oxygen species [23]. Thus our data indicate that BH4 availability is reduced due to elevated oxidized or inactive biopterins in these mice, thus leading to reduced nNOS activity and function. Therefore, in a setting of diabetes, reduced levels of E2 may augment impairment of BH4-nNOS function and elevate oxidative stress, thus promoting gastroparesis in women.
Estrogens bind to their receptors ERα or ERβ and regulate vascular functions through NO [24]. We measured the protein expression of gastric E2 receptors to investigate if FSH-R knock-out down regulates these receptors in FORKO mouse stomach. Our data show a small but significant decrease in ERα but not ERβ protein expression in FORKO mice gastric muscular tissue (Fig. 4). Collectively, these results suggest that chronic depletion of E2 and gastric ERα lead to decreased availability of BH4 and impaired nitrergic relaxation which promotes gastroparesis. All these results provide compelling evidence that E2 is necessary for optimal gastric motility function in women.
However, unlike results with diabetic animal models [3, 7], we noticed an increase in gastric nNOS dimerization, but not nNOSα protein expression per se, in FORKO mice at 12 weeks (Figs. 1b, c). Reports indicate that an increase in dimerization is the result of decreased proteasome degradation and that nNOS expression is regulated by ubiquitination and proteasome degradation paths [25]. Recently, we found that nNOS dimerization is also elevated in the NRF2 (transcriptional factor that influences Phase II antioxidant gene expression and regulates proteasome subunits) null female mouse stomach [26]. In rodent models of diabetes, E2 is known to protect neuronal survival as well as pancreatic beta cells against oxidative stress, amyloid polypeptide toxicity, lipotoxicity and apoptosis [27]. We speculate, therefore, that E2 deficiency is leading to diminished proteasome activity perhaps due to impaired NRF2 gene expression. The result being that the dimeric form of nNOSα is elevated in FORKO female mice. Even though there is an increase in nNOS dimer in FORKO mice gastric tissue the in vitro levels of NO were diminished in FORKO mice at both 24 and 48 h time points when compared to WT mice (Fig. 2). The decreased NO levels provide a strong support to the hypothesis that loss of nNOS function accounts for the impairment of stomach motility and delay in gastric emptying.
Verrengia et al. [28] demonstrated that symptoms associated with gastroparesis, in particular nausea and early satiety, were elevated in the luteal phase of the menstrual cycle suggesting that endogenous sex hormones may be somewhat harmful rather than beneficial [28]. In addition, these studies further suggested that a variation in the symptoms was not seen in gastroparesis female patients on hormonal contraception. The underlying mechanisms responsible for these discrepancies are unknown. Based on available data together with our recent findings, we suggest that the pathogenetic mechanisms of diabetic gastroparesis are common to both men and women; however, women appear to be disproportionately symptomatic because the motility of their stomachs is slower to begin with, perhaps due to elevated levels of circulating female sex hormones and nitric oxide [9].
Although diabetes induction decreases the circulatory sex steroid hormone levels in both women and female rodents [9, 19, 29]; other studies show increased or no change in these hormone levels [30, 31]. Circulatory estrogens bind to sex hormone-binding globulin and thereby lose its physiological activity. In addition, alterations in testosterone (T) levels may also influence or reduce the availability of physiologically active estrogen levels in the onset of diabetes [30]. The conflicting results observed in published reports could also be due to selection of stage of the estrus cycle in females, time of experimentation after diabetes induction, or measurement of free versus bound circulating estrogens. Eventually these procedures may reflect on myenteric estrogen receptor concentrations in female stomachs.
Acknowledgments
We thank Dr. Ayman Al-Hendy for the FORKO and SVE129 mice, and Dr. Diana Marver for review of the manuscript. Financial support for the original research was provided by NIH-NIDDK R21DKO76704 (PG), P60DK020593 pilot project funds (PG), and RCMI G12RR03032 (PG) start-up funds at Meharry Medical College, Nashville, TN, USA.
Footnotes
Conflict of interest Dr. Gangula has filed a patent application for the use of BH4 in gastroparesis subjects through the University of Texas Medical Branch, Galveston, TX.
Contributor Information
K. Ravella, Department of Physiology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA
A. Al-Hendy, Center for Women’s Health Research, Meharry Medical College, Nashville, TN 37208, USA. Department of Obstetrics and Gynecology, Meharry Medical College, Nashville, TN 37208, USA
C. Sharan, Center for Women’s Health Research, Meharry Medical College, Nashville, TN 37208, USA. Department of Obstetrics and Gynecology, Meharry Medical College, Nashville, TN 37208, USA
A. B. Hale, Department of Cardiovascular Medicine, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK
K. M. Channon, Department of Cardiovascular Medicine, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DU, UK
S. Srinivasan, Division of Gastroenterology and Hepatology, Emory University Medical Center, Atlanta, GA, USA
P. R. Gangula, Email: pgangula@mmc.edu, Department of Physiology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd, Nashville, TN 37208, USA. Center for Women’s Health Research, Meharry Medical College, Nashville, TN 37208, USA
References
- 1.Parkman HP, Yates K, Hasler WL, Nquyen L, et al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140:101–115. doi: 10.1053/j.gastro.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi T. Pathophysiological significance of neuronal nitric oxide synthase in the gastrointestinal tract. J Gastroenterol. 2003;38:421–430. doi: 10.1007/s00535-003-1094-y. [DOI] [PubMed] [Google Scholar]
- 3.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:725–733. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 5.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology. 2000;119:766–773. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 6.Klatt P, Pfeiffer S, List BM, Lehner D, et al. Characterization of heme-deficient neuronal nitric-oxide synthase reveals a role for heme in subunit dimerization and binding of the amino acid substrate and tetrahydrobiopterin. J Biol Chem. 1996;271:7336–7342. doi: 10.1074/jbc.271.13.7336. [DOI] [PubMed] [Google Scholar]
- 7.Gangula PR, Mukhopadhyay S, Ravella K, Cai S, et al. Tetrahydrobiopterin (BH4), a cofactor for nNOS, restores gastric emptying and nNOS expression in female diabetic rats. Am J Physiol Gastrointest Liver Physiol. 2010;298:692–699. doi: 10.1152/ajpgi.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 9.Gangula PR, Sekhar KR, Mukhopadhyay S. Gender bias in gastroparesis: is nitric oxide the answer? Dig Dis Sci. 2011;56:2520–2527. doi: 10.1007/s10620-011-1735-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in folliclestimulating hormone receptor knockout (FORKO) female mice. Endocrinology. 2000;141:4295–4308. doi: 10.1210/endo.141.11.7765. [DOI] [PubMed] [Google Scholar]
- 11.Gangula PR, Chinnathambi V, Hale AB, Mukhopadhyay S, Channon KM, Ravella K. Impairment of nitrergic system and delayed gastric emptying in low density lipoprotein receptor deficient female mice. Neurogastroenterol Motil. 2011;23:773–e335. doi: 10.1111/j.1365-2982.2011.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol. 2001;280:1546–1554. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 13.Kamada Y, Jenkins GJ, Lau M, Dunbar AY, Lowe ER, Osawa Y. Tetrahydrobiopterin depletion and ubiquitylation of neuronal nitric oxide synthase. Brain Res Mol Brain Res. 2005;142:19–27. doi: 10.1016/j.molbrainres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Omoto Y, Imamov O, Warner M, Gustafsson JA. Estrogen receptor α and imprinting of the neonatal mouse ventral prostate by estrogen. Proc Natl Acad Sci. 2005;102:1484–1489. doi: 10.1073/pnas.0409168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree MJ, Tatham AL, Al-Wakeel Y, Warrick N, et al. Quantitative regulation of intracellular endothelial nitric-oxide synthase (eNOS) coupling by both tetrahydrobiopterin-eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J Biol Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 16.Tayeh MA, Marletta MA. Macrophage oxidation of L-arginine to nitric oxide, nitrite, and nitrate: tetrahydrobiopterin is required as a cofactor. J Biol Chem. 1989;264:19654–19658. [PubMed] [Google Scholar]
- 17.Javeshghani D, Schiffrin EL, Sairam MR, Touyz RM. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. J Am Soc Hypertens. 2009;5:295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Javeshghani D, Touyz RM, Sairam MR, Virdis A, Neves MF, Schiffrin EL. Attenuated responses to angiotensin II in follitropin receptor knockout mice, a model of menopause-associated hypertension. Hypertension. 2003;42:761–767. doi: 10.1161/01.HYP.0000085331.22169.3F. [DOI] [PubMed] [Google Scholar]
- 19.Kim NN. Sex steroid hormones in diabetes-induced sexual dysfunction: focus on the female gender. J Sex Med. 2009;3:239–246. doi: 10.1111/j.1743-6109.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Serova LI, Filipenko M, Schilt N, Veerasirikul M, Sabban EL. Estrogen-triggered activation of GTP cyclohydrolase 1 gene expression: role of estrogen receptor subtypes and interaction with cyclic AMP. Neuroscience. 2006;140:1253–1263. doi: 10.1016/j.neuroscience.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki-Akita A, Hayashi T, Ding QF, Shiraishi H, et al. 17Beta-estradiol antagonizes the down-regulation of endothelial nitric-oxide synthase and GTP cyclohydrolase I by high glucose: relevance to postmenopausal diabetic cardiovascular disease. J Pharmacol Exp Ther. 2007;320:591–598. doi: 10.1124/jpet.106.111641. [DOI] [PubMed] [Google Scholar]
- 22.Wells CC, Riazi S, Mankhey RW, Bhatti F, Ecelbarger C, Maric C. Diabetic nephropathy is associated with decreased circulating estradiol levels and imbalance in the expression of renal estrogen receptors. Gend Med. 2005;2:227–237. doi: 10.1016/s1550-8579(05)80052-x. [DOI] [PubMed] [Google Scholar]
- 23.Cai S, Khoo J, Mussa S, Alp NG, Channon KM. Endothelial nitric oxide synthase dysfunction in diabetic mice: importance of tetrahydrobiopterin in eNOS dimerization. Diabetologia. 2005;48:1933–1940. doi: 10.1007/s00125-005-1857-5. [DOI] [PubMed] [Google Scholar]
- 24.Arnal JF, Fontaine C, Billon-Galés A, Favre J, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. doi: 10.1161/ATVBAHA.109.191221. [DOI] [PubMed] [Google Scholar]
- 25.Bender AT, Demady DR, Osawa Y. Ubiquitination of neuronal nitric-oxide synthase in vitro and in vivo. J Biol Chem. 2000;275:17407–17411. doi: 10.1074/jbc.M000155200. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyay S, Sekhar KR, Hale AB, et al. Loss of NRF2 impairs gastric nitrergic stimulation and function. Free Radic Biol Med. 2011;51:619–625. doi: 10.1016/j.freeradbiomed.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiano JP, Mauvias-Jarvis F. Importance of estrogen receptors to preserve functional β-cell mass in diabetes. Nat Rev Endocrinol. 2012;8:342–351. doi: 10.1038/nrendo.2011.242. [DOI] [PubMed] [Google Scholar]
- 28.Verrengia M, Sachdeva P, Gaughan J, Fisher RS, Parkman HP. Variation of symptoms during the menstrual cycle in female patients with gastroparesis. Neurogastroenterol Motil. 2011;23:1365–1368. doi: 10.1111/j.1365-2982.2011.01681.x. [DOI] [PubMed] [Google Scholar]
- 29.Mankhey RW, Bhatti F, Maric C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2005;288:F399–F405. doi: 10.1152/ajprenal.00195.2004. [DOI] [PubMed] [Google Scholar]
- 30.Maric C. Sex, diabetes and the kidney. Am J Physiol Renal Physiol. 2009;296:F680–F688. doi: 10.1152/ajprenal.90505.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Showkat Ali M, Tiscareno-Grejada I, Locovei S, Smiley R, Collins T, Sarosiek J, McCallum R. Gender and estradiol as major factors in the expression and dimerization of nNOS in rats with experimental diabetic gastroparesis. Dig Dis Sci. 2012;57:2814–2825. doi: 10.1007/s10620-012-2230-4. [DOI] [PubMed] [Google Scholar]