Abstract
During a T cell response, naïve CD8 T cells differentiate into effector cells. Subsequently, a subset of effector cells termed memory precursor effector cells (MPECs) further differentiates into functionally mature memory CD8 T cells. The transcriptional network underlying this carefully scripted process is not well understood. Here, we report that the transcription factor FoxO1 plays an integral role in facilitating effector to memory transition and functional maturation of memory CD4 and CD8 T cells. We find that FoxO1 is not required for differentiation of effector cells, but in the absence of FoxO1, memory CD8 T cells displayed features of senescence and progressive attrition in polyfunctionality, which in turn led to impaired recall responses and poor protective immunity. These data suggest that FoxO1 is essential for maintenance of functional CD8 T cell memory and protective immunity. Under competing conditions in bone marrow chimeric mice, FoxO1-deficiency did not perturb clonal expansion or effector differentiation. Instead, FoxO1-deficient MPECs failed to survive and form memory CD8 T cells. Mechanistically, FoxO1 deficiency perturbed the memory CD8 T-cell transcriptome, characterized by pronounced alterations in the expression of genes that encode transcription factors (including Tcf7), effector molecules, cell cycle regulators and proteins that regulate fatty acid, purine and pyramidine metabolism and mitochondrial functions. We propose that FoxO1 is a key regulator that reprograms and steers the differentiation of effector cells to functionally competent memory cells. These findings have provided fundamental insights into the mechanisms that regulate the quality of CD8 T-cell memory to intracellular pathogens.
Introduction
During T cell responses to vaccines or infections, TCR signaling occurring in concert with co-stimulatory and inflammatory signals stimulate naïve CD8 T cells to clonally expand and differentiate into effector CD8 T cells (1–2). The resulting heterogeneous population of effector cells consists of at least two subsets: the terminally differentiated short-lived effector cells (SLECs) and memory precursor effector cells (MPECs)(3). Upon resolution of the insult, the majority of the SLECs are lost by apoptosis but MPECs survive and eventually differentiate into long-lived memory CD8 T cells. Upon re-infection, memory CD8 T cells rapidly expand and/or develop effector functions, to clear the infection expeditiously; this is the basis of CD8 T cell memory-dependent protective immunity(4). The developmental pathway from naïve T cells to fully functional memory CD8 T cells encompasses two critical differentiation checkpoints. First, is the differentiation of naïve T cells to effector cells, and second is the differentiation of effector cells to functional memory CD8 T cells(3). The generation of a protective population of memory T cells depends upon successful passage through both checkpoints, therefore a thorough understanding of the cellular processes underlying these transitions is necessary for the rational development of strategies to manipulate CD8 T cell memory.
The differentiation to effector cells from naïve T cells is associated with installation of the effector transcription program driven by transcription factors (TFs) such as T-bet and Blimp-1 (5–8). This transcriptional program transforms quiescent cells into SLECs and MPECs. The subsequent differentiation of the MPECs into mature memory T cells occurs more slowly, over a period of several weeks. It is clear that the installation of a memory-specific transcriptional program must encompass substantial changes in gene expression (9). The MPECs, which are present among the effectors at the peak of the response do not yet possess the attributes that define competent memory CD8 T cells(9). These MPECs lack the ability to self-renew by cytokine-driven proliferation, do not proliferate upon re-exposure to the antigen or produce cytokines such as IL-2, and confer poor protective immunity to re-infection (9). Essentially, the new transcriptional program must facilitate a transition from a state of cell cycle arrest and highly active cytolytic function to a pseudo-quiescent state defined by heightened preparedness to rapidly proliferate and/or develop effector functions upon antigen re-exposure(9). Multiple transcription factors such as TCF-1 and Bcl-6 have been implicated in the regulation of effector to memory transition (10–12), however the full transcriptional network governing the functional maturation and maintenance of memory CD8 T cells remains poorly defined.
In mammals, members of the Forkhead box-O class of TFs collectively referred to as FoxOs include FoxO1, FoxO3, FoxO4 and FoxO6 (13). The FoxOs play a crucial role in regulating cellular processes such as proliferation, apoptosis, energy metabolism, and stress resistance in response to dynamic alterations in stress and abundance of nutrients and growth factors (14–16). In resting, quiescent cells, FoxOs exist in a hypo-phosphorylated state and localize to the nucleus to control the transcription of target genes that affect cellular proliferation or apoptosis (17). However, in response to stimulation with growth factors or cytokines that stimulate the PI3K/AKT signaling pathway, activated Akt phosphorylates FoxO leading to its exclusion from the nucleus and degradation by proteolysis in the cytoplasm (18–19). Consequently, the transcription of FoxO target genes is diminished, which facilitates cell cycle entry.
T cells express FoxO1 and FoxO3, which play key roles in the maintenance of T-cell homeostasis (20–21). FoxO3 downregulates the clonal expansion of CD8 T cells during an acute viral infection by T-cell intrinsic and extrinsic mechanisms (22–23). FoxO1 on the other hand plays a key role in promoting T-cell quiescence and peripheral tolerance(24–26). Inactivation of FoxO1 is obligatory for T cells to enter cell cycle and conditional deficiency of FoxO1 in T cells leads to spontaneous activation of T cells and autoimmunity. Additionally, FoxO1 protects against autoimmunity by promoting the development and function of regulatory T cells in the periphery (25, 27). Studies of in vitro-cultured cells indicate that FoxO1 might play a role in regulating CD8 T cell memory (28), but the biological significance of such studies has not been clarified yet. Moreover, it is clear that FoxO1 is important for maintaining T-cell quiescence and survival (24, 26), but its role in effector to memory transition and survival of memory CD8 T cells is yet to be determined in vivo. In this manuscript, we have examined the responses of antigen-specific FoxO1-deficient T cells to an acute viral infection in vivo, under competitive and non-competitive conditions. By doing so, we have delineated the pivotal roles of FoxO1 in guiding effector to memory transition, functional maturation and survival of memory CD4 and CD8 T cells and CD8 T cell memory-dependent protective immunity. Mechanistically, FoxO1 deficiency led to pronounced dysregulation in the transcriptome of memory CD8 T cells characterized by altered expression of genes that segregate into specific cellular processes including transcription (especially TCF-1), cytolytic effector function, proliferation, survival, energy homeostasis and mitochondrial metabolism. Based on the data presented in this manuscript along with published findings (24), we propose that FoxO1 is a key transcription factor that: (1) governs survival and quiescence of naïve and memory T cells; (2) reprograms the effector cell program and steers the “de-differentiation” of effector cells to functionally competent resting memory cells that share several features of naïve T cells. These findings have advanced our understanding of the transcriptional regulation of naïve and memory T-cell homeostasis and have implications in the development of vaccines that confer durable and effective cell-mediated immunity to intracellular pathogens.
Materials and Methods
Mice
Mice with T-cell-specific Foxo1 deletion, designated as FoxO1 −/− were generated by breeding CD4-Cre mice (Taconic Farms) with FoxO1f/f mice, which were a gift from Dr. A. DePinho (Dana-Farber Cancer Institute). FoxO1f/f mice were generated as previously reported (29). The CD4-Cre transgenic mouse strain expresses Cre recombinase under the control of the CD4 tissue-specific promoter. The presence of the Cre transgene and the absence of the FoxO1 protein were confirmed by PCR and flow cytometry respectively. All experiments were conducted in accordance with the approved protocols of the institutional animal care committee.
Viral infections
Mice that are 6 to 8 weeks old were infected intraperitoneally (i.p.) with 2 × 105 PFU Armstrong strain of LCMV to induce acute infection. LCMV-immunized mice were challenged with Clone 13 strain of LCMV intravenously at a dose of 2 × 106 PFU and infectious LCMV was quantified by a plaque assay on Vero cells, as described previously (30).
Flow cytometry and cell surface staining
Single-cell suspensions of mononuclear cells from spleen were prepared using standard procedures. MHC class I tetramers, specific for the LCMV epitopes Db/NP396–404 and Db/GP33–41, were prepared and used as previously described (31). Briefly, cells were stained with anti-CD8 (RPA-T8) and MHC class I tetramers. In some experiments, cells were co-stained with anti-CD44 (IM7), anti-CD62L (MEL-14), anti-KLRG-1 (2F1), anti-CD127 (A7R34), anti-CD122 (TM-Beta 1), anti-CD27 (LG.3A10), anti-LFA-1 (2D7), anti-CD45.1 (Ly5.1) (A20), and anti-CD45.2 (Ly5.2) (104). All Abs were purchased from BD Biosciences or eBioscience except the anti-KLRG-1 Ab (Southern Biotech). Samples were analyzed on a FACSCalibur or LSRII flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (Tree Star).
Intracellular staining for cytokines and granzyme B
For intracellular cytokine staining, splenocytes were stimulated ex vivo with LCMV epitope peptides NP396 or GP33 in the presence of IL-2 and Brefeldin A (BD Biosciences) for 5hr at 37°C. After culture, cells were stained for cell surface molecules and intracellular IFN-γ (XMG1.2), TNF-α (MP6-XT22), and IL-2 (JES6-5H4), using Cytofix/Cytoperm intracellular staining kit (BD Biosciences). To stain for intracellular Gznb, splenocytes were stained for cell surface molecules and subsequently permeabilized and stained for intracellular proteins using anti-Gznb (GB11) Ab (Invitrogen).
Staining for intracellular proteins
Splenocytes were stained with anti-CD8 in conjunction with MHC class I tetramers. Following surface staining, cells were fixed, lysed and washed using the PhosFlowKit (BD Biosciences). Cells were subsequently stained for intracellular proteins FoxO1 (C29H4), TCF1 (C63D9) (Cell Signaling Technology), EOMES (Dan11mag) and T-bet (eBIO-4B10; eBioscience), Bcl-6 (K112-91) and Bcl-2 (Bcl-2/100; BD Biosciences); and protein specific (BD Biosciences) or IgG isotype (DA1E) control Abs (Cell Signaling Technology).
BrdU staining
To assess in vivo proliferation of antigen-specific cells, LCMV-immune mice were administered BrdU (MP Biomedicals), 1.5 mg once i.p. and subsequently (0.8mg/ml) in drinking water for 8 days. On day 9 after the initiation of BrdU administration, splenocytes were stained with anti-CD8 in conjunction with MHC class I tetramers and BrdU, using a BrdU staining kit (BD Biosciences) according to the manufacturer’s recommendations.
Mitochondria and DiOC6 staining
To assess mitochondrial content and potential, single-cell suspensions of mononuclear cells from spleen of LCMV immune mice were stained with, anti-CD8, MHC class I tetramers and co-stained with MitoTracker and DiOC6 (Invitrogen) (32). Staining was according to manufacturer’s recommendations. Briefly, 100ul of 100nM MitoTracker and 40nM of DiOC6 was incubated with cells at 37°C for a half hour. This was followed by a half hour surface staining with anti-CD8 and Db/NP396 tetramers.
Generation of bone marrow chimeras
Bone marrow cells (BMCs) were collected by flushing the femurs and tibias from WT and FoxO1 −/− mice with RPMI 1640 media. Single-cell suspensions of BMCs were depleted of T cells using anti-CD5 microbeads (Milteny Biotec). A 1:1 mixture of 7.5 × 106 T cell-depleted BMCs from WT (Ly5.1) and WT (Ly5.2) or FoxO1 −/− (Ly5.2) mice were adoptively transferred into lethally irradiated (900 rads) WT Rag1-deficient (Rag1−/−) mice. Bone marrow-reconstituted Rag1−/− mice were treated with neomycin (0.025 mg/ml) and polymyxin B (.013 mg/ml; Sigma-Aldrich) in drinking water for up to 6 weeks. Reconstitution of the lymphoid system by the donor BMCs were assessed at 6 weeks and mice were infected with LCMV 8 weeks after cell transfer.
Transcriptome analysis by microarray
Single-cell suspensions from splenocytes of WT and FoxO1−/− LCMV-immune mice were prepared using standard procedures. CD8 T cells were then isolated using Thy1.2 (CD90.2) (30-H12) microbeads (Miltenyi Biotec). Cells were then stained with anti-CD8 and Db/NP396 MHC class I tetramers. Virus-specific CD8 T cells were sorted using FACSAria II instrument (BD Biosciences). The purity of the cells was >95%. Total RNA was extracted from the sorted cells by TRIzol Reagent. RNA samples were reverse transcribed and Cy3-labeled cDNAs were hybridized to Agilent whole Mouse Genome Oligo Microarrays. Fluorescence signals were detected using Agilent’s Microarray Scanner system, data was analyzed using the Rosetta Resolver gene expression data analysis system and genes with a fold change >2 and p-value≤0.01 were identified. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus and are accessible through GEO Series accession number GSE45673 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE45673). Gene clusters were identified using the DAVID functional annotation tool (33–34). Pathways that were significantly affected by the loss of FoxO1 were identified using the Partek® software, version 6.6 Copyright © 2012, Partek Inc., St. Louis, MO, USA.
Statistical methods
SEM and p-values were determined using Prism software (GraphPad Software, Inc.). P-values were calculated using Student’s t test and significance was defined at p≤0.05.
Results
Clonal expansion and effector differentiation of FoxO1-deficient CD8 T cells
Here, using T cell-specific conditional FoxO1-deficient (FoxO1−/−) mice, we investigated the extent to which FoxO1 regulates antigen-driven proliferative expansion and differentiation of effector CD8 T cells following an acute lymphocytic choriomeningitis virus (LCMV) infection in mice. At 6–8-weeks of age, FoxO1 deficiency in T cells did not significantly alter the size of the CD8 T-cell compartment in uninfected mice (Supplemental Fig. 1). At day 8 post-infection (PI) with LCMV, conditional deletion of FoxO1 in T cells minimally altered the numbers of CD8 T cells in spleens (Fig. 1A and 1B). Likewise the percentages and total number of CD8 T cells that are specific to the immunodominant epitopes, NP396–404 (NP396) and GP33–41 (GP33) were comparable in wild type (WT) and FoxO1−/− mice (Fig. 1C and 1D). These findings suggested that FoxO1 deficiency did not significantly affect the accumulation of LCMV-specific CD8 T cells in vivo, during an acute viral infection.
Figure 1. Activation and expansion of CD8 T cells in FoxO1−/− mice.
Groups of WT and FoxO1 −/− mice were infected with LCMV. On day 8 after infection, splenocytes were stained with anti-CD8 and anti-CD44 antibodies, Db/NP396 and Db/GP33 tetramers. A and B, Bar graphs show the percentages and total number of CD8 T cells in spleen on day 8 PI. C, Flow cytometry plots are gated on total splenocytes and the numbers are the percentages of tetramer-binding CD8 T cells among splenocytes. D, Bar graph shows the total number of LCMV-specific CD8 T cells. Data are the mean ± SEM and representative of at least 3 independent experiments.
Next, we evaluated the ability of LCMV-specific effector CD8 T cells from WT and FoxO1−/− mice to produce IFN-γ upon antigen stimulation ex vivo at day 8 PI. CD8 T cells from both WT and FoxO1−/− mice produced readily detectable levels of IFN-γ upon stimulation with the cognate antigenic peptide and the percentages or total number of IFN-γ-producing epitope-specific CD8 T cells were not significantly different between WT and FoxO1−/− mice (Fig. 2A and 2B). However, the amounts of IFN-γ produced (based on measurement of mean fluorescent intensity [MFI]) by FoxO1-deficient effector CD8 T cells were significantly lower than those produced by WT effector CD8 T cells (Fig. 2C). We also measured the ability of LCMV-specific CD8 T cells to produce TNF-α and IL-2. The fraction of IFN-γ-producing CD8 T cells that also produced TNF-α and IL-2 was similar (1–3% and 2–4% for NP396-specific and GP33-specific CD8 T cells respectively) for LCMV-specific CD8 T cells from WT and FoxO1−/− mice. As a surrogate index for the cytolytic function of effector CD8 T cells, we quantified granzyme B (Gznb) levels in LCMV-specific CD8 T cells directly ex vivo (Fig. 2D). Interestingly, the levels of Gznb in CD8 T cells from FoxO1−/− mice were substantially greater than in effector CD8 T cells from WT mice. Thus, T cell-specific loss of FoxO1 dysregulated the expression of effector molecules IFN-γ and Gznb in CD8 T cells. However, infectious LCMV was below the levels of detection in tissues of both WT and FoxO1−/− mice by day 8 PI.
Figure 2. Effector functions of FoxO1−/− CD8 T cells.
At day 8 after LCMV infection, splenocytes from WT and FoxO1 −/− mice were stimulated with NP396 and GP33 peptides. Antigen-induced production of IFNγ was quantified by intracellular staining and flow cytometry. Bar graphs in A show the percentage of IFN-γ-producing CD8 T cells; B, shows the total number of CD8 T cells producing IFN-γ and C illustrates the MFI of IFN-γ. D, Splenocytes were stained with anti-CD8, anti-granzyme B and Db/NP396 tetramers. Histograms are gated on tetramer-binding CD8 T cells, and the numbers in the histograms represent the MFI for granzyme B. Data are mean ± SEM from 3–5 mice/group and representative of 3–5 independent experiments. * p<.05
The population of LCMV-specific effector CD8 T cells that is present at the peak of clonal expansion (i.e. day 8 PI) consists of two distinct subsets based on the differential expression of CD127 (the IL-7R) and KLRG-1 (senescence marker): the SLECs (CD127Lo/KLRG-1Hi) and MPECs (CD127Hi/KLRG-1Lo) (6). At day 8 PI, as expected, a large fraction the WT and FoxO1−/− effector CD8 T cells expressed the SLEC phenotype. While 3–5% of effector CD8 T cells displayed the MPEC phenotype in the WT mice, prototypical MPECs were hardly detectable among effector CD8 T cells from FoxO1−/− mice (Fig. 3A). Our inability to detect MPECs in FoxO1−/− mice was due to loss of CD127 expression on effector CD8 T cells, and not linked to loss of MPECs per se. This is because MPECs in FoxO1−/− mice were still detectable as CD127Lo/KLRG-1Lo subset. The loss of CD127 expression on FoxO1−/− CD8 T cells was expected because FoxO1 is required for full expression of CD127 in T cells(26). Since, CD127 expression was defective in FoxO1−/− effector CD8 T cells, we used KLRG-1 expression alone to distinguish MPECs and SLECs (Fig. 3A). The total numbers of MPECs and SLECs in FoxO1−/− mice were comparable to those in WT mice. Additionally, FoxO1−/− effector CD8 T cells displayed only a slight increase in the expression of T-bet, compared to WT effector CD8 T cells (Fig. 3B). Further, FoxO1 deficiency had no detectable effect on EOMES protein levels in LCMV-specific effector CD8 T cells (Fig. 3C). These data led to the inference that FoxO1 deficiency did not significantly affect the differentiation of SLECs and MPECs during an acute LCMV infection. We also investigated whether FoxO1 deficiency affected the cell surface phenotype of LCMV-specific effector CD8 T cells at day 8 PI (Fig. 3D). As expected LCMV-specific effector CD8 T cells in WT mice displayed the CD44HiCD27HiCD127LoCCR7Lo phenotype (Fig. 3D). Notably, LCMV-specific effector CD8 T cells from FoxO1−/− mice expressed lower levels of CD44 and CD27, as compared to their WT counterparts; the expression levels of other molecules were largely similar in WT and FoxO1−/− effector CD8 T cells (Fig. 3D).
Figure 3. Effect of FoxO1 deficiency on differentiation of effector subsets.
WT and FoxO1−/− mice were infected with LCMV. At day 8 PI splenocytes were stained with anti-CD8, Db/NP396 tetramers, anti-KLRG1 and anti-CD127. A, Representative FACS plots are gated on NP396–specific CD8 T cells. The numbers are the percentages of the following subsets: KLRG-1HiCD127Lo, KLRG-1LoCD127Hi, and KLRG-1LoCD127Lo among NP396- specific CD8 T cells. The bar graph shows the total number of KLRG-1Hi or KLRG-1Lo subsets of NP396-specific CD8 T cells. B and C, At day 8 after LCMV infection, splenocytes were stained with anti-CD8, Db/NP396 tetramers and anti-T-bet or anti-Eomes antibodies. T-bet and Eomes levels in NP396-specific CD8 T cells were assessed by quantifying MFI on a flow cytometer. D, To characterize the cell surface phenotypes of NP396- and GP33-specific CD8 T cells, splenocytes were stained with Db/NP396 and Db/GP33 tetramers in conjunction with anti-CD44, anti-CD127, anti-CD62L, anti-CD122, anti-CD27 and anti-CCR7. The histograms are gated on tetramer-binding CD8 T cells, and the numbers in the histograms represent the MFI for the indicated protein. Data are the mean ± SEM from analysis of 3–5 mice/group, and representative of at least three independent experiments.
Impaired memory CD8 T cell differentiation in the absence of FoxO1
After day 8 PI, in the ensuing 3–4 weeks, MPECs embark on a differentiation program that guides effector to memory transition, which signifies a switch from a state of immediate cytolytic effector function to a resting but poised state that enables these cells to clonally expand and rapidly develop effector functions upon re-exposure to antigen(35). Additionally, effector to memory transition for CD8 T cells entails the acquisition of canonical memory features such as the high proliferative potential and cytokine-driven self-renewal (9). First, we assessed whether FoxO1 regulates differentiation of MPECs into fully functional memory CD8 T cells. At day 60 PI, the frequencies and total numbers of LCMV-specific memory CD8 T cells in FoxO1−/− mice were comparable to those in WT mice (Fig. 4A and 4B). Thus, FoxO1 deficiency did not affect the magnitude of CD8 T-cell memory.
Figure 4. CD8 T-cell memory in FoxO1−/− mice.
WT and FoxO1 −/− mice were infected with LCMV, and virus-specific CD8 T cells were quantified in spleen at day 60 PI. Splenocytes were stained with anti-CD8 and anti-CD44 antibodies, Db/NP396 and Db/GP33 tetramers. A, Flow cytometry plots are gated on total CD8 T cells and the numbers are the percentages of tetramer-binding CD8 T cells among CD8 T cells. B, Bar graph shows the total number of LCMV-specific CD8 T cells. C, At day 60 PI, splenocytes were stained with anti-CD8, Db/NP396 tetramers, anti-KLRG-1, and anti-CD127. Representative FACS plots are gated on NP396-specific CD8 T cells. The numbers are the percentages of the following subsets: KLRG-1HiCD127Lo, KLRG-1LoCD127Hi, and KLRG-1LoCD127Lo among NP396- specific CD8 T cells. The bar graph in C shows the KLRG-1 MFI for NP396-specific CD8 T cells. D, To characterize the cell surface phenotypes of NP396- and GP33-specific CD8 T cells, splenocytes were stained with Db/NP396 and Db/GP33 tetramers along with anti-CD44, anti-CD127, anti-CD62L, anti-CD122, anti-CD27, and anti-CCR7. The histograms are gated on tetramer-binding CD8 T cells, and the numbers in each of the histograms represent the MFI for the indicated protein. Data are the mean ± SEM from analysis of 3–5 mice/group, and representative of at least two to three independent experiments.
Effector to memory transition also includes enrichment for CD127Hi/KLRG-1Lo cells and it was of interest to determine whether FoxO1 deficiency disrupted transition into memory cells. At day 60 PI, as expected, 60–80% of LCMV-specific memory CD8 T cells in WT mice displayed the CD127Hi/KLRG-1Lo phenotype (Fig. 4C). In striking contrast, only 40–50% of memory CD8 T cells in FoxO1−/− mice expressed the CD127Hi/KLRG-1Lo phenotype, and the remaining 30–50% of the cells exhibited the terminally differentiated/senescent CD127Lo/KLRG-1Hi phenotype (Fig. 4C). Notably, regardless of CD127 expression, memory CD8 T cells from FoxO1−/− mice expressed significantly higher levels of KLRG-1, which is highly suggestive of a senescent state (Fig. 4C). Further, the expression of several cell surface molecules was dysregulated in memory CD8 T cells from FoxO1−/− mice (Fig. 4D). FoxO1−/− memory CD8 T cells displayed substantially reduced levels of cell surface CD127, CD62L and CCR7 as compared to their WT counterparts. Thus, as in naïve T cells(26), FoxO1 is required for full expression of CD127, CD62L and CCR7 on memory CD8 T cells. In contrast to CD127, the cell surface expression of CD122 was minimally altered on FoxO1−/− memory CD8 T cells. Interestingly, CD44 levels on FoxO1−/− memory CD8 T cells were considerably lower than in WT memory CD8 T cells. Significantly, the expression of CD27, a molecule implicated in protective immunity was also substantially reduced in FoxO1−/− memory CD8 T cells (Fig. 4D) (36). The overall cell surface phenotype of FoxO1−/− memory CD8 T cells is highly suggestive of disrupted memory differentiation (37). Collectively, these data suggested that FoxO1 plays a crucial role in facilitating effector to memory transition during an acute viral infection.
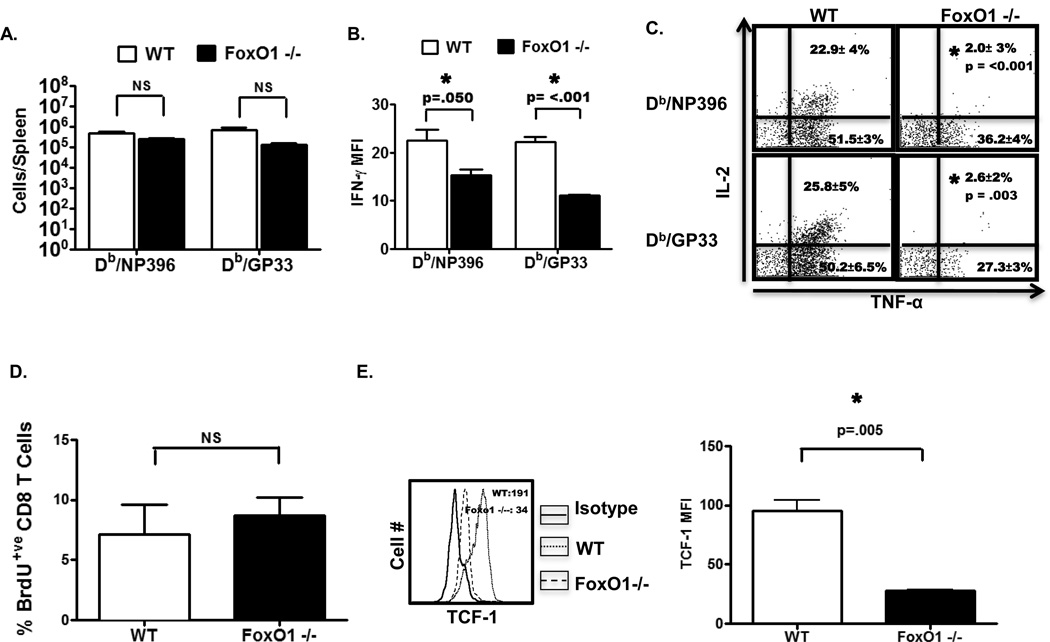
Functional maturation of memory CD8 T cells is associated with the acquisition of the ability to produce multiple cytokines including IFN-γ, TNF-α and IL-2. To assess the effect of FoxO1 deficiency on the cytokine-producing functions of memory CD8 T cells, we quantified antigen-induced cytokine production, at day 60 PI. The total numbers of epitope-specific IFN-γ-producing CD8 T cells in FoxO1−/− mice were not significantly different, as compared to those in WT mice (Fig. 5A). Notably, however, FoxO1-deficient memory CD8 T cells produced significantly lower levels of IFN-γ than WT memory CD8 T cells (Fig. 5B). Furthermore, while 20–30% of IFN-γ-producing CD8 T cells also produced TNF-α and IL-2 in the WT mice, only 2% of FoxO1−/− memory CD8 T cells co-produced all the three cytokines (Fig. 5C). Taken together, these data strongly suggested that FoxO1 plays a non-redundant role in promoting polycytokine production and functional maturation of memory CD8 T cells. A hallmark feature of memory CD8 T cells is their ability to self renew by IL-7 and/or IL-15-driven proliferation (38). LCMV-immune WT and FoxO1−/− mice were administered BrdU and cytokine-driven proliferation of memory CD8 T cells was assessed by flow cytometry. These in vivo BrdU incorporation studies showed that the proliferative renewal of LCMV-specific memory CD8 T cells in FoxO1−/− mice was similar to those in WT mice (Fig. 5D).
Figure 5. Effector functions of FoxO1−/− memory CD8 T cells.
On day 60 after LCMV infection, splenocytes from WT and FoxO1−/− mice were stimulated with NP396 and GP33 peptides and antigen-induced cytokine production was assessed by intracellular cytokine staining. A, Bar graph shows the total numbers of IFN-γ-producing NP396- and GP33-specific CD8 T cells. B, Bar graph illustrates the MFI for IFN-γ. C, Representative dot plots (gated on IFN-γ-producing CD8 T cells) show the percentage of IL-2+ and/or TNF-α+ cells among IFN-γ+ CD8 T cells. D, At day 60 PI, mice were administered BrdU for 8 days. After BrdU treatment, splenocytes were stained with Db/NP396 tetramers, anti-CD8, and anti-BrdU. The bar graph shows the percent of BrdU positive cells among NP396-specific CD8 T cells. Data are mean ± SEM from analysis of 3–5 mice/group and representative of 3 independent experiments. E. At day after LCMV infection, splenocytes were stained with anti-CD8 in conjunction Db/NP396 tetramers and anti-TCF-1 antibodies. The histogram is gated on Db/NP396-specific CD8 T cells from WT and FoxO1−/− mice, and the numbers in the histogram represents the MFI for TCF-1. The bar graph in E illustrates the MFI of TCF-1. Data are mean ± SEM from analysis of 3–5 mice/group.
FoxO1 actively maintains functional CD8 T cell memory and secondary responses in FoxO1−/− mice
To better understand the role of FoxO1 in regulating the generation and maintenance of functional CD8 T cell memory, we performed a detailed kinetic analysis of LCMV-specific CD8 T cells using MHC I tetramers and intracellular cytokine staining. Enumeration of LCMV-specific CD8 T cells by MHC I tetramers and intracellular staining for IFN-γ showed that the overall kinetics of the CD8 T cell response in WT and FoxO1−/− mice was largely similar (Fig. 6A and 6B). The numbers of polycytokine-producing LCMV-specific CD8 T cells (cells that produced IFN-γ, TNF-α and IL-2) remain relatively stable ensuing the resolution of an acute LCMV infection in WT mice (Fig. 6C). In striking contrast, there was a gradual attrition in the number of polycytokine-producing memory CD8 T cells in FoxO1−/− mice. These data suggested that FoxO1 is required for maintenance of functional CD8 T cell memory following an acute viral infection.
Figure 6. Maintenance of functional CD8 T cell memory in FoxO1−/− mice.
A, B and C. WT and FoxO1 −/− mice were infected with LCMV and at the indicated time days PI, the total number of NP396- and GP33-specific CD8 T cells were quantified by using MHC I tetramers (A) or intracellular staining for IFN-γ (B), TNF- α and IL-2. C, shows the total number of NP396- and GP33-specific CD8 T cells that produced IFN-γ, IL-2 and TNF-α. Data are representative of three to four independent experiments with three to five mice per group for each indicated time point. Error bars represent the SEM. * p<.05. D, Forty-five to sixty days after LCMV-Armstrong infection, WT and FoxO1−/− mice were challenged with LCMV-Clone 13. Mice were sacrificed at day 5 after challenge. Splenocytes were stained with Db/NP396, Db/GP33, Db/GP276 tetramers and anti-CD8. The numbers of tetramer-binding CD8 T cells were assessed by flow cytometry and LCMV titers in the kidney were quantified using a plaque assay; each data point in the scatter plot represents viral titer of an individual mouse.
Unlike an acute infection with Armstrong strain of LCMV, infection of immunocompetent mice with Clone 13 strain of LCMV results in a chronic infection lasting up to 6 months (30). However, in mice that have recovered from an acute infection with the Armstrong strain of LCMV, memory CD8 T cells undergo rapid secondary expansion, differentiate into effector cells and resolve LCMV-Clone 13 within 5 days after challenge (39). To assess the role of FoxO1 in regulating secondary expansion of memory CD8 T cells and protective immunity, WT and FoxO1−/− mice were immunized with LCMV-Armstrong. At 45–60 days after immunization, LCMV-immune mice were challenged with LCMV-Clone 13 and secondary CD8 T cell responses and viral control was assessed at day 5 after challenge. As shown in Fig. 6D, LCMV-specific memory CD8 T cells in WT mice exhibited robust secondary expansion. However, the numbers of LCMV-specific CD8 T cells in spleen (Fig. 6D) of FoxO1−/− mice were significantly lower than in WT mice. Importantly, only 1 of 8 WT mice had detectable infectious LCMV in the kidneys, but kidneys of 6 of 11 FoxO1−/− mice contained infectious LCMV at day 5 after challenge (Fig. 6D). Thus, the development and/or maintenance of functional CD8 T-cell memory, secondary expansion and protective immunity require FoxO1 in T cells.
FoxO1 regulates the function of effector and memory CD4 T cells
Results presented so far clearly illustrated a non-redundant role for FoxO1 in the maintenance of functional CD8 T cell memory. It was of interest to explore whether conditional deficiency of FoxO1 exerted similar effects on the functions of effector and memory CD4 T cells. The numbers of IFN-γ-producing LCMV-specific effector CD4 T cells in spleen of FoxO1−/− mice were comparable to those in WT mice at day 8 PI, which suggested that the expansion of CD4 T cells was largely unaffected by FoxO1 deficiency (Fig. 7A). Additionally, the levels of IFN-γ induced in FoxO1−/− effector CD4 T cells were not significantly different than in WT CD4 T cells (Fig. 7B). However, the percentages of polycytokine-producing LCMV-specific effector CD4 T cells in FoxO1−/− mice were lower than in WT mice (Fig. 7C). At day 60 PI, the numbers of IFN-γ-producing LCMV-specific memory CD4 T cells in spleen of FoxO1−/− mice were similar to those in WT mice (Fig. 7D). Notably however, in comparison to WT CD4 T cells, memory CD4 T cells in FoxO1−/− mice produced substantially lower levels of IFN-γ (Fig. 7E). A large fraction of WT IFN-γ-producing memory CD4 T cells also produced TNF-α and IL-2, but the ability of FoxO1−/− memory CD4 T cells to produce all the three cytokines was significantly diminished (Fig. 7F). Taken together, these findings suggested that FoxO1 controls the functions of memory CD4 T cells without affecting their number, at least under non-competitive conditions.
Figure 7. FoxO1 regulates function of effector and memory CD4 T cells.
WT and FoxO1 −/− mice were infected with LCMV. At days 8 and day 60 postinfection, splenocytes were stimulated with the I-Ab-restricted GP66 peptide for 5 hrs. A and D, shows the total numbers of IFN-γ-producing I-Ab-restricted GP66 CD4 T cells for day 8 and day 60 respectively. B and E, The levels of IFN-γ produced by I-Ab-restricted GP66-specific CD4 T cells were measured by flow cytometry and bar graphs illustrate the MFI for IFN-γ for day 8 and day 60 respectively. C and F, The percentages of CD4 T cells that produced IFN-γ, IL-2 and TNF-α were quantified by intracellular cytokine staining on day 8 and day 60 respectively. Data are mean ± SEM from analysis of 3–5 mice/group.
FoxO1 deficiency dysregulates the transcriptome of memory CD8 T cells
Although target genes for FoxO1 have been identified in naïve and regulatory T cells(24, 40), the constellation of genes controlled by FoxO1 in memory CD8 T cells is yet to be determined. To gain insight into the transcriptional basis of FoxO1 actions in memory CD8 T cells, we compared the transcriptome of WT and FoxO1−/− NP396-specific bonafide memory CD8 T cells by microarray analysis. Remarkably, >6,000 genes were downregulated and >5,000 genes were significantly (P<0.01) up-regulated >2-fold in FoxO1−/− memory CD8 T cells, as compared to those in WT memory CD8 T cells. In particular, loss of FoxO1 led to altered expression of a substantive number of transcription factors, cytolytic effector molecules, cell cycle regulators, molecules involved in the Wnt signaling pathway, cytokine receptors, cytokines and chemokines, energy homeostasis and mitochondria (Table 1). Consistent with the reported role for FoxO1 in inducing the expression of IL-7R (CD127), CD62L (Sell) and CCR7 in naïve CD8 T cells (24), expression of mRNAs encoding these molecules were significantly lower in FoxO1−/− memory CD8 T cells (Table 1). To further delineate the cellular processes or pathways that are potentially affected by FoxO1 deficiency, we performed gene expression pathway analysis for the set of genes that were downregulated or upregulated by at least 3-fold in FoxO1−/− memory CD8 T cells. The putative FoxO1-regulated genes were strongly represented in the PI3K/AKT, insulin and Wnt signaling pathways (Supplemental Fig. 2A–2C). Additionally, FoxO1 deficiency altered the expression of genes that are associated with pathways that regulate the metabolism of fatty acids (Supplemental Fig. 2D), purines (Supplemental Fig. 2E) and pyrimidines (Supplemental Fig. 2F). Taken together, these data suggested that FoxO1 regulate several facets of cellular functions including transcription, signaling and metabolism in memory CD8 T cells.
Table 1. FoxO1 deficiency dysregulates the transcriptome of memory CD8 T cells.
WT and FoxO1 −/− mice were infected with LCMV Armstrong. At day 60 post infection, splenocytes were stained with anti-CD8, anti-CD44 and Db/NP396 tetramers. Tetramer-binding CD8 T cells were purified using a FACSAria II sorter and cellular RNA was extracted using Trizol. RNA samples were reverse transcribed and Cy3-labeled cDNAs were hybridized to Agilent Whole Mouse Genome Oligo Microarrays. Table 1 shows the gene symbol, gene title, and the fold change (WT/FoxO1−/−) of the indicated genes in FoxO1−/− memory CD8 T cells relative to those in WT memory CD8 T cells in the clusters for effector molecules, transcription factors, cell cycle, Wnt signaling pathway, and cytokine/cytokine receptors and chemokine/chemokine receptors and mitochondrion.
| Regulated Genes | Gene Symbol |
Gene Description | Fold Change (WT/FoxO1 −/−) |
|---|---|---|---|
| Effector Molecules | |||
| FasL | Fas ligand (TNF superfamily, member 6) transcript variant 1 | 2.66615 | |
| Gzmb | granzyme B | 6.82551 | |
| Gzma | granzyme A | 23.06987 | |
| Prf1 | perforin 1 (pore forming protein) | 1.65348 | |
| Transcription Factors | |||
| AF4/AFF3 | AF4/FMR2 family, member 3 | −14.8698 | |
| Bach2 | BTB and CNC homology 2 | −10.0687 | |
| Ikzf5 | IKAROS family zinc finger 5 | −1.66228 | |
| Ikzf2 | IKAROS family zinc finger 2 | 13.1467 | |
| Klf4 | Kruppel-like factor 4 (gut) | −7.79951 | |
| Klf7 | Kruppel-like factor 7 (ubiquitous) | −11.1625 | |
| IRF5 | interferon regulatory factor 5 | −19.7053 | |
| STAT2 | signal transducer and activator of transcription 2 | −71.4931 | |
| STAT5b | signal transducer and activator of transcription 5B | −2.7923 | |
| Foxm1 | forkhead box M1 | −68.6891 | |
| Foxp4 | forkhead box P4 | −12.2277 | |
| Tcf7 | transcription factor 7 T-cell specific | −31.0128 | |
| Eomes | Eomesodermin homolog (Xenopus laevis) (Eomes), transcript variant 1 | −1.77244 | |
| Tbx21 | T-box 21 (Tbx21) | 1.99801 | |
| Cell Cycle | |||
| Ccna2 | cyclin A2 | −12.2245 | |
| Ccnd1 | cyclin D1 | −7.69436 | |
| Ccnf | cyclin F | −7.38653 | |
| Cdk5rap3 | CDK5 regulatory subunit associated protein 3 | 1.13118 | |
| CDKn2b | cyclin-dependent kinase inhibitor 2B | 11.3784 | |
| Cdkn1c | cyclin-dependent kinase inhibitor 1C (P57) (Cdkn1c),transcript variant 2 | 9.11104 | |
| Cdkn2c | cyclin-dependent kinase inhibitor 2C (p18, inhibits CDK4) | 4.31286 | |
| Cdkn2aipnl | CDKN2A interacting protein N-terminal like | −2.93969 | |
| Wnt Signaling Pathway | |||
| Lef1 | lymphoid enhancer binding factor 1 | −2.44659 | |
| Myc | myelocytomatosis oncogene (Myc), transcript variant 1 | −4.09162 | |
| Tcf7 | transcription factor 7 T-cell specific | −31.0128 | |
| Cytokines/Cytokine Receptors/Chemokine/ Chemokine Receptors | |||
| IL7R | interleukin 7 receptor | −5.97606 | |
| IL2Ra | interleukin 2 receptor, alpha chain | −5.1623 | |
| Il10ra | interleukin 10 receptor, alpha | −3.78897 | |
| Il17b | interleukin 17B | 2.73137 | |
| Il6ra | interleukin 6 receptor, alpha | −39.4913 | |
| Il15 | interleukin 15 | −19.3757 | |
| Ccr4 | chemokine (C-C motif) receptor 4 | 3.7626 | |
| Ccr5 | chemokine (C-C motif) receptor 5 | 2.74019 | |
| Ccr7 | Chemokine (C-C motif) receptor 7 | −5.72565 | |
| Ccr10 | chemokine (C-C motif) receptor 10 | 4.27874 | |
| Ccr8 | chemokine (C-C motif) receptor 8 | 18.48211 | |
| Cxcl14 | chemokine (C-X-C motif) ligand 14 | 2.40558 | |
| Cxcr5 | chemokine (C-X-C motif) receptor 5 | 1.37419 | |
| Cxcl16 | chemokine (C-X-C motif) ligand 16 | −2.94351 | |
| Cxcl10 | chemokine (C-X-C motif) ligand 10 | −22.0272 | |
| Cxcr4 | chemokine (C-X-C motif) receptor 4 | −33.3527 | |
| Ifngr1 | interferon gamma receptor 1 | −4.55352 | |
| Mitochondrion | |||
| Atp5b | ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit | −2.39264 | |
| Atp5b | ATP synthase, H+ transporting mitochondrial F1 complex, beta subunit | −2.39264 | |
| Atp5j | ATP synthase, H+ transporting mitochondrial F0 complex, subunit F psuedogene | −3.75671 | |
| Atp5g3 | ATP synthase, H+ transporting mitochondrial F- complex, subunit c | −4.28939 | |
| Atp5e | ATP synthase, H+ transporting mitochondrial F1 complex, epsilon subunit | −12.1938 | |
| Atp6v0a1 | ATP synthase, H+ transporting lysosomal V0 subunit A1 | −4.53032 | |
| Atp6v0b | ATP synthase, H+ transporting lysosomal V0 subunit B | −2.9928 | |
| Atp6v0d2 | ATP synthase, H+ transporting lysosomal V0 subunit D2 | −2.98129 | |
| Atp6v1d | ATP synthase, H+ transporting lysosomal V01subunit D | −3.04329 | |
| Bax | Bcl2 associated X protein | −2.65333 | |
| Bcl2 | B-cell leukemia/lymphoma 2 (Bcl2), transcript variant 2 | −2.90786 | |
| Bid | BH3 interacting domain death agonist | −3.36876 | |
| Ndufa12 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, 12 | −99.3623 | |
| Ndufa4 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, 4 | −2.44543 | |
| Ndufa7 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, 7 (B14.5a) | −14.1216 | |
| Ndufa8 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, 8 | −24.4832 | |
| Ndufa9 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, 9 | −3.17405 | |
| Ndufaf1 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 1 | −7.83086 | |
| Ndufaf2 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 2 | −2.37409 | |
| Ndufaf4 | NADH Dehydrogenase (ubiquinone) 1 alpha subcomplex, assembly factor 4 | −2.75539 | |
| Aifm2 | Apoptosis-inducing factor, mitochondrian- associated 2 | −3.24718 | |
| Mtcp1 | Mature T-cell proliferation 1 | −2.72502 | |
| Mavs | Mitochondrial antiviral signaling protein | −27.8605 | |
| Mrp63 | Mitochondrial ribosomal protein 63 | −2.10538 | |
| Mrpl14 | Mitochondrial ribosomal protein L14 | −4.51761 | |
| Mrpl34 | Mitochondrial ribosomal protein L34 | −9.05047 | |
| Mrpl45 | Mitochondrial ribosomal protein L45 | −10.62 | |
| Mrpl46 | Mitochondrial ribosomal protein L46 | −100 | |
| Mrpl47 | Mitochondrial ribosomal protein L47 | −9.24727 | |
| Mrpl55 | Mitochondrial ribosomal protein L55 | −4.7741 | |
| Mrps12 | Mitochondrial ribosomal protein S12 | −4.04583 | |
| Mrps14 | Mitochondrial ribosomal protein S14, similar to mitochondrial ribosomal protein S14 | −6.02143 | |
| Mrp18a | Mitochondrial ribosomal protein S18A | −2.5653 | |
| Mrp18c | Mitochondrial ribosomal protein S18C | −8.07069 | |
| Mrps2 | Mitochondrial ribosomal protein S2 | −2.24477 | |
| Mrps22 | Mitochondrial ribosomal protein S22 | −100 | |
| Mrps30 | Mitochondrial ribosomal protein S30 | −2.81149 | |
| Mrps7 | Mitochondrial ribosomal protein S7 | −3.67166 | |
| Cell Adhesion Molecules | |||
| Sell | selectin, lymphocyte (Sell), transcript variant 2 | −6.61415 | |
| Antigen | |||
| CD27 | CD27 antigen (Cd27), transcript variant 1 | −1.00049 | |
T cell-intrinsic FoxO1 is not required for clonal expansion but necessary for survival of memory CD8 T cells under competitive conditions
So far, studies in T cell-specific FoxO1 conditional knockout mice illustrated that FoxO1-deficient memory CD8 T cells can survive, albeit in a functionally compromised state indefinitely. It is noteworthy that the levels of transcription factor TCF-1 in FoxO1−/− memory CD8 T cells were markedly lower than in WT memory CD8 T cells (Table 1). Since TCF-1-deficient memory CD8 T cells fail to persist, especially under competitive conditions (i.e. in the presence of TCF-1-sufficient WT memory CD8 T cells) (10), we hypothesized that the impaired expression of TCF-1 in FoxO1−/− memory CD8 T cells (Table 1 and Fig. 5E) would compromise the survival of these cells under competitive conditions. To test this hypothesis, we generated mixed bone marrow chimeras by reconstituting lethally irradiated RAG-1-deficient or Ly5.1/B6 mice with a mixture of T cell-depleted bone marrow cells from WT/Ly5.1 and WT/Ly5.2 or FoxO1−/−/Ly5.2 mice. Control chimeras (CCs) harbor T cells derived from WT/Ly5.1 and WT/Ly5.2 mice and experimental chimeras (ECs) contain T cells derived from WT/Ly5.1 and FoxO1−/−/Ly5.2 mice. CCs and ECs were infected with LCMV and virus-specific CD8 T cells were quantified at days 8 and 45 PI. At day 8 PI, in the CCs, the percentages of LCMV-specific CD8 T cells among WT/Ly5.1 or WT/Ly5.2 CD8 T cells were comparable (Fig. 8A). Likewise, in ECs, the percentages of LCMV-specific CD8 T cells within the population of WT/Ly5.1 or FoxO1−/−/Ly5.2 CD8 T cells were similar at day 8 PI. Additionally Gznb levels in LCMV-specific FoxO1−/− CD8 T cells (MFI of 183) were similar to those in WT/Ly5.1 (MFI of 176) CD8 T cells. Taken together, these data suggested that clonal expansion and effector differentiation of FoxO1−/− CD8 T cells occurred normally in the competitive environment of the chimeric mice. Next, we quantified LCMV-specific CD8 T cells at day 45 PI to assess whether FoxO1 was required for generation and maintenance of memory CD8 T cells under competitive conditions. As shown in Fig. 8A, at day 45 PI, the percentages of LCMV-specific memory CD8 T cells among WT/Ly5.1 and WT/Ly5.2 in CCs and WT/Ly5.2 CD8 T cells in ECs were largely similar. Strikingly, however, the percentages of LCMV-specific memory CD8 T cells among FoxO1−/−/Ly5.2 CD8 T cells in ECs were significantly lower, as compared to WT/Ly5.1 or WT/Ly5.2 CD8 T cells (Fig. 8A). The diminished ability of FoxO1−/− memory CD8 T cells to persist was associated with significant reduction in the expression of Bcl-2 (Fig. 8B). Thus, under competitive conditions, clonal expansion and effector differentiation of FoxO1−/− CD8 T cells occurred normally, but memory CD8 T cells displayed poor survival in the absence of FoxO1.
Figure 8. T-cell intrinsic effects of FoxO1 analyzed in mixed bone marrow chimeras.
A and C, Bone marrow cells (BMCs) were harvested from the femurs and tibias of WT and FoxO1 −/− mice. A 1:1 mixture of 7.5 × 106 T cell-depleted BMCs from WT (Ly5.1) and WT (Ly5.2) (Control) or FoxO1 −/− (Ly5.2) (Experimental) mice was adoptively transferred into lethally irradiated Rag1−/− mice to generate Control or Experimental bone marrow chimeras. Six to eight weeks after reconstitution, Control and Experimental bone marrow chimeras were infected with LCMV. A, At days 8 and 45 PI, splenocytes were stained with anti-Ly5.1, anti-Ly5.2, anti-CD8, Db/NP396 and Db/GP33 tetramers. The bar graphs in A show the percentages of NP396- and GP33-specific CD8 T cells amongst either Ly5.1+ve or Ly5.2+ve CD8 T cells. Data are from analysis of 4 mice for each group. Error bars represent SEM. B, FoxO1 regulates Bcl-2 expression in memory CD8 T cells. WT and FoxO1 −/− mice were infected with LCMV Armstrong. At day 8 and day 95 PI, cells were stained with anti-CD8, Db/NP396 tetramer and anti-Bcl-2 antibodies. The histograms are gated on Db/NP396-specific CD8 T cells, and the numbers in the histogram represents the MFI for Bcl-2. Dotted lines in the FACS histograms represent staining with the isotype control antibody. The bar graphs illustrate the MFI for Bcl-2 at day 8 and day 95 PI respectively. Data are mean ± SEM from analysis of 3–5 mice/group. C, Control and Experimental bone marrow chimeras were infected with LCMV. At day 50 PI, The levels of IFN-γ, IL-2 and TNF-α produced by NP396 and GP33-restricted CD8 T cells were quantified by intracellular cytokine staining. Bar graphs illustrate the MFI for IFN-γ and the percentage of CD8 T cells that produced IFN-γ, IL-2 and TNF-α for CCs and ECs at day 50 PI. Data are from analysis of 4 mice for each group. Error bars represent SEM.
Next we determined the extent to which FoxO1 regulated the functional maturation of memory CD8 T cells by cell-intrinsic mechanisms. CCs and ECs were infected with LCMV and at day 50 PI, cytokine-producing ability of WT and FoxO1−/− memory CD8 T cells in CCs and ECs was assessed by intracellular cytokine staining. In the CCs, WT/Ly5.1 and WT/Ly5.2 LCMV-specific memory CD8 T cells produced comparable levels of IFN-γ, upon antigenic stimulation ex vivo (Fig. 8C); MFIs for IFN-γ were comparable for WT/Ly5.1 and WT/Ly5.2 CD8 T cells. IFN-γ production by WT/Ly5.1 memory CD8 T cells in the ECs were similar to their counterparts in the CCs. Notably, in the ECs, IFN-γ production by FoxO1−/−/Ly5.2 memory CD8 T cells was significantly lower, as compared to WT/Ly5.1/Ly5.2 memory CD8 T cells (Fig. 8C). In CCs the percentages of triple cytokine-producing cells among LCMV-specific memory CD8 T cells were comparable for WT/Ly5.1 and WT/Ly5.2. By contrast, the percentages of triple cytokine-producing cells among LCMV-specific FoxO1−/−/Ly5.2 memory CD8 T cells were substantially lower, in comparison to WT/Ly5.1 or WT/Ly5.2 memory CD8 T cells (Fig. 8C). Based on these findings, we inferred that FoxO1 regulates functional maturation of memory CD8 T cells by cell-intrinsic mechanisms.
Discussion
The activity of FoxO1/O3 in antigen-specific CD8 T cells is controlled in a dynamic fashion in vivo, during an acute viral infection (22) and FOXO3 limits the clonal expansion of CD8 T cells in vivo by promoting the apoptosis of proliferating cells (22–23). There is convincing evidence that FoxO1 is a master regulator of the survival, trafficking and quiescence of naïve T cells (13). Data presented in this manuscript ascribes crucial cell intrinsic roles for FoxO1 in governing two distinct aspects of CD8 T cell memory: functional maturation of memory CD8 T cells from effector cells and the survival of memory CD8 T cells.
Accruing evidence supports the linear differentiation model for development of memory CD8 T cells (1). This model posits that naïve CD8 T cells differentiate into effector cells, which subsequently transition into long-lived functionally competent memory CD8 T cells(41). The effector to memory transition involves dramatic alterations in gene expression, metabolism and functions (including loss of active cytolytic functions and acquisition of proliferative potential, poly-functionality and the ability to self renew) (9) culminating in the establishment of a distinct cellular state that appears to be intermediate between naïve and effector cells (42). We refer effector to central memory transition as ‘de-differentiation’ because, during this transition, memory cells reacquire several features of naïve T cells such as quiescence, lack of cytolytic activity, ability to traffic to the secondary lymphoid organs and catabolic metabolism by fatty acid oxidation (3). The results presented here illustrate that the de-differentiation of effector to memory CD8 T cells is important for recall responses and protective immunity, and that this process requires FoxO1. Therefore, we propose that FoxO1-dependent overlapping mechanisms control the homeostasis of naïve and memory CD8 T cells. In the absence of FoxO1, memory CD8 T cells display a multitude of phenotypic, proliferative and functional deficits that impair their ability to confer protective immunity.
Previous work show that FoxO1-deficient CD8 single-positive thymocytes express elevated levels of T-bet, IFN-γ and gznb upon TCR stimulation in vitro (28), which suggested an inhibitory role for FoxO1 in effector differentiation and function. However, in our studies, we find that FoxO1−/− effector CD8 T cells contained greater levels of Gznb but produced lower levels of IFN-γ, as compared to WT effector CD8 T cells. In the present study, however, altered effector functions in FoxO1−/− effector cells and dysregulation of CD8 T cell memory in FoxO1−/− mice can not simply be linked to either elevated expression of T-bet or diminished expression of EOMES. It is worth emphasizing that in the absence of FoxO1, memory CD8 T cells display progressive loss of polycytokine producing ability, which suggests that continuous FoxO1 activity might be necessary for the maintenance of the full spectrum of effector functions in memory CD8 T cells. This inference needs to be confirmed by utilizing a mouse model that will enable inducible ablation of FoxO1 expression in fully differentiated memory CD8 T cells.
The molecular mechanisms underlying the actions of FoxO1 in regulating the effector to memory transition and functional maturation of memory CD8 T cells is complex but the spectrum of genes dysregulated in FoxO1−/− memory CD8 T cells stratifies into distinct cellular processes such as transcription, survival, proliferation, effector function and cellular metabolism, which collectively define the distinct differentiation state of functionally competent memory CD8 T cells. Significantly, memory CD8 T cells in FoxO1−/− mice express higher levels of mRNA for cytolytic effector molecules including Gznb, granzyme A and perforin, as compared to those in WT memory CD8 T cells (Table 1). These data are consistent with the idea that FoxO1 is required for downregulation of effector functions and effector to memory transition following viral clearance during an acute infection. Although, higher induction of effector molecules in FoxO1−/− thymocytes has been attributed to elevated levels of T-bet (28), continued expression of effector molecules in in vivo-generated FoxO1−/− memory CD8 T cells did not include substantively altered expression of T-bet or EOMES mRNA (Table 1). This finding raises the possibility that other FoxO1-regulated transcription factors are involved in downregulating effector functions and enforcing effector to memory transition of CD8 T cells.
Among transcription factors, notably, the markedly diminished expression of Tcf7 gene (encodes TCF-1) in FoxO1−/− memory CD8 T cells could be of particular significance. This is because the effects of TCF-1 deficiency on memory CD8 T cells are remarkably similar to those of FoxO1 deficiency, especially the impairment in effector to memory transition (10). Similar to FoxO1−/− memory CD8 T cells, TCF-1-deficient memory CD8 T cells display poor proliferative potential, inability to produce IL-2, and elevated levels of Gznb (10–11). We propose that one mechanism by which FoxO1 promotes effector to memory transition and functional maturation of functional CD8 T cell memory is by inducing TCF-1. It is likely that Tcf7 is a direct target gene for FoxO1 in memory CD8 T cells because recent CHIP-seq analysis has demonstrated FoxO1 binding to the intergenic region of the Tcf7 gene in regulatory T cells (40). Bach2 is considered as a B-cell specific transcription factor that promotes the maintenance of B cell identity by opposing terminal differentiation of B cells to plasma cells (43). However, it has been recently reported that Bach2 might also be expressed in T cells and it is noteworthy that MPECs express higher levels of Bach2 than in SLECs (44–45). Additionally, our own inspection of the previously published microarray data from Badovinac and Harty’s group indicate that terminal differentiation of CD8 T cells induced by repetitive antigenic exposure leads to diminished Bach2 expression in memory CD8 T cells (46). It remains to be determined whether FoxO1 regulates effector to memory transition by inducing Bach2.
Interestingly, the expressions of FoxM1 and Foxp4 genes show substantial reduction in FoxO1−/− memory CD8 T cells (Table 1). FoxM1 appears to be important for T cell proliferation and impaired FoxM1 expression might diminish the proliferative potential of FoxO1−/− memory CD8 T cells (47). Loss of Foxp4 has been reported to impair cytokine production by LCMV-specific CD4 T cells (48), but the role of Foxp4 in regulating CD8 T cell memory is yet to be determined. The reduced recall response of FoxO1−/− memory CD8 T cells is also associated with diminished levels of cyclins (A2, D1 and F) and increased expression of anti-proliferative cyclin-dependent kinase inhibitors (p15INK4B, p18INK4c and p57Kip2) (Table 1).
As compared to apoptosis-prone effector cells, memory CD8 T cells possess greater mitochondrial respiratory capacity and mitochondrial metabolism might play a pivotal role in governing the health of memory CD8 T cells (49). Notably, we find that a substantial number of genes associated with mitochondrial metabolism including ATP synthases and NADH dehydrogenases are dysregulated in FoxO1−/− memory CD8 T cells (Table 1). Additionally, the mitochondrial mass and membrane potential in FoxO1−/− memory CD8 T cells are lower than in WT memory CD8 T cells (Supplemental Figure 3); lower mitochondrial potential is considered as a sign of poor mitochondrial health (32, 49). These data suggest that FoxO1 might promote the maintenance of memory CD8 T cells by enhancing mitochondrial biogenesis and membrane potential, which in turn would be expected to increase the mitochondrial spare capacity (49) that is critical for memory cells to rapidly proliferate and develop effector functions upon reinfection.
To reiterate, similar to FoxO1deficiency, loss of either TCF-1 or Bcl-6 disrupts effector to memory transition and functional maturation of memory CD8 T cells during acute viral or intracellular bacterial infections (10, 12). While Bcl6 expression appears to be regulated by the IL-21/STAT-3 signaling pathway, we have reported that the PI3K/AKT pathway controls TCF-1 expression in CD8 T cells (12, 45). Here, we demonstrate that TCF-1 expression is markedly diminished in FoxO1−/− memory CD8 T cells and it is likely that Tcf7 is a direct target gene for FoxO1 (40). TCF-1 is believed to promote CD8 T-cell memory by inducing the transcription factor EOMES (10), but we find that FoxO1 deficiency-induced loss of TCF-1 is not associated with substantively reduced EOMES expression in effector or memory CD8 T cells. Perhaps, the residual levels of TCF-1 in FoxO1−/− memory CD8 T cells is sufficient to stimulate transcription of EOMES or TCF-1 might regulate functional maturation of CD8 T cell memory by mechanisms independent of EOMES. Intriguingly, loss of FoxO1 did not affect Bcl-6 expression in memory CD8 T cells, which implies that the induction of Bcl6 expression by IL-21/STAT-3 signaling pathway may not require FoxO1. Since either FoxO1 or Bcl-6 deficiency is sufficient to abrogate functional maturation of memory CD8 T cells, we speculate that FoxO1 by itself or FoxO1-induced factor(s) cooperatively function with Bcl-6 to facilitate the development of competent memory CD8 T cells.
The persistence of naïve T cells in the periphery requires signaling via the IL-7R and TCR, but only IL-7R signaling and not TCR/MHC I interactions is obligatory for survival of memory CD8 T cells (38, 50). As established for naïve T cells, we find that full expression of IL-7 receptor on memory CD8 T cells also requires FoxO1, and FoxO1−/− memory CD8 T cells fail to persist alongside WT T cells in a competitive environment. Future studies will determine whether transgenic expression of IL-7R would be sufficient to restore the survival of FoxO1−/− memory CD8 T cells. Nevertheless, it is clear that FoxO1-induced IL-7R expression is a common denominator that promotes persistence of both naïve and memory CD8 T cells. Which signaling pathway regulates FoxO activity and survival of memory CD8 T cells? We have reported that in CD8 T cells, the serine threonine kinase AKT functions as a signal integrator for biochemical pathways triggered by the engagement of the TCR and exposure to cytokines such as IL-2 (45). Further, we have shown that sustained activation of AKT results in phosphorylation of FoxO1, downregulated expressions of IL-7R and TCF-1, and impaired survival of memory CD8 T cells (45). Here, we show that FoxO1 deficiency alone is sufficient to downregulate TCF-1/IL-7R expression and impair the survival of memory CD8 T cells. Thus, one mechanism by which AKT regulates TCF-1 expression and survival of memory CD8 T cells is by phosphorylation and inactivation of FoxO1. Future work will elucidate whether transgenic expression of TCF-1 would restore the survival of FoxO1−/− memory CD8 T cells and TCF-1 expression requires IL-7R signaling.
Does FoxO1 control functional maturation of memory CD8 T cells by cell-intrinsic mechanisms? This is an important question because CD4 T cells are known to promote the maintenance of protective memory CD8 T cells (51–52) and LCMV-specific memory CD4 T cells are dysregulated in FoxO1−/− mice. Therefore the possibility exists that impairment of memory CD8 T cells in FoxO1−/− mice could result from dysregulated CD4 T cell responses and not from cell-intrinsic loss of FoxO1. In our studies that employed mixed bone marrow chimeras, we find that only FoxO1−/− but not WT LCMV-specific memory CD8 T cells display substantial functional impairment (lower production of IFN-γ and loss of polycytokine-producing ability). Thus, it is clear that FoxO1 controls functional maturation of memory CD8 T cells largely by cell-intrinsic mechanisms and not indirectly via the CD4 T cells.
In summary, we report that FoxO1 functions as a key regulator of functional CD8 T cell memory by governing diverse cellular processes, which promote the survival, metabolism, functions, and recall responses of memory CD8 T cells. These findings have provided critical mechanistic insights that have advanced our fundamental understanding of the mechanisms underlying the homeostasis of memory CD8 T cells. These findings are expected to have significant implications for the development of strategies to modulate FoxO1 activity in T cells, in order to enhance vaccine-induced CD8 T cell memory.
Supplementary Material
Acknowledgments
We thank Dr. David Gasper for discussions and review of the manuscript.
This work was supported by PHS grants from the National Institutes of Health (AI48785 and AI101976) to Dr. M. Suresh. Melba Marie Tejera was supported by Virology Training Grant from the National Institutes of Health (T32AI078985).
References
- 1.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Bevan M. CD8+ T Cells: Foot Soldiers of the Immune System. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Cui W. Transcriptional control of effector and memory CD8(+) T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 8.Kallies A, Xin A, Belz GT, Nutt SL. Blimp-1 transcription factor is required for the differentiation of effector CD8(+) T cells and memory responses. Immunity. 2009;31:283–295. doi: 10.1016/j.immuni.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 9.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeannet G, Boudousquie C, Gardiol N, Kang J, Huelsken J, Held W. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci U S A. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, Stone EL. FOXO transcription factors throughout T cell biology. Nat Rev Immunol. 2012;12:649–661. doi: 10.1038/nri3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 15.Kousteni S. FoxO1, the transcriptional chief of staff of energy metabolism. Bone. 2012;50:437–443. doi: 10.1016/j.bone.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kousteni S. FoxO1: a molecule for all seasons. J Bone Miner Res. 2011;26:912–917. doi: 10.1002/jbmr.306. [DOI] [PubMed] [Google Scholar]
- 17.Boccitto M, Kalb RG. Regulation of Foxo-dependent transcription by post-translational modifications. Curr Drug Targets. 2011;12:1303–1310. doi: 10.2174/138945011796150316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hay N. Interplay between FOXO, TOR, and Akt. Biochim Biophys Acta. 2011;1813:1965–1970. doi: 10.1016/j.bbamcr.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunological Reviews. 2003;192:7–20. doi: 10.1034/j.1600-065x.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 20.Freitas AA, Rocha B. Homeostasis of naive T cells: the Foxo that fixes. Nat Immunol. 2009;10:133–134. doi: 10.1038/ni0209-133. [DOI] [PubMed] [Google Scholar]
- 21.Ouyang W, Li MO. Foxo: in command of T lymphocyte homeostasis and tolerance. Trends Immunol. 2011;32:26–33. doi: 10.1016/j.it.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan JA, Kim EH, Plisch EH, Peng SL, Suresh M. FOXO3 regulates CD8 T cell memory by T cell-intrinsic mechanisms. PLoS Pathog. 2012;8:e1002533. doi: 10.1371/journal.ppat.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30:358–371. doi: 10.1016/j.immuni.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 26.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, Hedrick SM. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10:176–184. doi: 10.1038/ni.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, Katayama CD, Hedrick SM. Foxo transcription factors control regulatory T cell development and function. Immunity. 2010;33:890–904. doi: 10.1016/j.immuni.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36:374–387. doi: 10.1016/j.immuni.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J Exp Med. 1984;160:521–540. doi: 10.1084/jem.160.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJD, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting Antigen-Specific CD8 T Cells: A Reevaluation of Bystander Activation during Viral Infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Grayson JM, Laniewski NG, Lanier JG, Ahmed R. Mitochondrial potential and reactive oxygen intermediates in antigen-specific CD8+ T cells during viral infection. J Immunol. 2003;170:4745–4751. doi: 10.4049/jimmunol.170.9.4745. [DOI] [PubMed] [Google Scholar]
- 33.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 34.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Youngblood B, Davis CW, Ahmed R. Making memories that last a lifetime: heritable functions of self-renewing memory CD8 T cells. Int Immunol. 2010;22:797–803. doi: 10.1093/intimm/dxq437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1:433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 37.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 38.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunological Reviews. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 39.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 40.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554–559. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 42.Holmes S, He M, Xu T, Lee PP. Memory T cells have gene expression patterns intermediate between naive and effector. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5519–5523. doi: 10.1073/pnas.0501437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ochiai K, Katoh Y, Ikura T, Hoshikawa Y, Noda T, Karasuyama H, Tashiro S, Muto A, Igarashi K. Plasmacytic transcription factor Blimp-1 is repressed by Bach2 in B cells. J Biol Chem. 2006;281:38226–38234. doi: 10.1074/jbc.M607592200. [DOI] [PubMed] [Google Scholar]
- 44.Rutishauser RL, Kaech SM. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol Rev. 2010;235:219–233. doi: 10.1111/j.0105-2896.2010.00901.x. [DOI] [PubMed] [Google Scholar]
- 45.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue L, Chiang L, He B, Zhao YY, Winoto A. FoxM1, a forkhead transcription factor is a master cell cycle regulator for mouse mature T cells but not double positive thymocytes. PLoS One. 2010;5:e9229. doi: 10.1371/journal.pone.0009229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiehagen KR, Corbo-Rodgers E, Li S, Staub ES, Hunter CA, Morrisey EE, Maltzman JS. Foxp4 is dispensable for T cell development, but required for robust recall responses. PLoS One. 2012;7:e42273. doi: 10.1371/journal.pone.0042273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pulendran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiesel M, Oxenius A. From crucial to negligible: functional CD8(+) T-cell responses and their dependence on CD4(+) T-cell help. Eur J Immunol. 2012;42:1080–1088. doi: 10.1002/eji.201142205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.