Abstract
Background
The ACOSOG Z0011 results provided convincing evidence that completion axillary lymph node dissection (CALND) was unnecessary in selected patients with 1–2 positive sentinel lymph nodes (SLNs). We hypothesized that preoperative axillary ultrasound (AUS) with fine needle aspiration is sufficiently sensitive to detect worrisome macrometastasis to preclude need for frozen section pathology of SLNs.
Study Design
A retrospective single institution study, tertiary academic referral center. 1,140 T1-2 breast cancer patients who underwent SLN biopsy ± CALND, from 1/1/07-12/31/10 were reviewed. All patients had negative preoperative AUS ± FNA.
Results
144 (13%) patients were node positive at surgery. Average age, tumor size, histology, ER and PR status were similar comparing 996 SLN negative to 144 (13%) SLN positive patients. Of the SLN positive patients, 25% were premenopausal, 9% were ER negative, and 19% had additional lymph nodes at CALND. Only 19 (2%) patients had SLN metastasis ≥6 mm, 10 (1%) had metastasis >7 mm, and only 1 patient had ≥3 positive SLNs.
Conclusions
The addition of preoperative AUS ± FNA to patients who meet ACOSOG Z0011 eligibility criteria reduced the risk of macrometastasis measuring ≥6 mm to only 2%, very few of whom would be premenopausal, have ER negative tumors, or ≥3 positive SLNs. With the addition of AUS ± FNA, we endorse the conclusions of the ACOSOG Z0011 trial in avoiding CALND, and see marginal gain in frozen section analysis of SLNs.
Nearly 120 years ago, the outlook for patients with breast cancer was bleak, summarized by Halsted’s admission that “most of us have heard our teachers in surgery admit that they have never cured a case of cancer of the breast.”1 Flash forward to the recent publications of the randomized trial, American College of Surgeons Oncology Group (ACOSOG) Z00112, 3. In contrast to the extensive resection of the Halsted radical mastectomy, the Z0011 study provides strong basis in many patients to limit the surgical intervention to wide local excision and sentinel lymph node (SLN) biopsy only, even if the SLNs contain metastatic deposits. Specifically, with a mean follow-up of 6.3 years, in breast cancer patients with T1-2 cancers, without preoperative palpable lymphadenopathy, treated with breast conservation surgery followed by usual breast radiotherapy and standard adjuvant medication, there was no benefit of completion axillary lymph node dissection (CALND) beyond sentinel lymph node (SLN) biopsy alone for patients with one or two positive sentinel lymph nodes with respect to disease-free survival (DFS), overall survival (OS), or overall local recurrence. Moreover, the axillary recurrence rate of patients who had only the positive SLN(s) removed was just 0.9%.
However, some bias in patient enrollment on the study was inevitable, and technology has progressed since the study was initiated. Patients enrolled had “favorable” disease, notably 70% had T1 tumors, 83% were estrogen receptor (ER) positive, 71% had a single SLN positive and 44% had only micrometastases, 38% were 50 years or younger, and only 27% of those patients undergoing CALND had additional positive lymph nodes (compared to a 53% positive rate in meta-analysis of over 8,000 patients4).
From 2007 axillary ultrasound (AUS) with percutaneous fine needle aspiration (FNA) biopsy of morphologically abnormal lymph nodes has been employed at Mayo Clinic in Rochester to identify the subset of patients with cytology-detected metastatic lymph nodes so as to avoid the time and expense of SLN mapping, SLN biopsy and frozen section. For these patients, with the enhancement of AUS+/−FNA to physical examination, we treat these patients as LN positive, and ALND has been incorporated into their operative management. ACOSOG Z0011 did not use preoperative AUS in the workup of the patients.
Following publication of the ACOSOG Z0011 results, we hypothesized that preoperative AUS was sufficiently sensitive to detect breast cancer with SLN disease that would warrant axillary dissection so as to preclude the need for intraoperative frozen section pathologic review for the remainder of patients with negative AUS who were undergoing SLN surgery. The aim of this study was to determine the frequency and size of macrometastases, and the number of patients with 3 or more positive SLNs, especially in the higher risk ER-negative and premenopausal patients. Ultimately, we intended to identify which breast cancer patients AUS +/− FNA was sufficiently sensitive to eliminate the attendant time and cost of intraoperative frozen section histology of SLNs removed in breast conservation patients.
METHODS
Study design and data collection
The single-center, retrospective study included clinical T1 and T2 breast cancer patients who underwent sentinel lymph node (SLN) biopsy with or without CALND from January 1, 2007 to December 31, 2010. Data were collected from two prospective databases maintained at the Mayo Clinic in Rochester: Cancer Registry (2007–2008) and Breast Surgery Database (2009-onwards). Medical records were reviewed for patient demographics, year of surgery, clinical history, imaging, primary tumor histology, grade, stage, hormone status, Her-2/neu status, results of AUS and of FNA, if performed, number of sentinel nodes retrieved, number of positive nodes by histological examination, outcomes of axillary dissection (if conducted), and size of the largest metastatic lymph node deposit (if node positive). AUS became institutional practice for all patients with a diagnosis of invasive breast carcinoma at the end of 2006, and we incorporated records from 2007 onwards. Only patients who had clinically node-negative breast cancer, preoperative negative AUS or suspicious lymph nodes on AUS but were FNA cytology negative, performed at Mayo Clinic in Rochester, and had SLN biopsy were included. All participants were women at least 18 years of age with clinical T1 or T2 N0 M0 breast cancer treated with SLN biopsy and either breast-conserving surgery or mastectomy. Only patients who were candidates for breast conservation but elected mastectomy were included. Patients treated with neoadjuvant chemo-or hormonal therapy, palpable and grossly involved adenopathy, T3 or T4 tumors, multicentric disease, preoperatively established metastases by AUS and positive FNA cytology or with previous axillary surgery were excluded from this study. This study has been approved by the institutional review board of the Mayo Clinic.
Axillary ultrasonography and sentinel lymph node biopsy
All AUS were performed by dedicated breast ultrasonographers using high resolution linear array transducers with a maximum frequency of at least 12 MHz with scanning in transverse and sagittal planes. Lymph nodes with hilar effacement, hilar replacement, node matting, perinodal edema, and unclear node margins and cortical thickening greater than 3mm, especially if nodular or asymmetric on AUS were considered abnormal. All suspicious lymph nodes were followed up by FNA biopsy that was performed with local anesthesia; a 25-gauge needle was used to obtain specimens for cytological examination. Cases with positive FNA cytology were excluded from this analysis.
SLNs were identified using radioactive colloid and/or blue dye according to the surgeon’s preference. Intraoperative frozen section and permanent hematoxylin-eosin (H&E) stains were performed on all SLNs. Immunohistochemical stains were performed on all SLNs that were negative by frozen section examination and permanent H&E. SLNs were considered positive if metastasis seen by immunohistochemical or H&E stains measured greater than 0.2 mm. Isolated tumor cells (ITCs) were classified as negative for metastases as per the American Joint Committee on Cancer 7th edition staging.
Study end points and reference standards
The primary end point was the pathology results from SLN biopsy as determined by frozen section and permanent pathology analysis. The study plan was to compare pathology results after SLN biopsy with preoperative AUS +/− FNA to determine sensitivity of AUS +/− FNA as a tool to eliminate the need for frozen section in patients meeting Z0011 criteria.
The size of the largest metastatic deposit was utilized to categorize lymph nodes as isolated tumor cells (ITC; <0.2mm), micrometastases (0.2–2mm), or macrometastases (>2mm).
Statistical analysis
Frequency distributions and univariate analysis were used to summarize and compare patients’ characteristics. Chi-square test, independent samples t-test, and logistic regression were used to compare effect of clinical characteristics and the correlation of outcomes from AUS +/− FNA and SLN biopsy pathology. All analyses used a 2-tailed significant level of 0.05 and were performed using SPSS® version 19 (SPSS, Chicago, Illinois, USA).
RESULTS
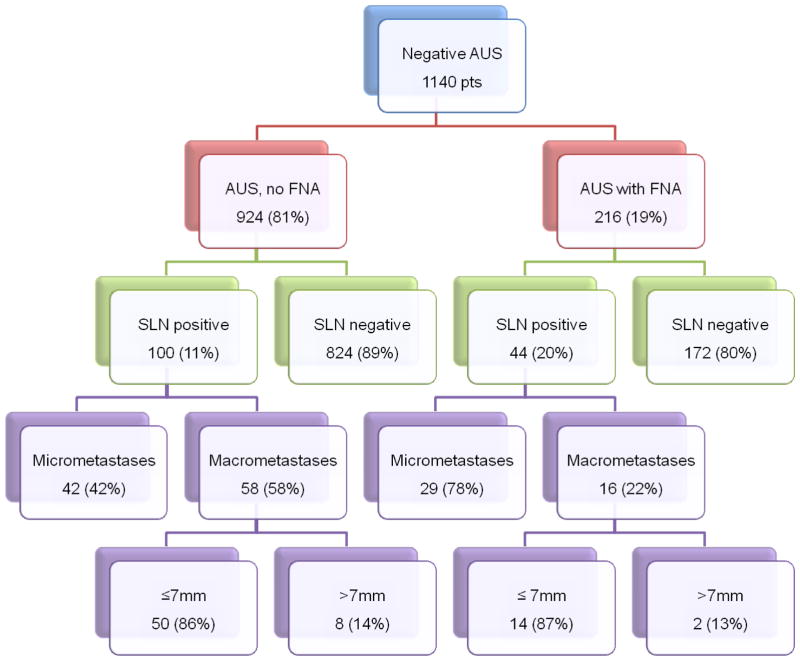
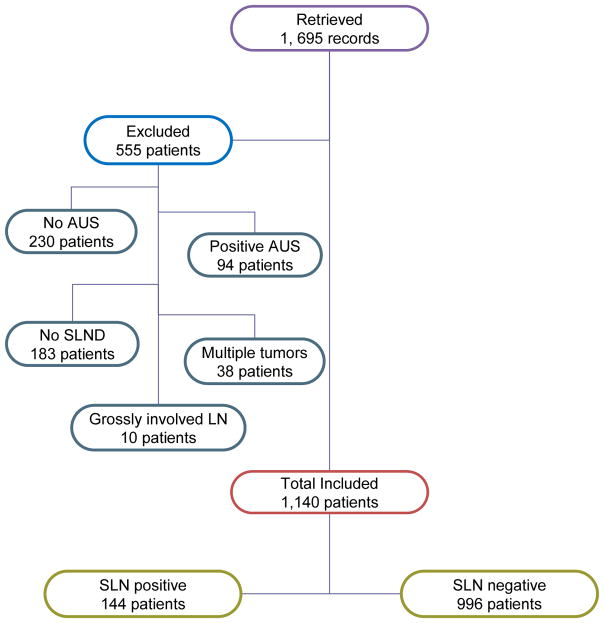
A total of 1,140 patients with negative axillary ultrasound +/− FNA were included in our study and Figure 1 shows a flow diagram of patients’ selection process. Positive nodes were found in 144 (12.6%) of patients and 996 had negative SLN. Patients’ demographics and tumor characteristics are shown in Table 1. There were 822 patients (72%) with invasive ductal carcinoma, 120 patients (11%) with invasive lobular carcinoma, 110 patients (10%) with mixed invasive ductal and lobular carcinoma, and 43 patients (4%) with other histologic types. There were 817 patients (71%) with T1 stage. Mean age on presentation was 62. The majority of patients were ER positive (71%) and PR positive (63%). Her 2 status was significantly different between the SLN positive and SLN negative patients (Her2 negative 88% vs. 30%, p < 0.0001, respectively). Two-hundred sixteen patients (19%) had abnormal lymph nodes on AUS and went on to US-directed FNA which was negative for metastatic disease, whereas the lymph nodes were deemed negative by US in 924 patients (81%). The mean number of SLNs excised in the node positive group was not significantly different from the node negative group (2.69 vs. 2.62, p=0.57). The correlation of AUS +/−FNA with the pathology results after SLN biopsy is summarized in Figure 2. In our study 87.4% of preoperative negative AUS +/− FNA were truly negative.
Figure 1.
Summary flow diagram of patients. AUS, axillary ultrasound; FNA, fine needle aspiration; SLN, sentinel lymph node.
Table 1.
Demographics, Cytology, and Tumor Characteristic of Patients with Negative Axillary Ultrasonography
| Variable | Total (n=1140) | SLN positive (n=144) | SLN negative (n=996) | p Value |
|---|---|---|---|---|
| Age, y, mean, SD | 62 ± 13.2 | 60 ± 12.7 | 62 ± 13.3 | 0.045 |
| Tumor classification, n (%) | 0.060 | |||
| T1 | 817 (71) | 95 (66) | 722 (72) | |
| T2 | 312 (27) | 46 (32) | 266 (27) | |
| Tumor size, mm, mean, SD | 1.7 ± 1.1 | 1.9 ± 1.2 | 1.7 ± 1.1 | |
| Tumor histology, n (%) | 0.575 | |||
| Ductal | 822 (72) | 111 (77) | 711 (71) | |
| Lobular | 120 (11) | 10 (7) | 110 (11) | |
| Ductal and lobular | 110 (10) | 10 (7) | 100 (10) | |
| Other | 10 (4) | 10 (7) | 33 (3) | |
| Tumor grade | 0.239 | |||
| 1 | 315 (28) | 41 (29) | 274 (28) | |
| 2 | 424 (37) | 71 (50) | 377 (38) | |
| 3 | 174 (15) | 29 (21) | 147 (15) | |
| Lymph node status, axillary ultrasound, n (%) | 0.001 | |||
| Negative, no FNA | 924 (81) | 102 (71) | 822 (83) | |
| Suspicious, neg FNA | 216 (19) | 42 (29) | 174 (17) | |
| ER, n (%) | 0.471 | |||
| Positive | 829 (71) | 101 (70) | 728 (73) | |
| Negative | 95 (8) | 16 (11) | 79 (8) | |
| PR, n (%) | 0.662 | |||
| Positive | 719 (63) | 91 (64) | 628 (63) | |
| Negative | 205 (18) | 26 (18) | 179 (18) | |
| Her2, n (%) | <0.0001 | |||
| Positive | 439 (39) | 9 (6) | 430 (43) | |
| Negative | 425 (38) | 127 (88) | 298 (30) | |
| Type of surgery, n (%) | 0.118 | |||
| BCS | 593 (47) | 65 (45) | 528 (53) | |
| Mastectomy | 541 (52) | 79 (55) | 462 (46) | |
| Menopause status, n (%) | <0.0001 | |||
| Postmenopausal | 896 (79) | 98 (68) | 798 (80) | |
| Premenopausal | 239 (21) | 41 (28) | 198 (20) | |
ER, estrogen receptor; Her2, human epidermal growth factor receptor; PR, progesterone receptor; BCS, breast conserving surgery.
Figure 2.
Correlation of axillary ultrasound with and without fine needle aspiration and subsequent sentinel lymph node (SLN) pathology. AUS, axillary ultrasound; SLN, sentinel lymph node; SNLD, sentinel lymph node dissection.
Table 2 shows a comparison of the node positive patients in this study to the patients enrolled in ACOSOG Z0011. Of the 144 node positive patients in this study, micrometastases were found in higher frequency, 49% (70 patients), than in the Z0011 study. Of the 144 patients with positive SLNs, 100 underwent CALND, 27 of them had additional non-SLNs with metastasis, which is similar to the findings in Z0011. Only 4 (3%) patients had 3 or more SLNs positive and retrospectively would have required CALND by the subsequently published Z0011 findings. Compared to the ACOSOG Z0011 study, this node positive group had fewer patients who were premenopausal (25%), ER negative (9%) and PR negative (18%) than the Z0011 study groups.
Table 2.
Mayo Clinic Lymph Node Positive Patients Compared to ACOSOG Z0011 Patients
| Criteria | Mayo Clinic | ACOSOG Z0011 (%)(2 patient groups) |
|---|---|---|
|
| ||
| n | 144 | 856 |
|
| ||
| Age, y, mean | 60 | 54–46 |
|
| ||
| Menopausal status, n (%) | ||
| Post | 108 (75) | 62–67 |
| Pre | 36 (25) | 33–38 |
|
| ||
| Tumor stage, n (%) | ||
| T1a | 5 (3) | T1 =68–71 |
| T1b | 17 (12) | |
| T1c | 68 (48) | |
| T2 | 53(37) | 30–32 |
|
| ||
| Tumor size, mean, cm | 1.9 | 1.6–1.7 |
|
| ||
| Histology, n (%) | ||
| Ductal | 111 (79) | 83–84 |
| Lobular | 10 (7) | 7–9 |
| Ductal/lobular | 10 (7) | |
| Other | 10 (7) | 8–11 |
|
| ||
| Tumor grade, n (%) | ||
| 1 | 41 (29) | 21–26 |
| 2 | 71 (50) | 46–49 |
| 3 | 29 (21) | 28–30 |
|
| ||
| ER status, n (%) | ||
| Positive | 130 (91) | 83 |
| Negative | 13 (9) | 17 |
|
| ||
| PR status, n (%) | ||
| Positive | 117 (82) | 70 |
| Negative | 26 (18) | 30 |
|
| ||
| Axillary US results, n (%) | ||
| Negative, no FNA | 100 (69) | |
| Suspicious, negative FNA | 44 (31) | |
|
| ||
| SLN, no. removed, n (%) | ||
| 1 | 21 (15) | 58–72 |
| 2 | 39 (28) | 18–20 |
| 3 | 34 (24) | |
| >3 | 46 (33) | 21 (CALND group) |
|
| ||
| Additional + LN, CALND, n (%) | 27 (19) | 97 (27) |
|
| ||
| SLN metastasis, n (%) | ||
| Micrometastasis | 70 (49) | 38–45 |
| Macrometastasis | 74 (51) | 55–63 |
| > 7 mm | 10 (7) | |
| ≥ 6mm | 19 (13) | |
CALND, completion axillary lymph node dissection; FNA, fine needle aspiration; LN, lymph node.
Only 10 patients demonstrated macrometastases larger than 7mm, and 19 patients with macrometastases of 6 mm or larger (Table 3). In patients with ≥6 mm SLN metastasis, only a single patient had three or more positive SLN on pathology. To determine whether patients with a positive SLN were similar in terms of disease burden to patients with negative SLN, they were compared with respect to age, tumor grade, histology, size, AJCC stage, hormone and menopausal status, and type of surgery (Table 1). The SLN positive group had a higher proportion of premenopausal women (28% vs. 20%, p <0.0001) and higher number of initially suspicious lymph nodes on preoperative AUS deemed negative by FNA (29% vs. 17%, p=0.001) as compared to SLN negative group.
Table 3.
Sentinel Lymph Node Macrometastases
| Criteria | SLN Metastasis ≥ 6 mm, | SLN Metastasis >7 mm |
|---|---|---|
|
| ||
| n | 19 | 10 |
|
| ||
| Menopausal status | ||
| Pre | 5 | 3 |
| Post | 14 | 7 |
|
| ||
| Tumor grade | ||
| 1 | 4 | 0 |
| 2 | 9 | 5 |
| 3 | 5 | 4 |
|
| ||
| Tumor size | ||
| T1a-b | 1 | 0 |
| T1c | 12 | 6 |
| T2 | 6 | 4 |
|
| ||
| ER status | ||
| Positive | 18 | 10 |
| Negative | 0 | 0 |
|
| ||
| PR status | ||
| Positive | 14 | 7 |
| Negative | 4 | 3 |
|
| ||
| SLN excised | ||
| 1 | 2 | 0 |
| 2 | 7 | 4 |
| 3 | 4 | 3 |
| >3 | 6 | 2 |
|
| ||
| Patients with ≥3 positive SLNs | 1 | 0 |
|
| ||
| Patients with additional + LNs on CALND | ||
| 0 | 10 | 5 |
| 1 | 3 | 0 |
| 2 | 4 | 3 |
| 3 | 0 | 0 |
| >3 | 2 | 2 |
CALND, completion axillary lymph node dissection; ER, estrogen receptor; PR, progesterone receptor; SLN, sentinel lymph node.
DISCUSSION
The key message from this study is that for patients who fulfill the ACOSOG Z0011 study criteria, the addition of a negative preoperative AUS +/− FNA should reduce the risk of SLN macrometastases of ≥6 mm to just 2%, and >7 mm to1%. Similarly, such SLN macrometastases in potentially high-risk premenopausal or ER negative patients would be found in only a fraction of 1%, and only 3% of patients had 3 or more positive SLNs. Additional residual non-SLN would be found in only about 0.5% of patients. We recognize that going forward, a positive preoperative US-FNA presently commits about 10% of our patients to axillary dissection, at least some of whom may be adequately treated with only whole breast radiation, sentinel node biopsy and adjuvant therapy.
The impact on survival of metastatic axillary LNs has been questioned for over 35 years. The National Surgical Adjuvant Breast Project (NSABP) B-04 study, predating the publication of the ACOSOG Z0011 study by 25 years, and actually initiated in 1971, reported ten-year results, including over 1,700 patients randomized to either radical mastectomy, total mastectomy with axillary radiation (instead of axillary lymphadenectomy), or total mastectomy alone with subsequent axillary lymphadenectomy for development of clinically evident axillary lymph node metastasis (without any adjuvant therapy).5 There were no differences in DFS or OS, which remained true after 25 years of follow-up.6 These data raised serious question regarding the survival impact of either surgically removing or irradiating occult positive axillary nodes in breast cancer. But the value of knowing the disease status of the lymph nodes has remained paramount in disease staging and been a key decision point for the use of adjuvant therapy.
In 1997, however, randomized studies demonstrated marked improvement in not only local recurrence, but also in DFS and OS in post-modified radical mastectomy, axillary node positive patients given postoperative adjuvant radiotherapy in addition to standard chemotherapy.7–9 Therefore, the need for and value of axillary dissection and perhaps radiation for node-positive disease, seemed solidly established.
Almost synchronously, Giuliano10 published the early experience of intraoperative lymphatic mapping and axillary sentinel lymph node (SLN) biopsies. This technique evolved and eventually seemed ideal to meet the need of precise axillary lymph node disease staging, avoidance of unnecessary axillary node dissection for node-negative disease thereby reducing the attendant surgical morbidity. Careful surgical/pathological correlation revealed that in only about 50% of patients with metastatically involved SLNs were non-SLNs involved. Contrary to the Fisher hypothesis11, there might actually be some barrier function to SLN as survival was better if only sentinel lymph nodes were positive compared to patients with both SLN and non-SLN involvement.12 Surgeons and oncologists have struggled with the best method to limit axillary dissection to just those with high enough risk of additional non-SLNs to justify CALND when the SLN contained this limited disease. Mathematical models to predict non-SLN involvement when the SLN is positive have been devised13–15 to facilitate decisions whether to recommend CALND. Implicit in this decision, however, is the recognition that some patients who choose to forego reoperation will actually have residual positive non-SLN.
Clearly there has been a trend to forego completion ALND even when the SLN is positive, especially when the disease is limited to micrometastasis. Review of the Surveillance, Epidemiology, and End Results (SEER) database in 2010 revealed that 40% of patients with SLN-micrometastases did not undergo completion ALND16. Further analysis of the SEER database, reviewing nearly 27,000 patients with positive nodes, demonstrated that over 16% had undergone SLN biopsy only whereas the remainder had proceeded to CALND.17 Impressively, there was no statistically significant difference in OS between the SLN biopsy only and the CALND groups. Similarly, Bilimoria18 reviewed the National Cancer Data Base from 1999 – 2005, comprising nearly 100,000 node positive patients in which about 20% had SLN biopsy only, and the remaining 80% had completion ALND. In patients with only microscopic nodal metastasis, there was no difference in axillary recurrence or survival between the two groups, and in macroscopic nodal disease, a non-significant trend was found for the CALND group compared to the SLN biopsy only patients.
Publication of the ACOSOG Z0011 trial solidified the evolving data-driven practice that CALND could be safely omitted in patients with T1-2 tumors, clinically node-negative, with 1–2 positive SLNs, having undergone breast conservation therapy and who would be treated with standard breast radiation and adjuvant therapy.
The response to the study was rapid and bold. For breast conservation patients, the Memorial Sloan-Kettering Cancer Center Breast Service eliminated intraoperative frozen section as well as CALND in patients with 1–2 positive SLN, regardless of metastasis size.19 Similarly, the multidisciplinary group at the University of Texas MD Anderson Cancer Center (MDACC) endorsed avoidance of CALND for patients who fulfill the Z0011 study criteria. Additionally, routine intraoperative SLN assessment no longer is undertaken for these patients. However, the radiation oncologists at MDACC intend to modify the opposing tangential whole breast radiation fields for women who do not undergo CALND. In contrast to usual breast radiation required in the Z0011 trial, high tangents will be added, increasing the coverage to more than 80% of axillary levels I and II.
Amid the strong endorsement of the Z0011 study results and recommendations, concern has been raised that the highly favorable results might not be achieved when applied to the usual cross-section of breast cancer patients, that the Z0011 study was a highly selected patient group. Underrepresented were cancers generally regarded as more virulent and with greater disease burden. These would include a much higher percentage of lymph node macrometastasis, ER-negative, and premenopausal females.
To counteract these, clinicians derive some confidence in potentially leaving microscopic disease in residual lymph nodes by the therapeutic effect of adjuvant therapy. The After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS) phase III study compares axillary lymph node dissection and axillary radiation therapy (ART) in early breast cancer with tumor-positive SLNs. While the principal results of the study have yet to be published, the influence of random assignment to completion ALND or ART on the choice of adjuvant treatment has been analyzed20 Importantly, the absence of knowledge regarding the extent of nodal involvement in the ART arm did not have a major impact on the administration of adjuvant therapy. Moreover, adjuvant therapy is progressively being advised on the basis of tumor biology rather than number of histologically positive nodes. Radiation oncologists, however, seem concerned that the therapeutic effect on levels I and II from radiation limited to whole breast fields might be inadequate21. Whereas using standard tangential fields, >50% of level I and 20–30% of level II nodes receive 95% of the prescribed radiation dose, the use of additional high tangential fields has been shown to include the majority of level I–II nodes.22 Adjusting the radiotherapy fields has been proposed based on as many as nine factors: breast cancer histology, tumor size, ER status, presence of lymphovascular invasion, multifocality, number and size of SLN metastasis, and total number and presence of extranodal extension of positive SLNs.22 Support for this position derives from the Early Breast Trialists’ Collaborative Group (EBCTCG) conclusion that over a 15 year period, avoidance of local recurrence in four patients would avoid one breast cancer death.23 Additionally, the recent MA-20 trial has added further evidence to prior studies7–9 that found survival as well as local recurrence benefits in patients with metastatic lymph nodes when regional nodal irradiation was added to whole breast radiation.24
Stunningly contrary to the complex adjustments proposed by the radiation oncologists, several upcoming clinical trials are being proposed to evaluate whether SLN staging overall can be abandoned. In Europe, Gentilini and Veronesi have proposed a new clinical trial: Sentinel node vs Observation after axillary UltraouND (SOUND). The premise for this study is that “not only wider surgery in the axilla is not improving outcome but also that the information achieved by removing lymph nodes does not change the prognosis of the disease.” Extending the thinking, they believe that because local control is excellent, survival is unchanged, presence and extent of nodal involvement does not change type of treatment, “do we even need to look for a SLN?” The SOUND study eligibility criteria would be rather similar to the Z0011 study: T1 tumors, clinically negative axillary nodes, breast conservation therapy including radiotherapy. However, they add axillary ultrasound as a means of screening for “clinically relevant nodal burden”. With either a negative axillary US or a negative US-FNA of any questionable node, the patient would be randomized to either SLN biopsy ± completion ALND, or no axillary surgical staging at all.
Axillary US with percutaneous FNA or core needle biopsy of suspicious lymph nodes has been used to identify patients with positive nodes preoperatively in order to prevent the time, expense, and any morbidity of the SLN localization and procedure and also to guide nodal staging for consideration of neoadjuvant chemotherapy. A positive US-FNA would imply need for axillary lymphadenectomy. Criteria such as lymph node cortical thickening, asymmetry, plus the addition of US and needle sampling rather than imaging criteria alone, were intended to improve the sensitivity.25 For non-palpable axillary nodes, sensitivity ranges from about 44–73%.26, 27 Without minimizing the importance of sensitivity, the principal emphasis of axillary US, and the reason of incorporating US-FNA, was to assure virtually 100% specificity. Axillary US +/− FNA was shown to be cost effective for invasive breast cancer due to decreasing the need for SLN biopsy for nodal staging.28
There is a new aim for axillary US, or at least the aim has shifted somewhat. To be applied across the entire spectrum of breast cancer patients who fulfill the Z0011 eligibility criteria, not just patients with favorable disease, the sensitivity needs to be sufficient to identify nodal disease burden that would be responsible for disease relapse. The Z0011 study clearly demonstrates that this sensitivity requirement is well below 100%, but the exact threshold remains unknown.
The value and accuracy of frozen section analysis of SLNs has been investigated. When integrated into an evaluation process including preoperative axillary US with FNA, SLN biopsy, and intraoperative frozen section, Genta et al29 found that about 30% of the positive nodes were identified by US-FNA, an additional 30% were confirmed by frozen section, and about 35% were identified only after final definitive permanent histologic analysis. It is no surprise that the fraction of patients in our study who had positive lymph nodes with CALND was similar to the 27% seen in the ACOSOG Z0011 study, and far fewer than the 53% in the meta-analysis of 8,000 patients. Patients with larger tumors often now receive neoadjuvant therapy; patients with multicentric cancers that would require mastectomy and those with US-FNA positive nodes were excluded from this study. We recognize that by committing patients with preoperative US-FNA positive lymph nodes to axillary dissection might encompass some who would be satisfactorily managed with SLN biopsy alone, relying on the combined radiation and adjuvant therapy to prevent axillary LN relapse. We do not have precise breakpoints for disease burden or markers of excessive disease virulence that might be best treated with CALND. We have gained additional confidence, however, that for small breast cancers, with no palpable lymphadenopathy or positive LNs by AUS, managed with BCS including whole breast radiation therapy and planned adjuvant treatment, that SLN metastasis ≥6 mm will be found in only 2% of patient, and >7 mm in only 1%. Furthermore, only 3% will have 3 or more positive SLNs, and it seems a very small percentage would be expected to be ER negative. Consequently, with the addition of AUS+/− FNA, we endorse the conclusions of the ACOSOG Z0011 trial in avoiding CALND, and we see marginal gain in continuing frozen section of SLNs in these patients.
Acknowledgments
This study was supported in part by NIH/NCRR-NCATS CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure information: Nothing to disclose.
Presented at the Western Surgical Association 120th Scientific Session, Colorado Springs, CO, November 2012.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halsted W. Clinical Society of Maryland. Vol. 2. Baltimore: The Johns Hopkins Press; 1894. The results of operations for the cure of cancer of the breast performed at the Johns Hopkins Hospital from June, 1889, to January, 1894; p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano A, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–433. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano A, Hunt K, Ballman K, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim T, Giuliano A, Lyman G. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a meta-analysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 5.Fisher B, Redmond C, Fisher ER, et al. Ten-year results of a randomized clinical trial comparing radical mastectomy and total mastectomy with or without radiation. New England Journal Medicine. 1985;312:674–681. doi: 10.1056/NEJM198503143121102. [DOI] [PubMed] [Google Scholar]
- 6.Fisher B, Jeong J-H, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 7.Overgaard M, Hansen P, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 8.Overgaard M, Jensen M-B, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 9.Ragaz J, Stewart S, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337:956–962. doi: 10.1056/NEJM199710023371402. [DOI] [PubMed] [Google Scholar]
- 10.Giuliano A, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B. Laboratory and clinical research in breast cancer--A personal adventure: The David A. Karnofsky Memorial Lecture Cancer Research. 1980;40:3863–3874. [PubMed] [Google Scholar]
- 12.Jakub J, Bryant K, Huebner M, et al. The number of axillary luymph nodes involved with metastatic breast cancer does not affect outcome as long as all disease is confined to the sentinel lymph nodes. Ann Surg Oncol. 2011;18:86–93. doi: 10.1245/s10434-010-1202-1. [DOI] [PubMed] [Google Scholar]
- 13.Mittendorf E, Hunt K, Boughey J, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting nonsentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann Surg. 2012;255:109–115. doi: 10.1097/SLA.0b013e318238f461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Zee K, Manasseh D, Bevilacqua J. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Degnim A, Reynolds C, Pantvaidya G, et al. Nonsentinel node metastasis in breast cancer patients: assessment of an existing and a new predictive nomogram. Am J Surg. 2005;190:543–550. doi: 10.1016/j.amjsurg.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Wasif N, Maggard M, Ko C, et al. Underuse of axillary dissection for the management of sentinel node micrometastases in breast cancer. Arch Surg. 2010;145:161–166. doi: 10.1001/archsurg.2009.269. [DOI] [PubMed] [Google Scholar]
- 17.Yi M, SHG, Meric-Bernstam F, et al. Trends in and outcomes from sentinel lymph node biopsy (SLNB) alone vs. SLNB with axillary lymph node dissection for node-positive breast cancer patients: experience from the SEER database. Ann Surg Oncol. 2010;17:S343–S351. doi: 10.1245/s10434-010-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bilimoria K, Bentrem D, Hansen N, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27:2946–2953. doi: 10.1200/JCO.2008.19.5750. [DOI] [PubMed] [Google Scholar]
- 19.Weber W, Barry M, Stempel M, et al. A 10-year trend analysis of sentinel lymph node frozen section and completion axillary dissection for breast cancer: are these procedures becoming obsolete? Ann Surg Oncol. 2012;19:225–232. doi: 10.1245/s10434-011-1823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straver M, Meijnen P, van Tienhoven G, et al. Role of axillary clearance after a tumor-positive sentinel node in the administration of adjuvant therapy in early breast cancer. J Clin Oncol. 2010;28:731–737. doi: 10.1200/JCO.2008.21.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caudle A, Hunt K, Kuerer H, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011;18:2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haffty B, Hunt K, Harris J, et al. Positive sentinel nodes without axillary dissection: implications for the radiation oncologist. J Clin Oncol. 2011;29:4479–4481. doi: 10.1200/JCO.2011.36.1667. [DOI] [PubMed] [Google Scholar]
- 23.(EBCTCG) EBCTCG . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 24.Whelan T, Olivotto I, Ackerman I, et al. NCIC-CTG MA.20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncology. 2011;29 (suppl; abstr LBA1003) [Google Scholar]
- 25.Solon J, Power C, Al-Azawi D, et al. Ultrasound-guided core biopsy: an effective method of detecting axillary nodal metastases. J Am Coll Surg. 2012;214:12–17. doi: 10.1016/j.jamcollsurg.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Cools-Lartigue J, Meterissian S. Accuracy of axillary ultrasound in the diagnosis of nodal metastasis in invasive breast cancer: a review. World J Surg. 2012;36:46–54. doi: 10.1007/s00268-011-1319-9. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez S, Anorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. Am J Roentgenol. 2006;186:1342–1348. doi: 10.2214/AJR.05.0936. [DOI] [PubMed] [Google Scholar]
- 28.Boughey J, Moriarty J, Degnim A, et al. Cost modeling of preoperative axillary ultrasound and fine-needle aspiration to guide surgery for invasive breast cancer. Ann Surg Oncol. 2010;17:953–958. doi: 10.1245/s10434-010-0919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genta F, Zanon E, Camanni M, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg. 2007;31:1155–1163. doi: 10.1007/s00268-007-9009-3. [DOI] [PubMed] [Google Scholar]