Abstract
Purpose
Following patients after prostatectomy can be expensive and stressful, therefore, a novel and reliable approach to improve stratification is needed both at diagnosis of PCa and following its treatment. We evaluate the association of both ERG and claudin-4, -5 and betacatenin expression in tumor tissues of patients with organ confined and advanced prostatic adenocarcinomas.
Methods
A total of 30 patients were included in the study. Nine men, who underwent radical prostatectomy for organ confined (pT2N0M0) cancer (OCC); 11 patients with clinically advanced cancer (CAC); and 11 controls with benign prostatic hypertrophy (BPH). Using immunohistochemistry applied to tissue microarrays, each group was evaluated for beta-catenin, claudin-4, -5 and ERG expression.
Results
The expression of ERG was higher in the CAC group as compared to OCC and BPH (p = 0.7684, p = 0.0224, respectively). Among these patients, 5 from the CAC (45%) and 5 from the OCC group (56%) stained positively for ERG (p = 1.0). The mean staining score for those with ERG+ advanced cancer was greater than that for the ERG+ organ-confined cancer (p = 0.0209). ERG staining correlated with Gleason score (Pearson correlation: 0.498, p = 0.0051), but not with serum PSA level (Pearson correlation: 0.404, p = 0.1202). When analyzing outcome data high ERG expressing tumors have shown a significantly worse overall survival (p = 0.0084).
Conclusions
Our results of presence or absence of claudin-4 and -5 and ERG staining intensities suggest their potential as prognostic factors for prostate cancer.
Keywords: prostate, prognosis, prediction, ERG, claudin
Introduction
Since the introduction of prostate-specific antigen (PSA) in the late 1980s, both the number of prostate biopsies performed and incidence of prostate cancer has risen [1,2]. Screening for PCa with PSA, though, is controversial. Some opinion leaders have questioned the role of PSA as a screening tool altogether, advocating that its worldwide use has led to increase in unnecessary biopsies, over-diagnosis of low-grade, low-stage tumors with subsequent over-treatment, without having a considerable impact on prostate cancer-related survival [3–7]. Additionally, control of cancer following prostatectomy in the PSA era has improved [8,9], although it is difficult to predict just who will recur [10]. Continually following patients after prostatectomy can be expensive and stressful [11–13]. Therefore, a novel and reliable approach to improve stratification is needed both at diagnosis of PCa and following its treatment.
In a clinically asymptomatic and normal man the key question to be answered is not only whether he has a cancer but also it is dangerous or not. To improve the specificity of total PSA, which is unfortunately expressed at similar level in benign and cancerous cells, several approaches based on PSA derivatives have been investigated. However, none of these alone have reached a significant improvement in PCa detection. Many prospective new biomarkers have been identified and are currently under investigation, with some showing substantial promise, like PCa biomarkers. Furthermore, the final aim of this study is to find prognostic markers for prostate cancer patients to improve their stratification for outcome and possibly, treatment options.
In general, a good biomarker has to be over-expressed only in prostate cancer cells and ideally should be reproducible, cost effective and also should be correlated with disease outcome. In these settings the future of cancer prognosis might rely on small panels of markers that can precisely predict PCa presence, stage, metastasis, and serve as prognostic factors and substitute end points of disease progression and response to therapy.
The potential of claudins, small tight junction proteins that are involved in intercellular architecture and communication was examined [14–16], both diagnostic and prognostic marker in PCa. Our results suggested that patients with organ-confined (OCC) and advanced cancer (CAC) were subsets with distinct claudin expression profiles, specifically, claudin-4 and -5 was expressed at higher levels in PCa with poor prognosis [17]. Elevated membranous claudin-4 expression was described by others as well to be a marker of poor prognosis in prostate cancer [18].
The ETS-Related Gene (ERG) is an oncogene that is over-expressed in PCa when a fusion event places it downstream of the androgen-dependent TMPRSS-2 promoter normally present 3 mB upstream [19]. Present in 50–70% of PCa patients in Western populations, the ERG-TMPRSS2 fusion is one of the most common genetic alterations in PCa [20–23]. Furusato et al. recently described a highly specific antibody able to detect the ERG oncoprotein expression with high concordance to genomic rearrangement [24]. In the present study, we evaluate the association of both ERG and claudin-4, -5 and beta-catenin expression in tumor tissues of patients with organ confined and advanced prostatic adenocarcinomas.
Materials and Methods
Patient samples
A total of 30 patients were included in the study (Table 1). Nine men, who underwent radical prostatectomy for organ confined (pT2N0M0) cancer (OCC); 11 patients with clinically advanced cancer (CAC); and 11 controls with benign prostatic hypertrophy following transurethral resection of the prostate (BPH). Tissue microarrays were used to evaluate the samples of the patients making it feasible to compare the same size of tissue areas in each case: two 2 mm wide cores were taken from each formalin-fixed and paraffin-embedded block for further immunohistochemical analysis.
Table 1.
Clinicopathological and immunohistochemical evaluation of the patients and respective tumors in the study.
| GROUP | CAC (n=10) | OCC (n=9) | BPH (n=11) | Sign. | |||
|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | ||
| Age | 70.09 | 2.80 | 62.13 | 2.29 | 67.82 | 0.99 | 0.055 |
| PSA (serum) | 703.45 | 254.87 | 22.76 | 12.12 | 11.92 | 2.23 | 0.001 |
| P.vol (ml) | 61.36 | 9.16 | 65.24 | 6.33 | 88.86 | 4.56 | 0.038 |
| Gleason score | 7.80 | 0.42 | 4.89 | 0.42 | NA | NA | 0.001 |
| Beta-catenin | 205.00 | 26.38 | 91.67 | 27.84 | 260.91 | 26.30 | 0.001 |
| Claudin-4 | 258.18 | 13.06 | 2.22 | 2.22 | 189.09 | 24.06 | 0.001 |
| Claudin-5 | 33.33 | 33.33 | 67.78 | 21.91 | 226.36 | 24.73 | 0.001 |
| ERG | 122.73 | 43.23 | 90.00 | 35.91 | 0.00 | 0.00 | 0.025 |
P.vol - Prostate volume
SE - Standard Error of Mean
Immunohistochemistry
Using immunohistochemistry applied to tissue microarrays, each group was evaluated for beta-catenin, claudin-4, -5 and ERG expression. After deparaffination, the slides were treated in pH9 Target Retrieval Solution (#S2368, DAKO, Carpinteria, CA, USA) in a microwave oven for 28 minutes, subsequently, incubation of the slides with primary antibody of ERG-Mab (Clone 9FY, dilution: 1:60, CPDR, Uniformed Services University, Bethesda, MD, USA; now available from Biocare Medical, Concord, CA, USA) diluted in Antibody Diluent (#251018; Ventana Medical Systems, Inc., Tucson, AZ, USA) was performed for 60 minutes at room temperature. The reactions were visualized with UltraView DAB Detection kit according to the manufacturer’s protocol (#760-500; Roche Diagnostics, Mannheim, Germany). ERG staining has shown a nuclear expression pattern.
Beta-catenin, claudin-4 and -5 staining were performed for an earlier study [17], where the expression was evaluated in an automated manner. Here, these membranous reactions were re-evaluated semi-quantitatively integrating intensity (0–3) and frequency (0–100) of staining on a scale of 0–300 [25].
Statistics
The analysis was performed with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Kaplan-Meier curves were used to plot the efficiency of the prediction on overall survival. Chi-squared test was performed to test relation of ERG and clinicopathological variables grouped into categorical variables. Continuous variables were compared with ANOVA test, and individual groups were tested with post-hoc analysis according to Tukey. Statistically significant results were accepted at p-values of 0.05.
Results
There were 10 patients with clinically advanced prostate cancer and 9 patients with organ confined prostate cancer compared to 11 BPH patients (Table 1).
The expression of ERG was higher in the CAC group as compared to OCC and BPH (p = 0.7684, p = 0.0224, respectively; Figure 1). Among these patients, 5 from the CAC (45%) and 5 from the OCC group (56%) stained positively for ERG (p = 1.0). The mean staining score for those with ERG+ advanced cancer was 270, which was significantly greater than that for the ERG+ organ-confined cancer (158; p = 0.0209). ERG staining correlated with Gleason score (Pearson correlation: 0.498, p = 0.0051), but not with serum PSA level (Pearson correlation: 0.404, p = 0.1202).
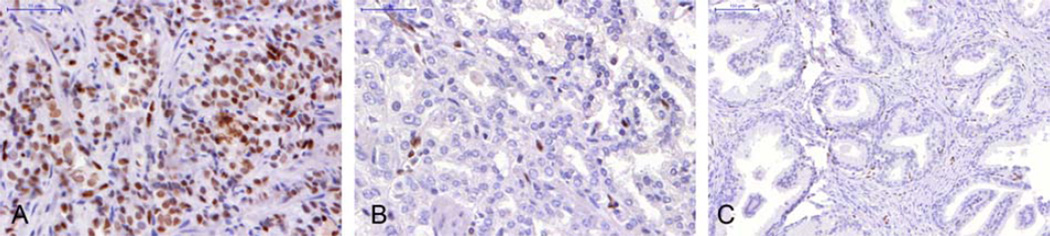
Figure 1.
Immunohistochemical images of ERG expression in advanced (A) and organ confined (B) tumors and benign hyperplastic (C) prostatic tissues (40×).
Difference was found between the three groups regarding claudin-4 (p=1.247e-10) and claudin-5 (p = 0.0001) expression regardless of age, PSA level, prostate volume and Gleason score. Claudin-4 and ERG were specifically present in the OCC and CAC, while claudin-5 was strongly expressed in BPH. All BPH specimens were negative for ERG. Expression of ERG did not correlate with expression of claudin-4 or beta-catenin (p = 0.9764, p = 0.8706, respectively). Claudin-5 has shown a significant correlation with ERG staining (Pearson correlation: -0.477, p = 0.0246).
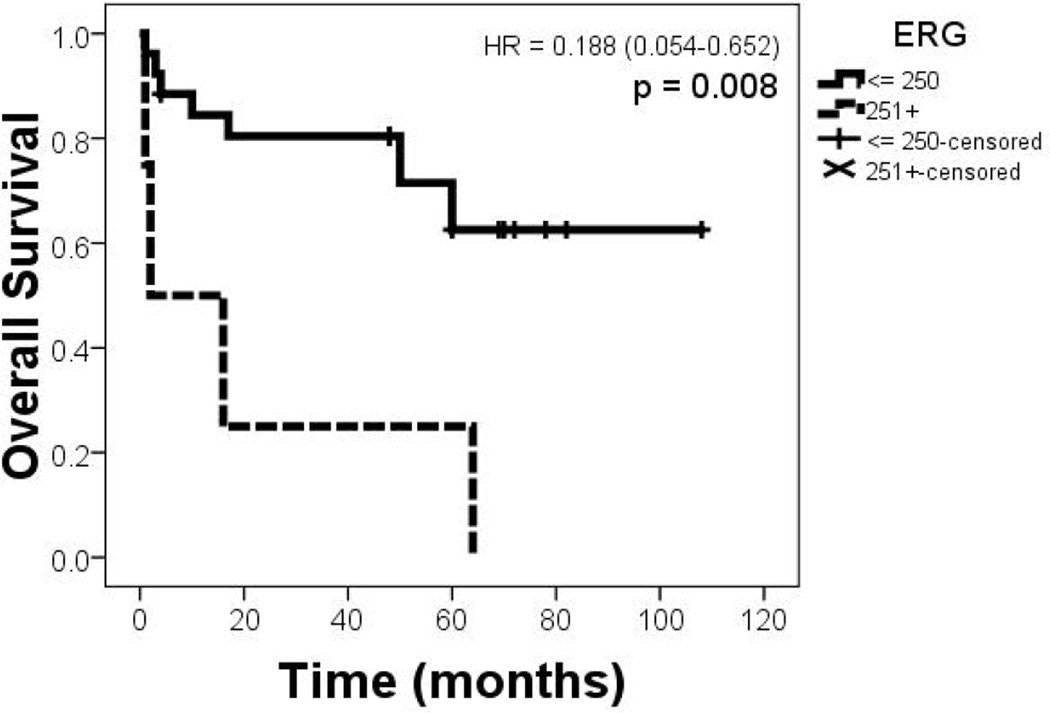
When analyzing outcome data high ERG expressing tumors have shown a significantly worse overall survival (p = 0.0084; Figure 2).
Figure 2.
Kaplan-Meier graph showing the survival curves of the groups based on lower vs. higher ERG expression (binned: 0-250 and 251-300).
Discussion
The potential role of claudins as potential markers of PCa prognosis has been reported previously [17]. We undertook a comparative approach to examine the prognostic potential of ERG in Central Eastern European patients in this pilot study, as claudin-4 has already been established as prognostic marker and the number of herein investigated samples is limited.
In the presented data, there was no statistically significant difference in ERG positivity rates between the organ-confined and advanced prostate cancers. Additionally, although the sample size was limited to this exploratory cohort, the rate of ERG positivity matches those previously reported for Western populations [20–22,19,23].
The expression of the ERG oncogene has been extensively studied as a prognostic marker, with inconsistent results[26]. Carefully designed multi-center studied with defined patients cohorts are needed to sort out these apparent differences. With the knowledge that the genomic rearrangement that places the TMPRSS2 promoter immediately upstream from the ERG oncogene can occur by the oncogene’s 5’ region or its insertion elsewhere, Attard et al. and Mehra et al. explored the prognostic significance of the mechanism of fusion. They determined that the deletion event conveys a poorer prognosis [27,28]. However, other studies suggested that ERG fusion of deletion type is the consequence of aneuploidy [29]. Hu et al. identified two splice variants of the ERG RNA: Type I, which codes for the full length ERG protein and type II, which codes for a truncated protein. A high type I to type II ratio, when quantitatively measured, was associated with PSA recurrence [30]. Overall prognostic utility of ERG fusions is uncertain and warrants further investigations [31].
To our knowledge, this is the first study to identify a correlation between intensity of ERG oncoprotein and PCa prognosis. Van Leenders et al. previously noted a correlation between strength of ERG IHC signaling and copies of ERG mRNA in prostate cancer cells. They found a statistically significantly higher amount of ERG mRNA in those cancer that expressed strong (3+) IHC signals compared to those that had moderate (2+) signaling, but no significant difference in quantitative mRNA levels between those cells with moderate and weak (1+) IHC signals. Contrary to the present results, in a set of 17 patients, they noted no correlation between ERG IHC intensity or mRNA levels with clinicopathological parameters at radical prostatectomy [32]. This study also reported the sensitivity, specificity, positive predictive value and negative predictive value of a positive ERG stain in biopsy was 61%, 94%, 92% and 72%, respectively [32]. Two other groups further explored the applicability of staining biopsy cores with ERG. Yaskiv et al. also sought to explore the diagnostic utility of ERG IHC, but they combined it with the basal cell marker p63, the loss of which is a very sensitive marker of prostate cancer. Because of the specificity of ERG staining, they concluded that ERG+/p63− cores should be diagnosed as prostate cancer [33].
Emerging studies underscore the robustness and ease of ERG IHC in biopsy and prostatectomy specimens, as well as the strong concordance of ERG protein expression in with ERG rearrangements in PCa [34]. These practical solutions are necessary if promising PCa biomarkers, such as claudin, beta-catenin and ERG are to be evaluated to help stratify patients prior to prostatectomy. Recent evidence also suggests that taxanes have a significant impact on androgen receptor signaling [35,36], thus, studies to evaluate the efficacy of taxanes guided by ERG status are of particular interest [37]. This proof of principle study provides new opportunities in further evaluations of prognostic and predictive utility of quantitative evaluation of ERG in PCa.
Conclusions
In this pilot study, the expression of ERG was higher in the CAC group as compared to OCC and BPH. ERG staining correlated with Gleason score, but not with serum PSA level. All BPH specimina were negative for ERG. When analyzing outcome data high ERG expressing tumors have shown a significantly worse overall survival. Claudin-4 and ERG were specifically present in the pCa groups, while claudin-5 was strongly expressed in BPH. Expression of ERG did not correlate with expression of claudin-4 or beta-catenin, but claudin-5 has shown a significant inverse correlation with ERG. Our results of presence or absence of claudin-4/5 and ERG staining intensities suggest their potential as prognostic factors for prostate cancer, which warrant further investigations in larger series of Central Eastern European population including biopsy samples and prostatectomy specimen comparisons.
Acknowledgments
Grant support: MTA-2012TKI643 grant to AMSz and JK. TÁMOP-4.2.2/B-10/1-2010-0013 grant to JK.
Contributor Information
A. Marcell Szász, Email: cac@korb2.sote.hu.
Attila Majoros, Email: majoros@urol.sote.hu.
Philip Rosen, Email: prosen@cpdr.org.
Shiv Srivastava, Email: ssrivastava@cpdr.org.
Albert Dobi, Email: adobi@cpdr.org.
Attila Szendrői, Email: aszendroi@gmail.com.
Janina Kulka, Email: kj@korb2.sote.hu.
Péter Nyirády, Email: nyiradyp@gmail.com.
References
- 1.Coldman AJ, Phillips N, Pickles TA. Trends in prostate cancer incidence and mortality: an analysis of mortality change by screening intensity. Canadian Medical Association Journal. 2003;168(1):31–35. [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RO, Bergstralh EJ, Peterson NR, Bostwick DG, Lieber MM, Jacobsen SJ. Positive and Negative Biopsies tn the Pre-Prostate Specific Antigen and Prostate Specific Antigen Eras, 1980 to 1997. The Journal of urology. 2000;163(5):1471–1475. [PubMed] [Google Scholar]
- 3.Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O'Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD. Mortality Results from a Randomized Prostate-Cancer Screening Trial. New England Journal of Medicine. 2009;360(13):1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SchrAder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A. Screening and Prostate-Cancer Mortality in a Randomized European Study. New England Journal of Medicine. 2009;360(13):1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 5.Daskivich T, Chamie K, Kwan L, Labo J, Palvolgyi R, Dash A, Greenfield S, Litwin M. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117(20):4805–4805. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 6.Bangma C, Roemeling S, Schröder F. Overdiagnosis and overtreatment of early detected prostate cancer. World Journal of Urology. 2007;25(1):3–9. doi: 10.1007/s00345-007-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao YH, Albertsen PC, Shih W, Roberts CB, Lu-Yao GL. The impact of PSA testing frequency on prostate cancer incidence and treatment in older men. Prostate Cancer Prostatic Dis. 2011;14(4):332–339. doi: 10.1038/pcan.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roehl KA, Han M, Ramos CG, Antenor JAV, Catalona WJ. Cancer Progression and Survival Rates Following Anatomical Radical Retropubic Prostatectomy in 3,478 Consecutive Patients: Long-Term Results. The Journal of urology. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 9.Han M, Partin AW, Piantadosi S, Epstein JI, Walsh PC. Era Specific Biochemical Recurrence-Free Survival Following Radical Prostatectomy for Clinically Localized Prostate Cancer. The Journal of urology. 2001;166(2):416–419. [PubMed] [Google Scholar]
- 10.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Yossepowitch O, Vickers AJ, Klein EA, Wood DP, Scardino PT. Prostate Cancer-Specific Mortality After Radical Prostatectomy for Patients Treated in the Prostate-Specific Antigen Era. Journal of Clinical Oncology. 2009;27(26):4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tollefson MK, Blute ML, Rangel LJ, Karnes RJ, Frank I. Lifelong Yearly Prostate Specific Antigen Surveillance is Not Necessary for Low Risk Prostate Cancer Treated With Radical Prostatectomy. The Journal of urology. 2010;184(3):925–929. doi: 10.1016/j.juro.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 12.Dale W, Hemmerich J, Bylow K, Mohile S, Mullaney M, Stadler WM. Patient Anxiety About Prostate Cancer Independently Predicts Early Initiation of Androgen Deprivation Therapy for Biochemical Cancer Recurrence in Older Men: A Prospective Cohort Study. Journal of Clinical Oncology. 2009;27(10):1557–1563. doi: 10.1200/JCO.2008.18.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chism K, Kunkel E. Prostate cancer: Issues in psychosomatic medicine. Current Psychiatry Reports. 2009;11(3):205–210. doi: 10.1007/s11920-009-0032-y. [DOI] [PubMed] [Google Scholar]
- 14.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. The Journal of Cell Biology. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto T, Kojima T, Murata M, Takano K-i, Go M, Chiba H, Sawada N. IL-1beta regulates expression of Cx32, occludin, and claudin-2 of rat hepatocytes via distinct signal transduction pathways. Experimental Cell Research. 2004;299(2):427–441. doi: 10.1016/j.yexcr.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Heiskala M, Peterson PA, Yang Y. The Roles of Claudin Superfamily Proteins in Paracellular Transport. Traffic. 2001;2(2):92–98. doi: 10.1034/j.1600-0854.2001.020203.x. [DOI] [PubMed] [Google Scholar]
- 17.Szasz AM, Nyirady P, Majoros A, Szendroi A, Szucs M, Szekely E, Tokes AM, Romics I, Kulka J. beta-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression. BJU international. 2010;105(5):716–722. doi: 10.1111/j.1464-410X.2009.08808.x. [DOI] [PubMed] [Google Scholar]
- 18.Landers KA, Samaratunga H, Teng L, Buck M, Burger MJ, Scells B, Lavin MF, Gardiner RA. Identification of claudin-4 as a marker highly overexpressed in both primary and metastatic prostate cancer. British journal of cancer. 2008;99(3):491–501. doi: 10.1038/sj.bjc.6604486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun X-W, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science (New York, NY) 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 20.Magi-Galluzzi C, Tsusuki T, Elson P, Simmerman K, LaFargue C, Esgueva R, Klein E, Rubin MA, Zhou M. TMPRSS2-ERG gene fusion prevalence and class are significantly different in prostate cancer of caucasian, african-american and japanese patients. The Prostate. 71(5):489–497. doi: 10.1002/pros.21265. [DOI] [PubMed] [Google Scholar]
- 21.Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul JW, Srivastava S. Frequent overexpression of ETS-related gene-1(ERG1) in prostate cancer transcriptome. Oncogene. 2005;24(23):3847–3852. doi: 10.1038/sj.onc.1208518. [DOI] [PubMed] [Google Scholar]
- 22.Soller MJ, Isaksson M, Elfving P, Soller W, Lundgren R, Panagopoulos I. Confirmation of the high frequency of the TMPRSS2/ERG fusion gene in prostate cancer. Genes, Chromosomes and Cancer. 2006;45(7):717–719. doi: 10.1002/gcc.20329. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto M, Joshua A, Chilton-MacNeill S, Bayani J, Selvarajah S, Evans AJ, Zielenska M, Squire JA. Three-color FISH analysis of TMPRSS2/ERG fusions in prostate cancer indicates genomic microdeletion of chromosome 21 is associated with rearrangement. Neoplasia (New York, NY) 2006;8(6) doi: 10.1593/neo.06283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, Thangapazham R, Chen Y, McMaster G, Sreenath T, Petrovics G, McLeod DG, Srivastava S, Sesterhenn IA. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis. 2010;13(3):228–237. doi: 10.1038/pcan.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arun P, Brown MS, Ehsanian R, Chen Z, Van Waes C. Nuclear NF-κB p65. Phosphorylation at Serine 276 by Protein Kinase A Contributes to the Malignant Phenotype of Head and Neck Cancer. Clinical Cancer Research. 2009;15(19):5974–5984. doi: 10.1158/1078-0432.CCR-09-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6(8):429–439. doi: 10.1038/nrurol.2009.127. [DOI] [PubMed] [Google Scholar]
- 27.Attard G, Clark J, Ambroisine L, Fisher G, Kovacs G, Flohr P, Berney D, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter V, De Bono JS, Scardino P, Cuzick J, Cooper CS. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2007;27(3):253–263. doi: 10.1038/sj.onc.1210640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra R, Tomlins SA, Yu J, Cao X, Wang L, Menon A, Rubin MA, Pienta KJ, Shah RB, Chinnaiyan AM. Characterization of TMPRSS2-ETS Gene Aberrations in Androgen- Independent Metastatic Prostate Cancer. Cancer research. 2008;68(10):3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gopalan A, Leversha MA, Satagopan JM, Zhou Q, Al-Ahmadie HA, Fine SW, Eastham JA, Scardino PT, Scher HI, Tickoo SK, Reuter VE, Gerald WL. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer research. 2009;69(4):1400–1406. doi: 10.1158/0008-5472.CAN-08-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y, Dobi A, Sreenath T, Cook C, Tadase AY, Ravindranath L, Cullen J, Furusato B, Chen Y, Thangapazham RL, Mohamed A, Sun C, Sesterhenn IA, McLeod DG, Petrovics G, Srivastava S. Delineation of TMPRSS2-ERG Splice Variants in Prostate Cancer. Clinical Cancer Research. 2008;14(15):4719–4725. doi: 10.1158/1078-0432.CCR-08-0531. [DOI] [PubMed] [Google Scholar]
- 31.Spencer ES, Johnston RB, Gordon RR, Lucas JM, Ussakli CH, Hurtado-Coll A, Srivastava S, Nelson PS, Porter CR. Prognostic value of ERG oncoprotein in prostate cancer recurrence and cause-specific mortality. The Prostate:n/a-n/a. 2013 doi: 10.1002/pros.22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Leenders GJLH, Boormans JL, Vissers CJ, Hoogland AM, Bressers AA, Furusato B, Trapman J. Antibody EPR3864 is specific for ERG genomic fusions in prostate cancer: implications for pathological practice. Mod Pathol. 2011;24(8):1128–1138. doi: 10.1038/modpathol.2011.65. [DOI] [PubMed] [Google Scholar]
- 33.Yaskiv O, Zhang X, Simmerman K, Daly T, He H, Falzarano S, Chen L, Magi-Galluzzi C, Zhou M. The Utility of ERG/P63 Double Immunohistochemical Staining in the Diagnosis of Limited Cancer in Prostate Needle Biopsies. The American Journal of Surgical Pathology. 2011;35(7):1062–1068. doi: 10.1097/PAS.0b013e318215cc03. [DOI] [PubMed] [Google Scholar]
- 34.Rosen P, Sesterhenn IA, Brassell SA, McLeod DG, Srivastava S, Dobi A. Clinical potential of the ERG oncoprotein in prostate cancer. Nat Rev Urol. 2012;9(3):131–137. doi: 10.1038/nrurol.2012.10. [DOI] [PubMed] [Google Scholar]
- 35.Seruga B, Tannock IF. Chemotherapy-Based Treatment for Castration-Resistant Prostate Cancer. Journal of Clinical Oncology. 2011;29(27):3686–3694. doi: 10.1200/JCO.2010.34.3996. [DOI] [PubMed] [Google Scholar]
- 36.Reid AHM, Attard G, Ambroisine L, Fisher G, Kovacs G, Brewer D, Clark J, Flohr P, Edwards S, Berney DM, Foster CS, Fletcher A, Gerald WL, Moller H, Reuter VE, Scardino PT, Cuzick J, de Bono JS, Cooper CS. Molecular characterization of ERG, ETV1 and PTEN gene loci identifies patients at low and high risk of death from prostate cancer. British journal of cancer. 2010;102(4):678–684. doi: 10.1038/sj.bjc.6605554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Small EJ, de Bono JS. Prostate Cancer: Evolution or Revolution? Journal of Clinical Oncology. 2011;29(27):3595–3598. doi: 10.1200/JCO.2011.37.8653. [DOI] [PubMed] [Google Scholar]