Abstract
Objectives
To compare asymmetric dimethylarginine (ADMA) among HIV-infected and uninfected individuals and to evaluate predictors of ADMA in HIV infection.
Background
HIV-infected individuals have high rates of atherosclerosis. Endothelial dysfunction is central to atherogenesis and is one possible mechanism underlying this increased cardiovascular risk. ADMA is an endogenous inhibitor of endothelial nitric oxide synthase. Among uninfected individuals, higher ADMA levels predict cardiovascular events and mortality. The association between HIV infection, HIV-related factors, and ADMA has not been well described.
Methods
We compared ADMA in 248 HIV-infected individuals and 50 uninfected controls. We performed multivariable analysis using traditional cardiovascular and HIV-specific factors as covariates to identify factors associated with ADMA.
Results
HIV-infected men were older, less often Caucasian, more hypertensive, and had lower HDL than uninfected men. The median duration of HIV infection was 13 years, median CD4+ count was 592 cells/μL, 76% had an undetectable viral load, and 76% were on antiretroviral therapy. ADMA levels were modestly higher in HIV-infected individuals than controls [median (IQR): 0.46μM (0.41–0.52) vs. 0.44μM (0.38–0.46), p=0.019], but the association lost statistical significance after controlling for cardiovascular risk factors (+0.028μM, p=0.054). Lower CD4+ count and both detectable and higher viral load were independently associated with increased ADMA.
Conclusions
ADMA levels were modestly elevated in the setting of HIV infection. Notably, a greater HIV-associated inflammatory burden, as evidenced by lower CD4+ counts and higher viral loads, was associated with increased ADMA levels. Our findingssuggest that HIV infection impairs endothelial function and predisposes to atherosclerosis through chronic inflammation and subsequent accumulation of ADMA.
Keywords: HIV, Asymmetric dimethylarginine, Endothelial dysfunction, Nitric oxide
Introduction
Human immunodeficiency virus (HIV)-infected individuals have higher rates of atherosclerosis and cardiovascular disease in comparison to uninfected individuals.[1, 2] Chronic inflammation resulting in endothelial activation and dysfunction has been proposed as a possible mechanism underlying this increased cardiovascular risk.[3] Nitric oxide (NO) is a potent vasodilator and a key mediator of vascular homeostasis. Endothelium-derived NO inhibits leukocyte recruitment, platelet adhesion and aggregation, and smooth muscle cell proliferation. Diminished biological activity of NO is accompanied by other alterations in endothelial phenotype (e.g. expression of inducible endothelium-leukocyte adhesion molecules) that further increase the propensity for vasoconstriction, inflammation, thrombosis, and cellular proliferation in the vascular wall.[4]
Asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of endothelial nitric oxide synthase (eNOS). ADMA accumulates through 1) degradation of nuclear proteins containing methylated arginine residues (primarily heterogeneous nuclear ribonucleoproteins), which also generates symmetric dimethylarginine (SDMA), and 2) impairment of dimethylarginine dimethylaminohydrolase (DDAH), the enzyme responsible for its metabolism. The biological activity of DDAH is reduced by the oxidative stress generated from chronic inflammation. Unlike ADMA, SDMA does not inhibit eNOS and is not degraded by DDAH.[5, 6] Several traditional cardiovascular risk factors that induce oxidative stress through chronic inflammation such as age, cigarette smoking, diabetes, hyperhomocysteinemia, hyperlipidemia, hypertension, and insulin resistance have been implicated with increased ADMA levels.[7–12] Elevated ADMA levels have been shown to independently predict cardiovascular events and mortality in non-HIV infected populations.[13–15]
Recent studies have reported increased ADMA levels in HIV-infected individuals in comparison to uninfected individuals.[16, 17] However, the impact of HIV-associated disease characteristics on ADMA has not been fully described. The purpose of this study was to specifically evaluate the association between ADMA levels and HIV-related characteristics including antiretroviral therapy (ART), CD4+ count, and both detectable and level of HIV viral load. We hypothesized that a larger HIV disease burden as evidenced by a lower CD4+ count and a higher viral load—both of which are known predictors of chronic inflammation—would result in elevated levels of ADMA, and therefore, greater impairment of endothelial function.
Methods
Study Population
HIV-infected individuals were recruited from the UCSF-based SCOPE cohort. SCOPE is an ongoing prospective clinic-based cohort of over 1,500 HIV-infected and uninfected adults at San Francisco General Hospital and the San Francisco Veteran's Affairs Medical Center. The inclusion criterion was documented HIV infection; individuals were not recruited on the basis of cardiovascular risk factors, symptoms, or HIV-related factors. Uninfected participants were recruited through study flyers posted in clinics at the aforementioned hospitals in an effort to establish a comparable control group. They were screened for HIV infection with HIV-1 Rapid Antibody testing and documented as HIV-antibody negative prior to study entry. The UCSF Committee on Human Research approved this study and all study subjects provided written informed consent.
Study Design
All participants completed a comprehensive study intake including assessment of demographic characteristics, traditional cardiovascular risk factors, medication usage, illicit drug use, and HIV-related factors. An extensive chart review was performed to ascertain duration of HIV infection, nadir CD4+ count, and ART history including duration of exposure to protease inhibitors, nucleoside/nucleotide reverse transcriptase inhibitors including abacavir-containing regimens, and nonnucleoside reverse transcriptase inhibitors.
Measurements
Fasting blood was obtained from each individual. Lipid panel and high sensitivity C-reactive protein (hsCRP, Dade Behring, Deerfield, Illinois) were measured at the SFGH clinical laboratory. The nadir CD4+ count was the lowest laboratory value documented prior to study entry. For plasma ADMA and SDMA levels, blood samples were immediately centrifuged at 4°C and subsequentl y stored at −80°C. ADMA and SDMA levels were analyzed using a modified high-performance liquid chromatography protocol (Oxonon BioAnalysis, Emeryville, CA) that has been previously described.[18] The coefficient of variation of plasma ADMA was 4.1%. Additional markers of inflammation (IL-6 and fibrin fragment D-Dimer) and immune activation (T cell activation) were measured in a subset of HIV-infected subjects. Serum IL-6 was measured using an ELISA assay (R&D Systems HS600B, Minneapolis, MN) and D-dimer was measured in EDTA plasma using an immune-turbidimetric assay (Liatest D-DI; Diagnostic Stago, Parsippany, NJ). T cell activation was defined as the percentage of CD4+ and CD8+ T cells expressing CD38 and HLA-DR using flow cytometry.[19]
Statistical Covariates
Candidate covariates of ADMA were comprised of demographic characteristics, traditional cardiovascular risk factors, and HIV-specific risk factors. Cardiovascular risk factors included age, body mass index, cigarette smoking (pack years), hsCRP levels, diabetes mellitus, hyperlipidemia, hypertension, and a history of coronary artery disease. HIV-related factors included current and nadir CD4+ count, HIV viral load, duration of HIV infection, duration of ART, and co-infection with hepatitis C virus (HCV). Multiple imputation with the Markov chain Monte Carlo method was used to impute missing covariates, with 5 imputations yielding approximately 95% relative efficiency. A complete case approach was utilized in a sensitivity analysis and produced similar results (Supplementary Appendix Tables 1S and 2S).
Statistical Analysis
We restricted the comparison of HIV-infected individuals to controls to men only since none of the uninfected participants were women. However, similar to a prior study [16], we compared ADMA among the entire HIV-infected cohort to a reference range based on healthy men and women in order to include our female and transgendered HIV-infected subjects in the primary analysis. We compared demographic and clinical characteristics of HIV-infected and uninfected men using the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables. Smoothing splines were constructed using generalized additive models in order to test the assumption of linearity between continuous candidate covariates and ADMA.
Multivariable linear regression with robust standard errors was used to compare levels of ADMA in HIV-infected and uninfected men. We utilized multivariable models sequentially adjusted for 1) demographics alone, and 2) demographics plus traditional cardiovascular risk factors in order to determine whether HIV infection was independently associated with ADMA. We also conducted an analysis comparing ART-suppressed (on ART with an HIV RNA level < 75 copies/mL) men with uninfected men to ascertain if ADMA levels remained elevated in individuals with well-controlled disease.
Both men and women were included in the analysis of ADMA in HIV-infected individuals alone. Separate models were constructed adjusting for demographic, cardiovascular, and HIV-specific factors. Follow-up ADMA levels were available for 45 HIV-infected participants; these values were incorporated into the analysis using linear mixed models with random intercepts and slopes using a first-order autoregressivecovariance structure. Factors forced into the full model included age, gender, and race. We used stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that were not associated with the outcome. We also used a Bayesian model averaging as an alternative model building approach; predictors with posterior probabilities > 35% were retained in the model. Models constructed using these two approaches were very similar.
Bayesian model averaging was performed using the BMA package for the R statistical computing language (R Development Core Team, Vienna, Austria). All other analyses were conducted using the SAS system, version 9.2 (SAS Institute, Inc., Cary, NC).
Results
ADMA levels were measured in 248 HIV-infected individuals (212 men, 32 women, 4 transgender male to female) and 50 uninfected men (Table 1). HIV-infected men were older, less likely to be Caucasian, more hypertensive, and had lower HDL and higher triglycerides than uninfected men. Among HIV-infected men, the median duration of HIV infection was 13 years (interquartile range [IQR] 5–20), the current CD4+ count was 592 cells/μL (IQR 372–775), 76% were on antiretroviral therapy, and 76% had undetectable HIV RNA levels.
Table 1.
Baseline Characteristics of HIV-Infected and Uninfected Participants
| Parameter | HIV+ Men (N = 212) | Control Men (N = 50) | P-value | All HIV+ participants (N = 248) |
|---|---|---|---|---|
| Age (yr) | 49 (42–55) | 42 (33–55) | 0.0049 | 49 (43–55) |
| Female | 0 | 0 | 32 (13%) | |
| Transgendered | 0 | 0 | 4 (2%) | |
| Race | ||||
| Caucasian | 143 (67%) | 39 (78%) | 0.0047 | 150 (60%) |
| African American | 42 (20%) | 4 (8%) | 68 (27%) | |
| Latino | 16 (8%) | 0 | 16 (6%) | |
| Other | 11 (5%) | 7 (14%) | 14 (6%) | |
| History of CAD | 10 (5%) | 0 | 0.22 | 14 (6%) |
| Cigarette smoking | ||||
| Current | 54 (25%) | 13 (27%) | 0.99 | 71 (29%) |
| Past | 66 (31%) | 15 (31%) | 78 (31%) | |
| Never | 92 (43%) | 21 (43%) | 99 (40%) | |
| Diabetes mellitus | 19 (9%) | 1 (2%) | 0.14 | 21 (8%) |
| Hypertension | 79 (37%) | 5 (10%) | <.0001 | 93 (38%) |
| Hyperlipidemia | 65 (31%) | 10 (20%) | 0.16 | 68 (27%) |
| LDL (mg/dL) | 102 (83–125) | 116 (91–131) | 0.021 | 102 (85–126) |
| HDL (mg/dL) | 44 (36–56) | 49 (42–58) | 0.0049 | 45 (36–56) |
| Triglyceride (mg/dL) | 110 (77–192) | 83 (60–101) | <.0001 | 110 (78–181) |
| Total Cholesterol (mg/dL) | 178 (149–202) | 182 (159–213) | 0.17 | 178 (150–204) |
| hsCRP (mg/L) | 1.6 (0.8–3.1) | 1.3 (0.5–3.0) | 0.28 | 1.6 (0.8–3.2) |
| Creatinine (mg/dL) | 0.9 (0.8–1.0) | 0.9 (0.8–1.0) | 0.91 | 0.9 (0.8–1.0) |
| eGFR (Cockroft-Gault) | 91 (79–105) | 90 (81–105) | 0.86 | 90 (79–106) |
| Chronic Kidney Disease | 10 (5%) | 2 (4%) | 0.99 | 13 (5%) |
| Body Mass Index (kg/m2) | 26 (23–28) | 25 (23–28) | 0.65 | 26 (23–30) |
| Testosterone (ng/dL) | 589 (387–752) | 529 (420–603) | 0.79 | 576 (352–744) |
| Duration of HIV infection (y) | 13 (5–20) | 13 (5–20) | ||
| HAART use (ever) | 167 (79%) | 192 (77%) | ||
| HAART use | 161 (76%) | 184 (74%) | ||
| HAART duration (yr)* | 6.1 (2.7–9.5) | 5.4 (2.5–9.4) | ||
| NRTI use | 165 (78%) | 190 (77%) | ||
| NRTI duration (yr)* | 6.6 (2.7–10.9) | 6.0 (2.6–10.6) | ||
| NNRTI use | 99 (47%) | 110 (44%) | ||
| NNRTI duration (yr)* | 3.1 (1.8–6.2) | 3.1 (1.9–5.9) | ||
| PI use | 122 (58%) | 139 (56%) | ||
| PI duration (yr)* | 5.2 (2.3–10.2) | 4.8 (2.3–9.8) | ||
| Current CD4+ (cells/μL) | 592 (372–775) | 599 (405–786) | ||
| Nadir CD4+ (cells/μL) | 250 (95–439) | 250 (100–438) | ||
| HIV RNA (Viral Load) | ||||
| <75 (copies/mL) | 161 (76%) | 187 (76%) | ||
| 75–1999 (copies/mL) | 15 (7%) | 23 (9%) | ||
| 2000–9999 (copies/mL) | 10 (5%) | 10 (4%) | ||
| >10000 (copies/mL) | 25 (12%) | 27 (11%) | ||
| HCV† | 28 (13%) | 44 (18%) | ||
Data are presented as median (IQR) or numbers (percent).
Duration of antiretroviral use among ever users.
One HCV+ control excluded.
Abbreviations: HAART, highly active antiretroviral therapy; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Median (IQR) ADMA levels were modestly higher in HIV-infected men than in uninfected men [0.46μM (0.41–0.52) vs. 0.44μM (0.38–0.46), p=0.019, Table 2, Supplementary Appendix Figure 1S]. ADMA levels were similarly elevated among all HIV-infected individuals in comparison to an established ADMA reference range based on healthy male and female adults (0.48μM ± 0.09 vs. 0.42μM ± 0.06, p<0.0001).[20] SDMA levels were similarly elevated in HIV-infected men compared to controls [0.50μM (0.46–0.58) vs. 0.48μM (0.45–0.52), p=0.025, Table 2]. Among all HIV-infected individuals, the mean ADMA:SDMA ratio (a correlate of DDAH bioactivity) was 0.92. HIV infection remained associated with increased ADMA levels (+0.031μM, p=0.027) after multivariable adjustment for age and race. After further adjustment for traditional cardiovascular risk factors, the association between HIV infection and elevated ADMA levels was no longer statistically significant (+0.028μM, p=0.054). Factors contributing to the attenuation of the HIV effect included low HDL and hypertension. In multivariable analysis of all HIV-infected participants (Table 3), factors independently associated with elevated ADMA levels included female gender (+0.047μM, p=0.0057), cigarette smoking (+0.0072μM, p=0.027), detectable viremia (+0.035μM, p=0.0045), and lower current CD4+ count (−0.014μM per doubling of CD4+, p=0.033). Replacing detectable viremia with the level of HIV viral load in the model yielded similar results (+0.018μM per 10-fold increase, p=0.0006).
Table 2.
Association Between HIV Infection and Levels of ADMA and SDMA
| Parameter | HIV+ Men (N = 212) | Control Men (N = 50) |
|---|---|---|
| ADMA (μM) | ||
| Median (IQR) | 0.46 (0.41–0.52) | 0.44 (0.38–0.46) |
| Mean ± SD | 0.47 ± 0.09 | 0.44 ± 0.07 |
| Range | 0.27–0.74 | 0.29–0.62 |
| Estimate for HIV-lnfected Men vs. Control Men (95% CI) | ||
| Unadjusted | 0.032 (0.0053, 0.059), p=0.019 | |
| Adjusted for demographics* | 0.031 (0.0035, 0.059), p=0.027 | |
| Fully adjusted† | 0.028 (−0.00047, 0.057), p=0.054 | |
| SDMA (μM) | ||
| Median (IQR) | 0.50 (0.46–0.58) | 0.48 (0.45–0.52) |
| Mean ± SD | 0.52 ± 0.09 | 0.49 ± 0.08 |
| Range | 0.32–0.76 | 0.34–0.71 |
| Estimate for HIV-lnfected Men vs. Control Men (95% CI) | ||
| Unadjusted | 0.030 (0.0038, 0.057), p=0.025 | |
| Adjusted for demographics* | 0.026 (−0.0016, 0.053), p=0.065 | |
| Fully adjusted† | 0.023 (−0.0047, 0.051), p=0.10 | |
Adjusted for age and race.
Adjusted for age, race, smoking status, diabetes, blood pressure, total cholesterol, HDL cholesterol, and hsCRP.
Abbreviations: ADMA, asymmetric dimethylarginine; CI, confidence interval; IQR, interquartile range; SD, standard deviation; SDMA, symmetric dimethylarginine.
Table 3.
Factors Associated with ADMA in HIV Infection
| Parameter | Unadjusted Estimate (95% CI), p-value | Adjusted Estimate (95% CI), p-value |
|---|---|---|
| Age (per decade) | −0.00098 (−0.013, 0.011), p=0.87 | −0.0019 (−0.014, 0.010), p=0.75 |
| Female vs. Male | 0.041 (0.0095, 0.073), p=0.011 | 0.047 (0.014, 0.081), p=0.0057 |
| African-American vs. Caucasian | 0.0042 (−0.020, 0.029), p=0.74 | −0.018 (−0.044, 0.0079), p=0.17 |
| Other/Latino vs. Caucasian | −0.011 (−0.044, 0.022), p=0.52 | −0.011 (−0.043, 0.022), p=0.52 |
| Pack Years (per 10 yr increase) | 0.0071 (0.00077, 0.014), p=0.028 | 0.0072 (0.00083, 0.014), p=0.027 |
| VL > 75 vs. VL ≤ 75 (copies/mL) | 0.037 (0.014, 0.061), p=0.0017 | 0.035 (0.011, 0.058), p=0.0045 |
| Current CD4+ (cells/μL per doubling) | −0.015 (−0.028, −0.0020), p=0.024 | −0.014 (−0.027, −0.0011), p=0.033 |
Analysis includes all 248 HIV-infected participants
Abbreviations: ADMA, asymmetric dimethylarginine; CI, confidence interval; VL, viral load.
After restricting the analysis to ART-suppressed HIV-infected men, HIV infection remained associated with higher ADMA levels in comparison to uninfected men (+0.029μM, p=0.038, Table 4). SDMA levels were similarly elevated in ART-suppressed men compared to controls (+0.029μM, p=0.035, Table 4). However, the association between ART-suppressed HIV infection and increased ADMA levels similarly weakened after controlling for demographic factors (+0.029μM, p=0.048) and lost statistical significance in the fully adjusted analysis (+0.025μM, p=0.11). Separate multivariable analysis demonstrated that the association between a lower CD4+ count and higher ADMA levels persisted in the ART-suppressed cohort (−0.017μM per doubling of CD4+, p=0.035, Supplementary Appendix Table 3S).
Table 4.
Association Between Treated, Virally Suppressed HIV Infection and Levels of ADMA and SDMA
| Parameter | Treated, Virally Suppressed Men (N = 148) | Uninfected Controls (N = 50) |
|---|---|---|
| ADMA (μM) | ||
| Median (IQR) | 0.46 (0.41–0.52) | 0.44 (0.38–0.46) |
| Mean ± SD | 0.47 ± 0.09 | 0.44 ± 0.07 |
| Range | 0.27–0.74 | 0.29–0.62 |
| Estimate for Well-Controlled HIV-lnfected Men* vs. Control Men (95% CI) | ||
| Unadjusted | 0.029 (0.0016, 0.057), p=0.038 | |
| Adjusted for demographics* | 0.029 (0.0002, 0.059), p=0.048 | |
| Fully adjusted† | 0.025 (−0.0054, 0.056), p=0.11 | |
| SDMA (μM) | ||
| Median (IQR) | 0.50 (0.45–0.56) | 0.48 (0.45–0.52) |
| Mean ± SD | 0.52 ± 0.08 | 0.49 ± 0.08 |
| Range | 0.32–0.72 | 0.34–0.71 |
| Estimate for Well-Controlled HIV-lnfected Men* vs. Control Men (95% CI) | ||
| Unadjusted | 0.029 (0.0021, 0.056), p=0.035 | |
| Adjusted for demographics* | 0.026 (−0.0019, 0.054), p=0.068 | |
| Fully adjusted† | 0.022 (−0.0079, 0.051), p=0.15 | |
Adjusted for age and race.
Adjusted for age, race, smoking status, diabetes, blood pressure, total cholesterol, HDL cholesterol, and hsCRP.
Abbreviations: ADMA, asymmetric dimethylarginine; CI, confidence interval; IQR, interquartile range; SD, standard deviation; SDMA, symmetric dimethylarginine.
Among the untreated cohort (including 29% “elite controllers” who are able to maintain undetectable HIV RNA levels without ART), the presence of detectable viremia and the level of HIV RNA were strongly associated with increased ADMA levels (+0.071, p=0.0024, Supplementary Appendix Table 4S and +0.028 per 10-fold increase, p=0.0019, respectively). However, in this setting, CD4+ count was no longer associated with ADMA (+0.0031, p=0.85). Notably, individuals with detectable HIV RNA levels had higher ADMA levels than individuals without detectable viremia irrespective of CD4+ count (Supplementary Appendix Figure 2S). We did not find a significant difference in ADMA levels between HIV elite controllers (n=19) and uninfected controls [median (IQR): 0.42μM (0.39–0.46) vs. 0.44μM (0.38–0.46), p=0.76]. However, elite controllers had significantly higher current CD4+ counts [median (IQR): 748 (570–976) vs. 627 (410–802) cells/μL, p=0.026] and lower ADMA levels compared to ART-suppressed individuals [0.42μM (0.39–0.46) vs. 0.46μM (0.41–0.52), p=0.036].
The relationship between ADMA and hsCRP was not statistically significant (+0.0060 per doubling of hsCRP, p=0.13). Additional markers of inflammation (IL-6 and D-dimer) and immune activation (CD4+CD38+HLA-DR+ and CD8+CD38+HLA-DR+) were only available in a subset of our HIV-infected cohort. The correlations between ADMA and these markers were similarly weak and did not reach statistical significance: IL-6 (n=126, r=0.098, p=0.28), D-dimer (n=190, r=0.024, p=0.74) CD4+CD38+HLA-DR+ (n=79, r=0.14, p=0.21), and CD8+CD38+HLA-DR+ (n=79, r=0.16, p=0.16).
Discussion
Despite recent advances in the knowledge of cardiovascular manifestations of HIV infection, the role of ADMA in atherogenesis and, in particular, its relationship to HIV-specific factors is not well understood. ADMA has previously been reported to be independently elevated in HIV infection.[17]We observed a similar but only modest increase in ADMA among HIV-infected men compared to uninfected men. However, we expand upon these findings by demonstrating that ADMA levels remain high even in ART-suppressed HIV-infected individuals, and that HIV disease characteristics, namely, lower CD4+ count and both detectable and higher HIV RNA levels, are independently associated with increased ADMA levels. These findings suggest that a larger HIV-associated inflammatory burden as evidenced by a lower CD4+ count and a higher viral load results in a greater degree of endothelial dysfunction and increased atherosclerotic potential through an accumulation of ADMA.
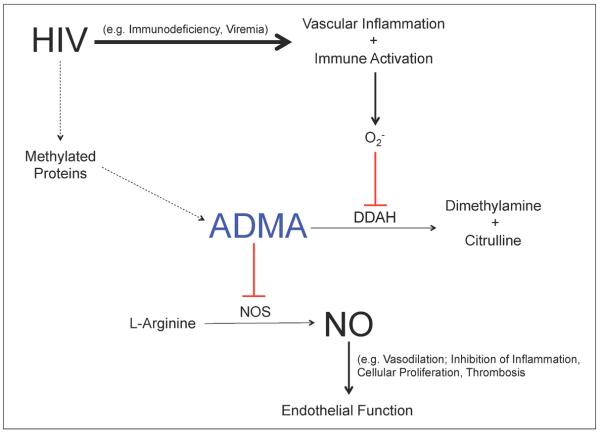
Elevated levels of ADMA among HIV-infected individuals most likely occur via two mechanisms: 1) chronic inflammation of the vascular endothelium, which generates oxidative stress and ultimately inhibits DDAH, and 2) proteolysis of methylated proteins, which has previously been linked to maximal HIV infectivity (Figure 1).[21] HIV infection causes inflammation through several mechanisms including viral replication, immunoregulatory dysfunction, diminished T cell regenerative potential, concurrent copathogen infections, and gut microbial translocation.[3] The ratio of ADMA to SDMA in healthy individuals is approximately 0.80–0.90.[16, 18] Among HIV-infected men in our study, the ADMA:SDMA ratio was slightly higher, namely 0.92. A recent study reported an ADMA:SDMA ratio of 0.98 among an HIV-infected cohort with advanced disease (median CD4+ count 185 cells/μl as compared to the median CD4+ count of 599 cells/μl in our study).[16] The disproportionate increase in ADMA compared to SDMA as evidenced by these ratios supports impaired DDAH bioactivity via chronic inflammation as the underlying cause of ADMA accumulation in HIV infection since only ADMA, and not SDMA, is metabolized by DDAH. Furthermore, we detected significant associations between elevated levels of ADMA and HIV-specific markers of chronic inflammation—immunoregulatory dysfunction (i.e. lower CD4+ count) and viral replication (i.e. detectable and higher viral load). Taken together, these findings support our hypothesis that HIV-induced inflammation leads to an accumulation of ADMA. However, similar to prior studies, we did not observe a significant relationship between ADMA and general inflammatory markers [16, 17]. In contrast, a more recent study in untreated HIV-infected patients reported an association between ADMA and both IL-6 and D-dimer but not hsCRP.[22] Our study included mostly ART-suppressed individuals whereas the study by Baker et al. (2012) consisted of all untreated individuals at baseline; this key difference in patient populations and thus HIV-associated inflammatory burden most likely underlies the discrepancies between the two studies.[22] Additionally, we only had D-dimer and IL-6 data available in a small subset of participants, which limited the power of our statistical analyses.(
Figure 1. Mechanism of ADMA Accumulation in HIV Infection.
Solid line = primary pathway; Dashed line = secondary pathway; Red = inhibits. Abbreviations: O2 −, oxidative stress; DDAH, dimethylarginine dimethylaminohydrolase; ADMA, asymmetric dimethylarginine; NOS, nitric oxide synthase; NO, nitric oxide.
A growing number of studies demonstrate that inflammatory, immunologic, and coagulation markers remain elevated among ART-suppressed HIV-infected individuals as compared to uninfected controls. A recent study found that hsCRP, IL-6, D-dimer, and cystatin C remained elevated even after HIV RNA levels were suppressed with treatment.[23] Similarly, while ART decreased levels of T cell activation as compared to untreated individuals, ART-suppressed individuals still had higher levels of T cell activation when compared to uninfected controls.[24] Interestingly, even among elite controllers, hsCRP levels remain elevated as compared to controls.[25] Many of these same markers have been independently associated with subclinical vascular disease as assessed by carotid intima media thickness[26]. Brachial artery flow-mediated dilation (FMD), a physiologic measure of endothelial function, is reduced in HIV-infected individuals compared to uninfected controls, and improves but remains impaired with ART[27–29]. Furthermore, as HIV disease directly results in CD4+ depletion, the strong association between a lower CD4+ count and cardiovascular events as demonstrated in the HIV Outpatient study[30] and among individuals with acute myocardial infarction[31] mechanistically links HIV infection to cardiovascular disease. The results of our study implicate ADMA as a marker of advanced HIV infection as evidenced by its association with lower CD4+ counts and higher HIV RNA levels. Taken together, these findings suggest that endothelial function may play a central role in HIV-associated atherosclerosis.
Three studies have evaluated the relationship between HIV infection and ADMA. All HIV-infected participants in these studies were either antiretroviral naïve or off ART for at least 6 months.[16, 17, 22] The first study in a more advanced HIV disease population (n=112, median: CD4+ 185 cells/μL, viral load 158,489 copies/mL) reported significantly elevated levels of ADMA in HIV-infected subjects compared to a reference range established from a prior study of healthy volunteers. ADMA was associated with neopterin (macrophage inflammatory marker) but not hsCRP.[16] The second smaller study (n=37, CD4+ and viral load not reported) reported significantly higher ADMA levels among HIV-infected participants than uninfected controls after adjusting for traditional cardiovascular risk factors. ADMA was not associated with hsCRP or fibrinogen.[17]However, neither of these studies identified a significant association between ADMA and CD4+ count, HIV viral load, or gender.[16, 17]The third and most recent study demonstrated that ART lowers ADMA levels in HIV-infected patients, and that immediate as opposed to deferred therapy leads to a greater reduction of ADMA. Baseline levels of IL-6 and D-dimer but not hsCRP, CD4+ count, and viral load were associated with entry ADMA levels; only baseline hsCRP and D-dimer levels predicted a reduction in ADMA following ART.[22]
We also report modestly elevated ADMA levels in HIV-infected mencompared to uninfected controls, but in contrast to a prior study by Jang et al. (2011), this relationship lost statistical significance after fully adjusting for traditional cardiovascular risk factors.[17] This important finding likely reflects 1) substantial differences in baseline cardiovascular risk factors between our HIV-infected and uninfected men (HDL and hypertension contributed most to the attenuation), and 2)reduced ADMA levels among the HIV-infected men due to a high rate of ART, which has recently been reported to lower ADMA levels[22], and a diminished HIV-associated inflammatory burden as evidenced by higher CD4+ counts and lower viral loads, which our present study has shown to correspond with decreased ADMA levels. In contrast to the previously described studies in which all HIV-infected subjects were untreated at baseline with low CD4+ counts and high viral loads (e.g. median CD4+ and viral load of 185 cells/μL and 158,489 copies/mL, respectively[16]), 76% of our HIV-infected male cohortwas on ART at study entry,the median CD4+ count was 592 cells/μL, and 76% had undetectable viremia. Nonetheless, future studies with better-matched HIV-infected individuals and controls are needed to better understand the impact of HIV infection on ADMA.
The association between HIV infection and ADMA remained significant when restricting the analysis to ART-suppressed subjects, though it also lost statistical significance in the adjusted analysis. This finding suggests that even low levels of viremia confer appreciable inflammation andthat although ART lowers ADMA, itdoes not completely reverse HIV-induced endothelial dysfunction, a conclusion that is also supported by other studies of FMD in HIV-infected individuals on ART[27–29].
In contrast to the aforementioned studies, we report independent associations between ADMA and both lower CD4+ count and higher HIV viral load. These inconsistent findings likely reflect the previously described substantial differences in baseline ART among the patient populations. Notably, other studies in HIV-infected individuals on ART have demonstrated an association between viremia and impaired endothelial function as assessed by FMD.[28, 29] In the overall untreated subgroup, which approximates the cohorts in the previously noted studies, higher viral load but not lower CD4+ count was associated with elevated ADMA levels. In contrast, among the ART-suppressed cohort, a lower CD4+ count was associated with increased ADMA levels. Furthermore, in comparison to elite controllers, ART-suppressed individuals had significantly lower CD4+ counts and higher ADMA levels. Collectively, these findings suggest that among untreated individuals, the effect of viremia may outweigh the impact of immunodeficiency on ADMA, whereas among individuals without detectable viremia, immunodeficiency is a key mechanism of endothelial dysfunction. Future studies are needed to better understand these dual mechanisms of inflammation and ADMA accumulation in various subcategories of HIV infection.
Previous studies differ with regards to gender-related differences in ADMA levels.[8, 14] We observed an association between ADMA and female gender in our study. This finding was likely driven by the higher prevalence of smoking and reduced use of ART among female compared with male participants. Although female participants had a higher rate of HCV co-infection compared to male participants (42% vs. 13%), female gender remained significantly associated with higher ADMA levels (+0.048 μM, p=0.0059) even after forcing HCV status into the model in a sensitivity analysis.
Although the mean difference in ADMA levels that we and others report between HIV-infected individuals and uninfected controls (0.03–0.05 μM) is modest, similar changes in magnitude of ADMA in non-HIV populations have been correlated with an increased risk of cardiovascular disease. In a study of nearly 1,000 uninfected patients referred for coronary angiography, similar differences in ADMA levels of 0.02–0.03 μM were associated with greater degrees of coronary occlusion as determined by cardiac catheterization, suggesting that even small elevations in ADMA have clinical significance.[13]
Our study has important limitations. First, our control group did not include any women. However, similar to prior studies, we used an established ADMA reference range to extend the primary analysis to our entire HIV-infected cohort and expand the generalizability of our findings. Second, we did not observe a statistically significant relationship between ADMA and both general inflammatory and immunologic markers. However, we investigated HIV-specific markers of inflammation—CD4+ count and viral load—and found associations between them and higher levels of ADMA, with lower CD4+ count maintaining this association even in ART-suppressed individuals. Furthermore, although we did not measure DDAH levels, the increased ADMA:SDMA ratio we observed among HIV-infected individuals indicates reduced biological activity of DDAH, which is known to be inhibited by inflammation. Taken together, these findings support our hypothesis that HIV-associated inflammation results in elevated levels of ADMA. Lastly, our study lacks direct measures of endothelial function (i.e. FMD) or surrogate markers of atherosclerosis (i.e. coronary artery calcium). Nonetheless, several studies have shown that ADMA is inversely correlated to FMD in healthy adults[32] as well as in populations with cardiovascular risk factors including diabetes mellitus[33] and hyperlipidemia.[10] Additionally, a recent study in HIV-infected individuals showed that elevated ADMA levels were also associated with higher coronary artery calcium scores.[17] Collectively, these studies support our conclusion that an accumulation of ADMA results in impaired endothelial function and increased atherogenesis.
In conclusion, our study expands upon previously published reports of elevated levels of ADMA in HIV infection by demonstrating that a lower CD4+ count and a higher viral load—both of which are established markers of HIV-associated inflammation—are associated with higher ADMA levels. These findings support our hypothesis that HIV infection leads to NO-mediated endothelial dysfunction and subsequent atherosclerosis via chronic inflammation and an accumulation in ADMA. Future studies are needed to determine if ADMA is similarly predictive of coronary atherosclerosis and cardiovascular events/mortality among HIV-infected individuals as it is among uninfected individuals. If so, ADMA may hold promise as both a novel marker and therapeutic target for HIV-associated cardiovascular disease.
Supplementary Material
Highlights
ADMA levels are modestly higher in HIV-infected men than uninfected men.
ADMA remains high even among ART-suppressed individuals
ADMA is independently associated with lower CD4+ count and detectable/higher viral load.
HIV infection causes endothelial dysfunction via chronic inflammation and subsequent accumulation of ADMA.
Acknowledgments
*P.Y.H. has received grant support from GSK.
‡Grant Support:NIH 5R01 HL095130 (PYH), NIH 5R01 HL095126 (PYH), SCOPE
‡The SCOPE cohort was supported by: NIAID (RO1 AI087145, K24AI069994), UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), UCSF Clinical and Translational Research Institute Clinical Research Center (UL1 RR024131), Center for AIDS Prevention Studies (P30 MH62246), and CFAR Network of Integrated Systems (R24 AI067039).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
†This work was presented in part at the American College of Cardiology 61st Annual Scientific Session, March 24–27, 2012, Chicago, IL.
References
- 1.Triant VA, et al. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors Among Patients with HIV Disease. J Clin Endocrinol Metab. 2007 doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsue PY, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109(13):1603–8. doi: 10.1161/01.CIR.0000124480.32233.8A. [DOI] [PubMed] [Google Scholar]
- 3.Deeks SG. HIV Infection, Inflammation, Immunosenescence, and Aging. Annual Review of Medicine. 2011;62(1):141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganz P, Vita JA. Testing Endothelial Vasomotor Function. Circulation. 2003;108(17):2049–2053. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 5.Cooke JP. Asymmetrical Dimethylarginine. Circulation. 2004;109(15):1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 6.Cooke JP, Ghebremariam YT. DDAH Says NO to ADMA. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(7):1462–1464. doi: 10.1161/ATVBAHA.111.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbasi F, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. Am J Cardiol. 2001;88(10):1201–3. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 8.Schwedhelm E, et al. Asymmetric dimethylarginine reference intervals determined with liquid chromatography-tandem mass spectrometry: results from the Framingham offspring cohort. Clin Chem. 2009;55(8):1539–45. doi: 10.1373/clinchem.2009.124263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang WZ, et al. Adverse effects of cigarette smoke on NO bioavailability: role of arginine metabolism and oxidative stress. Hypertension. 2006;48(2):278–85. doi: 10.1161/01.HYP.0000231509.27406.42. [DOI] [PubMed] [Google Scholar]
- 10.Boger RH, et al. Asymmetric Dimethylarginine (ADMA): A Novel Risk Factor for Endothelial Dysfunction : Its Role in Hypercholesterolemia. Circulation. 1998;98(18):1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- 11.Stuhlinger MC, et al. Relationship Between Insulin Resistance and an Endogenous Nitric Oxide Synthase Inhibitor. JAMA: The Journal of the American Medical Association. 2002;287(11):1420–1426. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara N, et al. Study on the Relationship Between Plasma Nitrite and Nitrate Level and Salt Sensitivity in Human Hypertension : Modulation of Nitric Oxide Synthesis by Salt Intake. Circulation. 2000;101(8):856–861. doi: 10.1161/01.cir.101.8.856. [DOI] [PubMed] [Google Scholar]
- 13.Lu TM, et al. Plasma asymmetric dimethylarginine predicts death and major adverse cardiovascular events in individuals referred for coronary angiography. Int J Cardiol. 2011;153(2):135–40. doi: 10.1016/j.ijcard.2011.06.120. [DOI] [PubMed] [Google Scholar]
- 14.Boger RH, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119(12):1592–600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnabel R, et al. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res. 2005;97(5):e53–9. doi: 10.1161/01.RES.0000181286.44222.61. [DOI] [PubMed] [Google Scholar]
- 16.Kurz K, et al. Plasma concentrations of the cardiovascular risk factor asymmetric dimethylarginine (ADMA) are increased in patients with HIV-1 infection and correlate with immune activation markers. Pharmacological Research. 2009;60(6):508–514. doi: 10.1016/j.phrs.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Jang JJ, et al. Asymmetric dimethylarginine and coronary artery calcium scores are increased in patients infected with human immunodeficiency virus. Atherosclerosis. 2011;217(2):514–517. doi: 10.1016/j.atherosclerosis.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Leone A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. The Lancet. 1992;339(8793):572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 19.Hsue PY, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. Aids. 2006;20(18):2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 20.Teerlink T, et al. Determination of Arginine, Asymmetric Dimethylarginine, and Symmetric Dimethylarginine in Human Plasma and Other Biological Samples by High-Performance Liquid Chromatography. Analytical Biochemistry. 2002;303(2):131–137. doi: 10.1006/abio.2001.5575. [DOI] [PubMed] [Google Scholar]
- 21.Willemsen N, et al. Protein methylation is required to maintain optimal HIV-1 infectivity. Retrovirology. 2006;3(1):92. doi: 10.1186/1742-4690-3-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker JV, et al. HIV Replication, Inflammation, and the Effect of Starting Antiretroviral Therapy on Plasma Asymmetric Dimethylarginine, a Novel Marker of Endothelial Dysfunction. J Acquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e318252f99f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhaus J, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 25.Hsue PY, et al. Role of viral replication, antiretroviral therapy, and immunodeficiency in HIV-associated atherosclerosis. Aids. 2009;23(9):1059–67. doi: 10.1097/QAD.0b013e32832b514b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsue PY, et al. Carotid intima-media thickness progression in HIV-infected adults occurs preferentially at the carotid bifurcation and is predicted by inflammation. J Am Heart Assoc. 2012;1:e000422. doi: 10.1161/JAHA.111.000422. doi: 10.1161/JAHA.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsue PY, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. Aids. 2009;23(15):2021–7. doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torriani FJ, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52(7):569–76. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solages A, et al. Endothelial Function in HIV-Infected Persons. Clinical Infectious Diseases. 2006;42(9):1325–1332. doi: 10.1086/503261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein KA, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51(4):435–47. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 31.Triant VA, et al. Association of Immunologic and Virologic Factors With Myocardial Infarction Rates in a US Healthcare System. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juonala M, et al. Brachial Artery Flow-Mediated Dilation and Asymmetrical Dimethylarginine in the Cardiovascular Risk in Young Finns Study. Circulation. 2007;116(12):1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
- 33.Fard A, et al. Acute Elevations of Plasma Asymmetric Dimethylarginine and Impaired Endothelial Function in Response to a High-Fat Meal in Patients With Type 2 Diabetes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20(9):2039–2044. doi: 10.1161/01.atv.20.9.2039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.