Abstract
Purpose
It is well known acuity slowly decreases in later decades of life. We wish to determine the extent 4 year longitudinal acuity changes can be accounted for by changes in optical quality, or combination of optical quality metrics and age between 50 and 80.
Methods
High contrast logMAR acuity, 35 image quality metrics, 4 intraocular scatter metrics, and 4 Lens Opacification Classification System-III metrics and entry age were measured on one eye of each of 148 subjects. Acuity change between baseline and the last visit was regressed against change in each metric for all eyes and a faster changing subset of 50 eyes with a gain or loss of 4 or more letters.
Results
Average change across 148 subjects was a 1.6 ± 4 letter loss (t148 = 4.31, p < 0.001) and loss for the faster changing subset was 3.4 ± 6.1 letters (t50 = 2.73, p = 0.008). The multiple-regression model for faster changing eyes included change in point spread function entropy, posterior subcapsular cataract, and trefoil and baseline age (sequential r2-adj values 0.19, 0.27, 0.32, 0.34 respectively p = 1.48×10−4 for the full 4 factor model). The same variables entered the multiple-regression model for the full 148 data set where the majority of the acuity measurements were within test re-test error and accounted for less of the variance (r2-adj = 0.15, p = 2.37×10−5).
Conclusions
Despite being near noise levels for the measurement of acuity, change in optical quality metrics were the most important factors in eyes that lost or gained 4 or more letters of acuity. These findings should be generalizable given our four year acuity change is essentially identical to other studies, and indicate these optical quality markers can be used to help identify those on a faster track to an acuity change.
Keywords: cataract, retinal image quality, vision, aging, longitudinal study
The need for optical quality metrics that are correlated with visual performance (e.g., visual acuity) continues to grow in the face of existing and developing methods of applying wavefront error (WFE) correction, such as refractive surgery, custom contact and intra-ocular lenses, adaptive optics correction, and the general need to better understand how age-related optical change impacts visual performance as measured by visual acuity.1,2 For abrupt changes in aberrations, good progress has been made demonstrating that change in acuity is well correlated with change in image quality.3-6 More specifically, research reveals the visual Strehl (the optical Strehl ratio weighted by the neural contrast sensitivity function for interference fringes)5 is a particularly good metric, accounting for about 80% of variance in the resulting induced change in high contrast logMAR acuity.4,7 Despite these gains, it is not known if the naturally occurring change in optical quality over years8-14 within an individual is as well correlated with change in acuity and the extent such measures can help identify individuals on a fast track to an acuity loss.
Several factors and conditions are expected to impact the correlation between longitudinal change in optical quality and longitudinal change in acuity, including: 1) adaptation15-18 to one’s slowly changing retinal image quality; 2) the repeatability of both wavefront error19-21 and acuity measurements22 is not perfect; and 3) changes in acuity may be related to other factors, such as neurological changes (e.g., neural cell loss or functional decline not measured by optical quality metrics calculated from WFE alone), that may subsequently be accounted for when age is included as a metric.23-26
Several studies, including the Beaver Dam,27 and Blue Mountain Eye Studies28 and the Smith-Kettlewell Eye Research Institute29 study, have reported on the longitudinal change in acuity and its relationship to age. However, age is a catch-all factor that does not identify the cause of acuity loss. As optical quality factors are expected to be related to visual performance and on average get worse with age,8-14 we seek here to determine to what extent change in acuity over time can be accounted for by change in any given optical quality metric or a combination of optical quality metrics and age for a population between 50 and 80 years of age over a 4 year time period.
METHODS
Given that it is expected to take about 6-10 years for a sample population between 50 and 80 years of age to lose on average 3 letters of acuity,30-35 a large number of subjects were enrolled in order to detect the anticipated average loss of one to two letters of acuity assuming a standard deviation of test re-test of 2-3 letters within the 4 year study period. Given the anticipated small change, we analyzed the data for the cohort as a whole and for a subset of the cohort who had a change in acuity of at least 4 letters, exceeding the 2-3 letter standard deviation for the test re-test measurement of acuity.22
Subjects
One hundred and sixty subjects (93 female, 67 male) between 50 and 80 years of age were recruited for a 4 year, 5 visit Longitudinal Early Nuclear Cataract Study (LENCS). Subjects were included in the study if they had best corrected acuity of 20/30 or better, Lens Opacities Classification Systems-III36 grades for cortical cataracts < 2, no or trace amounts of posterior subcapsular cataract, or mild nuclear cataract consistent with age. Subjects were excluded if they had any other ocular or systemic disease that would interfere with visual function. Of the 160 subjects, 2 dropped out for personal reasons unrelated to the study, 4 had cataract surgery on the study eye, and 2 passed away during the course of the study. At the end of 4 years 152 subjects had completed all 5 visits and 4 did not dilate to the 6 mm over which wavefront error and acuity were measured, leaving 148 LENCS subjects meeting all analysis criteria. One eye of each subject was used, where the right or left eye was randomly chosen except when poorer visual function in one eye was the deciding factor, in which case the better eye was the study eye.
Protection of Human Subjects
The study followed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Houston. An informed consent was signed by each subject.
Eye Dilation
Tropicamide 1% and phenylephrine 2.5% ophthalmic solutions were used for pupil dilation and to minimize any residual accommodation.
Wavefront Sensing
Wavefront error (WFE) was measured 3 times within 1-2 minutes and the average of the three measurements was used as the best estimate of the eye’s WFE. All measurements were obtained using a custom Shack-Hartmann wavefront sensor, the design of which has previously been described in detail37 and quantified using the Z80.28 – 2004 ANSI standard for specifying the normalized Zernike expansion through the 10th radial order.38
Optical Quality Metrics Calculated from WFE
The 4 year change in 31 optical quality metrics was calculated from WFE, each of which has been described in detail previously.5 Of these 31 metrics, 10 correspond to wavefront quality metrics measured in the pupil plane, and 21 correspond to image quality metrics measured in the retinal plane. In addition, high order root mean square wavefront error (RMS-HOA; calculated from the Zernike coefficients as the square root of the sum of the squares for coefficients in the 3rd through 10th radial orders), coma (calculated from the Zernike coefficients as ), spherical aberration (calculated from the Zernike coefficients as ), and trefoil (calculated from the Zernike coefficients as ) were included among optical quality metrics because they have been described to change with age and/or cataract development.8,39-42 In all, 35 optical quality metrics based on WFE were calculated.
Lens Opacification Classification System III (LOCS-III) Measurements
We used in our analyses the 4 year change in the Lens Opacification Classification Systems-III metrics 36 for nuclear opalescence (NO), nuclear color (NC), cortical cataract (C), and posterior subcapsular cataract (P). For all 5 visits the same four graders scored each LOCS-III metric. The average score of the graders for each metric defined the final annual score for each metric.
Forward Scatter Metrics
The 4 year change in 4 forward scatter metrics (Max_SD, Max_Max, Max_Mean, and Mean_Mean) were used in our analyses. The 4 measures of forward scatter were extracted from the spot pattern of the Shack-Hartmann wavefront measurement, and have been described previously.43 In short, metrics of forward scatter are derived by analyzing the individual lenslet point spread functions (PSF) within the Shack-Hartmann multiple spot wavefront sensing image. More specifically, Max_SD is the maximum standard deviation of pixel values of all PSFs in the Shack-Hartmann spot image, Max_Max is the maximum pixel value in the spot image, Max_Mean is the maximum mean pixel value of all PSFs in the spot image, and Mean_Mean is the mean of mean pixel values of all PSFs in the spot image.
Measurement of Visual Acuity
Visual Acuity was measured following a trial frame cycloplegic refraction viewing through a 6 mm artificial pupil at 12 feet. The chart was a high contrast (96% Weber), logMAR chart as designed by Bailey-Lovie44, 45 consisting of 5 letters per line and a 100.1 change in letter size between lines. Charts were uniformly illuminated (285 cd/m2 as measured with a Minolta LS-110 luminance meter) in an ETDRS acuity display box. Subjects were instructed to begin reading at the smallest line of letters which they could read in full, and to continue to read until 5 letters were missed. The total number of letters read up to the 5th miss was recorded and converted into logMAR acuity and used to calculate the 4 year change in acuity.
Data Analysis
First, for the 148 eye test population the 95% confidence limit for the random inclination to lose or gain acuity was calculated (i.e. the limits on the binomial probability of either gaining or losing acuity). Second, the 148 eye test population was divided into two sub-groups: eyes that did not have at least a 4 letter change in acuity and those that did. Percent regression to the mean (Prtm, i.e. the tendency for those at the extremes of the baseline distribution to regress toward the mean on subsequent measurement47-49) was calculated for each of these two sub-groups using the following formula:
| Eq.1 |
Where r is the correlation between baseline and four year HCA for the two subgroups. As with the full population the 95% confidence limit for the random inclination to lose or gain acuity was calculated for the two subgroups.
Third, for both the full complement of eyes and the faster changing subset four year change in logMAR high contrast acuity (ΔHCA) was regressed against change in each optical quality metric calculated from WFE, change in the LOCS-III metrics, change in the scatter metrics and baseline age. For each regression, the coefficient of determination (r2) was calculated and used to rank order all metrics as well as age to determine the individual regression variables that accounted for the most variability in ΔHCA. Finally, for both the full set and subset of eyes the best variables were included in a stepwise regression analysis (alpha to enter/exit = 0.15; Minitab 15, Minitab Inc., Pennsylvania) to determine the combination of variables that accounted for the most variability in ΔHCA over 4 years using a Bonferroni correction for large number of comparisons within multiple regression.
RESULTS
After 4 years, and of the 148 eyes of 148 subjects, 48 gained at least 1 letter of acuity, 89 lost at least 1 letter of acuity, and 11 had no change from base line. The 95% confidence limits for the random inclination to lose or gain acuity were 74±12. The average 4 year change in acuity for all eyes was a loss of 1.6 letters, standard deviation ± 4.1 letters (t148 = 4.31, p < 0.001). The greater number of eyes losing acuity, and the average loss of acuity are both consistent with the expected tendency for a population in this age group to be slowly losing acuity, as expected from prior literature.30-35
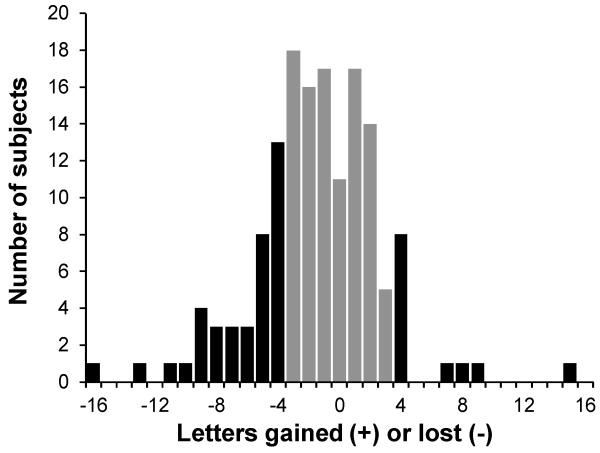
Within the 148 eye sample population there were 50 eyes that gained or lost at least 4 letters of acuity (a 4 letter loss is about 1.5 times the reported standard deviation of repeated acuity measurement22). The average 4 year change in acuity in this faster changing subset was a loss of 3.4 letters, standard deviation ± 6.1 letters (t50 = 2.73, p = 0.008). Of these eyes, 38 lost and 12 gained at least 4 letters of acuity. The 95% confidence limit for the random inclination to lose or gain acuity was 25±6.9. When evaluating smaller subsets within a larger sample it is necessary to take into account regression to the mean.46-48 The percent regression to the mean for those losing and gaining at least 4 letters was 17% and 16%, respectively. Figure 1 displays the number of subjects gaining or losing acuity as a function of the magnitude of the gain or loss for the entire group as well as the subset that gained or lost at least 4 letters of acuity.
Figure 1.
Number of eyes that gained or lost 4 or more letters of acuity (n = 50, black bars) or less than 4 letters of acuity (n = 98, grey bars). Of those eyes gaining or losing 4 or more letters of acuity, 38 eyes lost acuity and 12 eyes gained acuity. Of those eyes that gained or lost 3 letters or less 11 eyes had no change, 51 lost and 36 gained letters of acuity. The 95% confidence limits for the random inclination for loss or gain in the two subsets are 25±6.9 and 49±9.7, respectively. Positive kurtosis but not skew was significant for the full dataset, failing Kolmogorov-Smirnov test for normalcy (KS = 0.095, p < 0.01).
For both the full set and faster changing subset of eyes the 4 year change in acuity was regressed against the 4 year change for all 35 metrics of retinal image quality calculated from WFE, the 4 LOCS-III lens opacification metrics, the 4 scatter metrics and baseline age. In both cases the top individual regression variables included 7 image quality metrics calculated from WFE, 3 LOCS-III lens quality metrics, 2 forward scatter metrics, and patient age. These variables are listed separately for the full set and faster changing subset in the top and bottom halves of Table 1 in rank order based on the coefficient of determination, r2, of the regression against change in visual acuity.
Table 1.
Rank order of top individual regression variables. Age and 4 year change in 12 optical quality metrics incorporated into multiple regression analyses with ΔHCA as the response variable for all eyes (top half) and the faster changing subset (bottom half). Predictor variables are in descending order relative to their coefficients of determination, r2. Variables marked with (*) are metrics of image quality, with (**) are measures of high-order aberration calculated from the Zernike coefficients, with (#) are LOCS-III cataract grading metrics, and with (##) are forward scatter metrics. Each of the variables is defined in Table A1 of the Appendix, available online at [LWW insert link].
| 148 of 148 eyes | |||
|---|---|---|---|
| Variable | r 2 | Variable | r 2 |
| Age | 0.088 | SM* | 0.033 |
| ENT* | 0.053 | P# | 0.030 |
| HOA** | 0.049 | NO# | 0.028 |
| Trefoil** | 0.048 | C# | 0.021 |
| AreaMTF* | 0.038 | MAX_SD## | 0.016 |
| VSMTF* | 0.034 | MEAN_MEAN## | 0.007 |
| D50* | 0.033 | ||
| 50 of 148 eyes (faster changing subset) | |||
|---|---|---|---|
| Variable | r 2 | Variable | r 2 |
| ENT* | 0.213 | Trefoil** | 0.126 |
| D50* | 0.180 | P# | 0.076 |
| AreaMTF* | 0.180 | MAX SD## | 0.057 |
| HOA** | 0.170 | C# | 0.028 |
| STD* | 0.146 | NO# | 0.022 |
| PatientAge | 0.141 | MEAN_MEAN## | 0.018 |
| SRMTF* | 0.130 | ||
Notice in Table 1 for both the entire cohort of 148 eyes from 148 individuals and for the subset of eyes where acuity changed 4 or more letters that change in image quality metrics are always high in the rank order compared to backscatter as measured by LOCS-III scores or scatter metrics. However, all of the image quality metrics as well as the measures of high-order aberrations are calculated using Zernike coefficients of the measured wavefront error and are therefore likely to be highly correlated. To weed out the variables that best account for change in acuity, a stepwise multiple regression was performed to identify from the metrics listed in Table 1 those that uniquely account for variance in change in acuity.
For both the full set and the faster changing subset of eyes the variables displayed in Table 1 were entered into forward stepwise regression analyses (alpha to enter/exit = 0.15) to determine the combination of variables that are most predictive of ΔHCA over 4 years. In both cases the same four variables best explained the 4 year change in high contrast acuity (ΔHCA) and are described in order of importance according to the following equations: For the entire cohort:
| Eq.2 |
For the fast changing subset:
| Eq.3 |
Where:
ΔHCA’ is the predicted 4 year change in high contrast acuity
Age is age at study entry
ENT is an optical quality metric describing the entropy of the point spread function5, 49
P is a LOCS-III lens quality metric quantifying the amount of posterior subcapsular cataract36
Trefoil is the RMS WFE for the trefoil terms.
As indicated, equation 2 incorporates all subjects tested on the first and last visits (r2-adj = 0.15, p = 2.37×10−5; significant after Bonferroni correction for large number of comparisons within multiple regression), and Equation 3 incorporates only those subjects whose acuity changed by 4 or more letters between the first and last visits (r2-adj = 0.34, p = 1.48×10−4; significant after Bonferroni correction for large number of comparisons within multiple regression). The sequential partial r2-adj values with the addition of each factor to regression equation 2 are 0.07, 0.11, 0.13 and 0.15, and the sequential partial r2-adj values with the addition of each factor to regression equation 3 are 0.19, 0.27, 0.32, and 0.34 respectively. Here, the partial r2 values of the multiple regression analyses, unlike the full correlations, reflect the unique contribution of each factor in the regression. That these four variables are able to enter Equations 2 and 3 suggests ENT, P, trefoil and age contain fundamentally different information from the other variables, the significance of which is addressed in the Discussion. For fast changing eyes, age is least important variable adding 0.02 to the total r2-adj of 0.34. The increase in r2 found for the faster changing subset relative to the full set of eyes is significant50 (2-tailed p-value = 0.02).
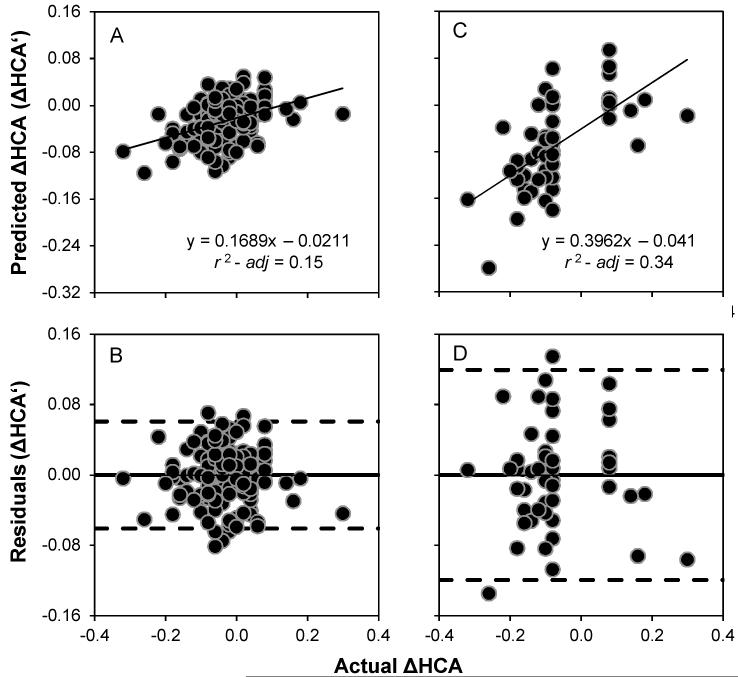
For the full and faster changing sets of eyes Figure 2, panels A and C, linearly regress ΔHCA acuity predicted by Equations 2 and 3 (ΔHCA’) against actual ΔHCA, and panels B and D plot the residuals. Given that there is no systematic trend revealed in the residuals in either case suggests that only noise remains.
Figure 2.
Panel A) Baseline age and change in ENT, PSC, and Trefoil (i.e., ΔHCA’ according to Equation 2) are able to account for ~15% of the variance in actual ΔHCA in the full set of eyes. Panel B) There are no systematic trends in the residuals revealed when the residuals are plotted as a function of actual ΔHCA. Panel C) Baseline age and change in ENT, PSC, and Trefoil (i.e., ΔHCA’ according to Equation 3) are able to account for ~34% of the variance in actual ΔHCA in the faster changing subset of eyes. Panel D) There are no systematic trends in the residuals revealed when the residuals are plotted as a function of actual ΔHCA. The solid lines in panels B and D are the mean residual error (effectively zero), and the dashed lines are ±2 SD. Negative values in all panels refer to loss of logMAR acuity and positive values a gain in logMAR acuity.
The impact of change in posterior subcapsular cataract on change in acuity is emphasized by the fact that the study design attempted to control for posterior subcapsular cataract (P) at enrollment because of its known impact on visual acuity.51-54 Nonetheless, P remained the third most important contributor to change in visual acuity for the study cohort and the second most important variable in the subset that lost 4 or more letters of acuity. Posterior subcapsular cataracts are located close to the visual axis, and are known for their impact on visual performance.51-54 Among all subjects completing the 4 year study gradable P (above the minimum LOCS-III score of 0.1) was found in 26 of 152 subjects on the first visit and 53 of 152 subjects on the final visit, a 104% increase compared to an 18% increase in gradable cortical cataracts (from 121 to 143 subjects).
For the faster changing subset of eyes Equation 3 showed that the same four variables remained in the multiple regression model. Importantly, Equation 3 showed that in this faster changing subset age is now the least important variable remaining in the equation (adding only 2% to the total 34% of variance in acuity change accounted for), whereas ENT is the most important variable and accounts for 19% of the variance in acuity change. That age is a relatively weak predictor of faster changes in acuity is not particularly surprising given individual changes in image quality metrics calculated from wavefront error are more likely to change in 4 years than the large collection of factors captured by age. Taken together these results suggest: 1) image quality factors contributing to 4 year change in this subset of faster changing eyes are the major driving force for the significant correlation found in the full set of eyes; 2) age is a poor predictor of fast change; and 3) an adverse as opposed to positive change in the key optical factors (ENT, P and trefoil) can be used to help identify patients at risk of being on the fast track to acuity loss.
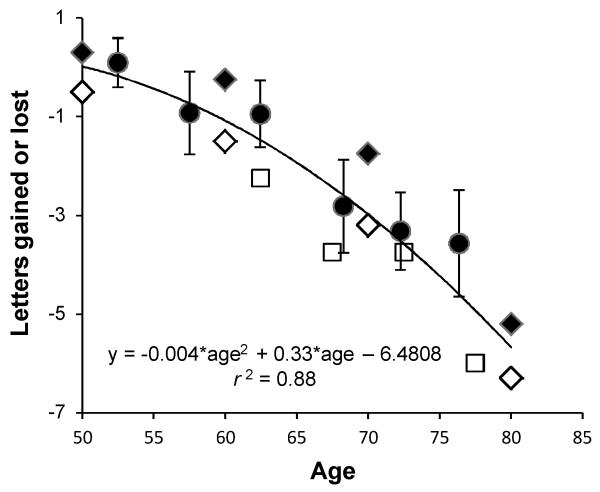
The age related change in acuity is not new, and the results reported here for all eyes (solid circles) are compared in Figure 3 to Beaver Dam27 (solid diamonds), Blue Mountain28 (open diamonds), and Smith-Kettlewell Eye Research Institute (SKI)29 data (open squares). The data of all four studies are similar and reveal an acuity loss with age that is well fit with an accelerating function. Unlike the Beaver Dam, Blue Mountain and SKI studies, the results presented here shed light on the optical factors contributing to loss of acuity with age. Since the loss of acuity as a function of age is essentially identical in all 4 studies the optical factors found here contributing to the acuity change are most likely generalizable to the population as a whole.
Figure 3.
Four year change in high contrast acuity as a function of baseline age for all 152 subjects (solid circles) is reported as letters gained (positive values) or lost (negative values) and compared to 5 year change reported in the Beaver Dam27 (solid diamonds) and Blue Mountain28 (open diamonds) Eye Studies, and an average of 4.4 year change reported in the SKI29 (open square) study. Data points represent the average age per bin (LCS) or the midpoint of the bin range (Blue Mountain, Beaver Dam and SKI). Error bars are one standard error of the mean (LCS). The solid curve is nonlinear least squares fit to all plotted data (i.e., LCS, Beaver Dam, Blue Mountain and SKI), and represents the expected change in acuity over 4 to 5 years as a function of age.
DISCUSSION
For eyes 50 to 80 years of age where acuity has changed at least 4 letters in 4 years optical metrics ENT, P and Trefoil account for 32% of the variance in acuity change with entry age adding an additional 2%. These findings suggest that change in quantifiable optical metrics can help identify suspect individuals on a fast track to an acuity change near the noise limits of the acuity measurement. For the cohort as a whole the average loss in acuity in 4 years was small but significant (1.6 letters) largely driven by the fast changing subset given the average acuity change for eyes whose acuity changed less than 4 letters was a loss of 0.5 letters.
As noted in the introduction, studies examining the impact of sudden changes in image quality on acuity reveal that individual retinal image quality metrics can by themselves account for approximately 80% of the variance in acuity. This raises the question, why does change in retinal image quality metrics account for much less of the variance in a 4 year longitudinal study? There are several factors that contribute. First, the abrupt change studies typically vary image quality over a range large enough to decrease acuity at least 25 letters (5 lines). In the current longitudinal study the logMAR acuity changes are much smaller (less than 1 line) for the majority of patients. If we reanalyze the abrupt change data of the Ravikumar report7 limiting the range to 10 letters (2 lines, 97% of LCS subjects reported here), the percentage of the variance in acuity the retinal image quality metrics account for is on the order of 60%. If we limit the Ravikumar data to 5 letters (1 line, 86% of LCS subjects reported here), the percentage of variance in acuity accounted for by their retinal image quality metrics is on the order of 40%. Here, for those eyes gaining or losing 4 or more letters of acuity, the single most significant optical quality metric (ENT) in the stepwise multiple regression accounts for 19% of the variance alone with trefoil adding another 8%, age accounting for only 2%, and P making up the remaining 5%.
What other factors can account for this discrepancy between studies examining abrupt acuity change and our study? Subjects in the abrupt change studies are asked to read letters distorted by aberrations with which they are not familiar and with essentially no time to adapt, whereas in the longitudinal study subjects are constantly adapting to their slowly changing aberration structure. As described in the introduction, it has been reported that subjects tend to adapt to their own slowly changing aberrations.15-18 For example, Artal et al.15 demonstrated simply rotating an individual’s aberration structure without changing its magnitude decreased visual performance, suggesting that we are each adapted to our own unique aberration structure. Thus any neural adaptation will act to increase the time to detect significant changes in acuity.
Another factor limiting the measurement of acuity change in a longitudinal study is noise. After accounting for baseline age and change in ENT, PSC and trefoil the residual error (Figure 2A) shows no systematic tendencies. That is, the residual error is likely the sum of different sources of nonsystematic measurement noise. Here the average 4 year change in acuity of 1.6 letters is within the noise limits for the measurement of acuity and the average magnitude of change of 3.4 letters for the faster changing group is near the noise limits in measuring acuity reported in the literature.22 Similarly, there is noise in WFE measurements19-21 which, in combination with the noise in acuity measurements, further reduces the ability to predict change when the change is small. Given these two factors (adaptation and measurement noise), we looked also at those variables accounting for 4 year change in acuity among those subjects who lost or gained at least 4 letters of acuity. For this smaller subgroup the same 4 metrics remained in the final regression model accounting for acuity change. However, 34% of the variance in change in acuity is now accounted for and age is the least important factor (only accounting for 2% of the variance in change in acuity), and retinal image quality measures became the most important factors. This makes sense given that the collective factors captured by age are not anticipated to change quickly over a short time span, whereas optical qualities can. Accounting for 34% of the change in acuity near the noise limits of measuring acuity is impressive. Such sensitivity suggests that the correlation will only increase as both the change in acuity and the metrics of retinal image quality increase with age.
CONCLUSIONS
For 50 to 80 year olds that had a change in acuity of 4 or more letters in a 4 year time span, change in entropy of the point spread function (ENT), posterior subcapsular cataract (P), trefoil, and study entry age accounted for 34% of the variance in change in acuity. For the entire cohort of 148 eyes change in the same variables accounted for 15%, with age entering first, and with the optical quality metrics otherwise entering in the same order. The different roles age plays in each analysis makes sense given that, unlike individual optical qualities, factors captured by age are not anticipated to change quickly over short periods of time. Correlations like those found here in fast changing eyes allow the identification of those at risk of being on a fast track to acuity loss. In the data set presented here, the significant correlations were found at or near the test re-test limits of acuity measurement which suggests the correlations will increase as the change increases.
Supplementary Material
ACKNOWLEDGMENTS
Support: NIH/NEI R01 EY08520 (RAA), NIH/NEI R01 EY019105 (RAA), NIH/NEI P30 EY07551 (Core Grant to the College of Optometry), and the Borish Endowment funding for the Chair of Optometry (RAA).
The authors are grateful for the software and mechanical support of Hope Queener and Chris Kuether and for the graciousness and commitment of our study subjects.
APPENDIX
The Appendix (Table A1, a summary of individual regression variables) is available online at [LWW insert link].
Footnotes
Conflicts of interest: RAA has patent interest in retinal image quality metrics through the University of Houston and scatter metrics through the University of Texas Health Science Center San Antonio. No other author has a proprietary interest in any material or method mentioned.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chen L, Singer B, Guirao A, Porter J, Williams DR. Image metrics for predicting subjective image quality. Optom Vis Sci. 2005;82:358–69. doi: 10.1097/01.opx.0000162647.80768.7f. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X, Thibos LN, Bradley A. Estimating visual quality from wavefront aberration measurements. J Refract Surg. 2003;19:S579–84. doi: 10.3928/1081-597X-20030901-14. [DOI] [PubMed] [Google Scholar]
- 3.Cheng X, Bradley A, Thibos LN. Predicting subjective judgment of best focus with objective image quality metrics. J Vis. 2004;4:310–21. doi: 10.1167/4.4.7. [DOI] [PubMed] [Google Scholar]
- 4.Marsack JD, Thibos LN, Applegate RA. Metrics of optical quality derived from wave aberrations predict visual performance. J Vis. 2004;4:322–8. doi: 10.1167/4.4.8. [DOI] [PubMed] [Google Scholar]
- 5.Thibos LN, Hong X, Bradley A, Applegate RA. Accuracy and precision of objective refraction from wavefront aberrations. J Vis. 2004;4:329–51. doi: 10.1167/4.4.9. [DOI] [PubMed] [Google Scholar]
- 6.Williams DR. Subjective image quality metrics from the wave aberration; Paper presented at the 4th International Congress of Wavefront Sensing and Aberration-Free Refractive Correction; San Francisco, California. February 16, 2003; [Accessed April 5, 2013]. PowerPoint presentation available at: http://cfao.ucolick.org/pubs/presentations/eyedesign/07_Metrics_DW.pdf. [Google Scholar]
- 7.Ravikumar A, Applegate RA, Shi Y, Bedell HE. Six just-noticeable differences in retinal image quality in 1 line of visual acuity: toward quantification of happy versus unhappy patients with 20/20 acuity. J Cataract Refract Surg. 2011;37:1523–9. doi: 10.1016/j.jcrs.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Applegate RA, Donnelly WJ, 3rd, Marsack JD, Koenig DE, Pesudovs K. Three-dimensional relationship between high-order root-mean-square wavefront error, pupil diameter, and aging. J Opt Soc Am (A) 2007;24:578–87. doi: 10.1364/josaa.24.000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Artal P, Ferro M, Miranda I, Navarro R. Effects of aging in retinal image quality. J Opt Soc Am (A) 1993;10:1656–62. doi: 10.1364/josaa.10.001656. [DOI] [PubMed] [Google Scholar]
- 10.Brunette I, Bueno JM, Parent M, Hamam H, Simonet P. Monochromatic aberrations as a function of age, from childhood to advanced age. Invest Ophthalmol Vis Sci. 2003;44:5438–46. doi: 10.1167/iovs.02-1042. [DOI] [PubMed] [Google Scholar]
- 11.Calver RI, Cox MJ, Elliott DB. Effect of aging on the monochromatic aberrations of the human eye. J Opt Soc Am (A) 1999;16:2069–78. doi: 10.1364/josaa.16.002069. [DOI] [PubMed] [Google Scholar]
- 12.Guirao A, Gonzalez C, Redondo M, Geraghty E, Norrby S, Artal P. Average optical performance of the human eye as a function of age in a normal population. Invest Ophthalmol Vis Sci. 1999;40:203–13. [PubMed] [Google Scholar]
- 13.Kuroda T, Fujikado T, Ninomiya S, Maeda N, Hirohara Y, Mihashi T. Effect of aging on ocular light scatter and higher order aberrations. J Refract Surg. 2002;18:S598–602. doi: 10.3928/1081-597X-20020901-20. [DOI] [PubMed] [Google Scholar]
- 14.McLellan JS, Marcos S, Burns SA. Age-related changes in monochromatic wave aberrations of the human eye. Invest Ophthalmol Vis Sci. 2001;42:1390–5. [PubMed] [Google Scholar]
- 15.Artal P, Chen L, Fernandez EJ, Singer B, Manzanera S, Williams DR. Neural compensation for the eye’s optical aberrations. J Vis. 2004;4:281–7. doi: 10.1167/4.4.4. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Artal P, Gutierrez D, Williams DR. Neural compensation for the best aberration correction. J Vis. 2007;7(9):1–9. doi: 10.1167/7.10.9. [DOI] [PubMed] [Google Scholar]
- 17.Sawides L, de Gracia P, Dorronsoro C, Webster M, Marcos S. Adapting to blur produced by ocular high-order aberrations. J Vis. 2011;21(7):1–11. doi: 10.1167/11.7.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawides L, de Gracia P, Dorronsoro C, Webster MA, Marcos S. Vision is adapted to the natural level of blur present in the retinal image. PLoS One. 2011;6(11):1–6. doi: 10.1371/journal.pone.0027031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng X, Himebaugh NL, Kollbaum PS, Thibos LN, Bradley A. Test-retest reliability of clinical Shack-Hartmann measurements. Invest Ophthalmol Vis Sci. 2004;45:351–60. doi: 10.1167/iovs.03-0265. [DOI] [PubMed] [Google Scholar]
- 20.Koenig DE, Applegate RA, Marsack JD, Sarver EJ, Nguyen LC. Detecting significant change in wavefront error: how long does it take? Clin Exp Optom. 2009;92:246–52. doi: 10.1111/j.1444-0938.2009.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Applegate RA, Marsack JD, Sarver EJ. Noise in wavefront error measurement from pupil center location uncertainty. J Refract Surg. 2010;26:796–802. doi: 10.3928/1081597X-20100921-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raasch TW, Bailey IL, Bullimore MA. Repeatability of visual acuity measurement. Optom Vis Sci. 1998;75:342–8. doi: 10.1097/00006324-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Werner JS, Peterzell DH, Scheetz AJ. Light, vision, and aging. Optom Vis Sci. 1990;67:214–29. doi: 10.1097/00006324-199003000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Hollyfield JG. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1992;33:1–17. [PubMed] [Google Scholar]
- 25.Spear PD. Neural bases of visual deficits during aging. Vision Res. 1993;33:2589–609. doi: 10.1016/0042-6989(93)90218-l. [DOI] [PubMed] [Google Scholar]
- 26.Knau H, Werner JS. Senescence of the human cone photoreceptor pathways. In: Lakishminarayanan V, editor. OSA Trends in Optics and Photonics, vol. 35: Vision Science and Its Applications. Optical Society of America; Washington, DC: 2000. pp. 382–392. [Google Scholar]
- 27.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103:1169–78. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 28.Foran S, Mitchell P, Wang JJ. Five-year change in visual acuity and incidence of visual impairment: the Blue Mountains Eye Study. Ophthalmology. 2003;110:41–50. doi: 10.1016/s0161-6420(02)01295-2. [DOI] [PubMed] [Google Scholar]
- 29.Schneck ME, Haegerstrom-Portnoy G, Lott LA, Brabyn JA, Gildengorin G. Low contrast vision function predicts subsequent acuity loss in an aged population: the SKI study. Vision Res. 2004;44:2317–25. doi: 10.1016/j.visres.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Weale RA. Senile changes in visual acuity. Trans Ophthalmol Soc U K. 1975;95:36–8. [PubMed] [Google Scholar]
- 31.Pitts DG. The effects of aging on selected visual functions: dark adaptation, visual acuity, steriopsis, and brightness contrast. In: Sekuler R, Kline D, Dismukes K, editors. Aging and Human Visual Function. Vol. 2. Alan R. Liss, Inc; New York: 1982. pp. 131–59. [Google Scholar]
- 32.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98:1310–5. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 33.Elliott DB, Yang KC, Whitaker D. Visual acuity changes throughout adulthood in normal, healthy eyes: seeing beyond 6/6. Optom Vis Sci. 1995;72:186–91. doi: 10.1097/00006324-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103:357–64. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 35.Pesudovs K, Marsack JD, Donnelly WJ, 3rd, Thibos LN, Applegate RA. Measuring visual acuity--mesopic or photopic conditions, and high or low contrast letters? J Refract Surg. 2004;20:S508–14. [PubMed] [Google Scholar]
- 36.Chylack LT, Jr., Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, Friend J, McCarthy D, Wu SY. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 37.Liang J, Grimm B, Goelz S, Bille JF. Objective measurement of wave aberrations of the human eye with the use of a Hartmann-Shack wave-front sensor. J Opt Soc Am (A) 1994;11:1949–57. doi: 10.1364/josaa.11.001949. [DOI] [PubMed] [Google Scholar]
- 38.American National Standards Institute (ANSI) Ophthalmics: Methods for Reporting Optical Aberrations of the Eye. ANSI; Washington, DC: 2004. ANSI Z80, 28-2004. [Google Scholar]
- 39.Wang L, Santaella RM, Booth M, Koch DD. Higher-order aberrations from the internal optics of the eye. J Cataract Refract Surg. 2005;31:1512–9. doi: 10.1016/j.jcrs.2004.01.048. [DOI] [PubMed] [Google Scholar]
- 40.Sachdev N, Ormonde SE, Sherwin T, McGhee CN. Higher-order aberrations of lenticular opacities. J Cataract Refract Surg. 2004;30:1642–8. doi: 10.1016/j.jcrs.2004.02.048. [DOI] [PubMed] [Google Scholar]
- 41.Qu J, Sasaki H, Sakamoto Y, Kawakami Y, Sasaki K, Jonasson F. Higher-order ocular aberrations caused by crystalline lens waterclefts. J Cataract Refract Surg. 2010;36:799–805. doi: 10.1016/j.jcrs.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 42.Rocha KM, Nose W, Bottos K, Bottos J, Morimoto L, Soriano E. Higher-order aberrations of age-related cataract. J Cataract Refract Surg. 2007;33:1442–6. doi: 10.1016/j.jcrs.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly WJ, 3rd, Pesudovs K, Marsack JD, Sarver EJ, Applegate RA. Quantifying scatter in Shack-Hartmann images to evaluate nuclear cataract. J Refract Surg. 2004;20:S515–22. [PubMed] [Google Scholar]
- 44.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. Am J Optom Physiol Opt. 1976;53:740–5. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Lovie-Kitchin JE. Validity and reliability of visual acuity measurements. Ophthalmic Physiol Opt. 1988;8:363–70. doi: 10.1111/j.1475-1313.1988.tb01170.x. [DOI] [PubMed] [Google Scholar]
- 46.Galton F. Regression towards mediocrity in hereditary stature. J Anthropol Inst. 1886;15:246–63. [Google Scholar]
- 47.Bland JM, Altman DG. Some examples of regression towards the mean. BMJ. 1994;309:780. doi: 10.1136/bmj.309.6957.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bland JM, Altman DG. Regression towards the mean. BMJ. 1994;308:1499. doi: 10.1136/bmj.308.6942.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guirao A, Williams DR. A method to predict refractive errors from wave aberration data. Optom Vis Sci. 2003;80:36–42. doi: 10.1097/00006324-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Good PI. Resampling Methods: A Practical Guide to Data Analysis. Birkhäuser; Boston: 2006. [Google Scholar]
- 51.Baraldi P, Enoch JM, Raphael S. Vision through nuclear and posterior subcapsular cataract. Int Ophthalmol. 1986;9:173–8. doi: 10.1007/BF00159846. [DOI] [PubMed] [Google Scholar]
- 52.Lempert P, Hopcroft M, Lempert Y. Evaluation of posterior subcapsular cataracts. With spatial contrast acuity. Ophthalmology. 1987;(Pt 2):14–8. doi: 10.1016/s0161-6420(87)33605-x. [DOI] [PubMed] [Google Scholar]
- 53.Stifter E, Sacu S, Weghaupt H. Functional vision with cataracts of different morphologies: comparative study. J Cataract Refract Surg. 2004;30:1883–91. doi: 10.1016/j.jcrs.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 54.Vasavada AR, Mamidipudi PR, Sharma PS. Morphology of and visual performance with posterior subcapsular cataract. J Cataract Refract Surg. 2004;30:2097–104. doi: 10.1016/j.jcrs.2004.02.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.