Abstract
Regulated actin dynamics play a central role in modulating signaling events at the immunological synapse (IS). Polymerization of actin filaments at the periphery of the IS, coupled to depolymerization near the center, generates a centripetal flow of the actin network and associated movement of signaling molecules. A recent flurry of papers addresses the role of myosin II in facilitating these events. Investigators agree that myosin II is present at the IS, where it forms actomyosin arcs within the peripheral supramolecular activation cluster, a region corresponding to the lamellum of migrating cells. However, there is substantial disagreement about the extent to which myosin II drives IS formation and signaling events leading to T cell activation.
Introduction
Upon contact with an antigen presenting cell (APC), a T cell undergoes a series of rapid cytoskeletal rearrangements that culminate in formation of an immunological synapse (IS) [1,2]. The most notable of these is the initiation of robust actin polymerization at the outermost edges of the contact [3,4]. Given the radial symmetry of the contact, this polymerization, coupled with depolymerization near the center of the interface, creates a dramatic centripetal flow of actin just under the T cell’s plasma membrane in the plane of the IS. Actin flow is widely thought to provide much of the motive force for the centripetal movement of signaling microclusters (MCs) [5–7]. This leads, over a period of several minutes, to the formation of a mature IS containing an outer ring rich in integrins (termed the peripheral supramolecular activation cluster or pSMAC [8]) and an inner region rich in TCRs and associated signaling molecules (the central supramolecular activation cluster or cSMAC [9]).
At the leading edge of other cell types, the pushing force of actin polymerization-driven retrograde flow is coupled with a pulling force generated by myosin II-dependent actin arc contraction [10–13]. These two forces reside in structurally distinct zones, with actin polymerization in the outer lamellipodium (LP) and actomyosin II arc contraction behind the LP, in the lamellum (LM). The LP is composed largely of a branched actin network created by Arp2/3-dependent nucleation at the plasma membrane:cytoplasm interface [14,15]. The LM, on the other hand, is composed of linear actin arcs or fibers aligned roughly parallel to the leading edge. These are probably created by formin-dependent nucleation and the rearrangement of LP actin [11,16–18]. Dynamic imaging of GFP-actin in Jurkat T cells engaged on planar stimulatory surfaces reveals robust Arp2/3-dependent actin retrograde flow principally in a ring surrounding the pSMAC, termed the distal SMAC (dSMAC) [3,19–22]. Based in part on these observations, Dustin hypothesized that the IS might represent a radially symmetric version of the leading edge of a fibroblast, with the dSMAC corresponding to the LP and the pSMAC corresponding to the LM [23,24] (Figure 1). In comparison with these outer regions of the IS, the cSMAC is F-actin-poor, albeit not devoid of F-actin [25,26]. Importantly, the LM is a zone of actomyosin II arc contraction, raising the possibility that the centripetal transport of TCR and other signaling MCs is driven not only by the pushing force of actin retrograde flow, but also by the pulling force of myosin II-dependent contraction. Here, we briefly review a series of recent studies that provide evidence for and against the existence of this latter mechanism and its contribution to MC transport, IS formation and T cell signaling.
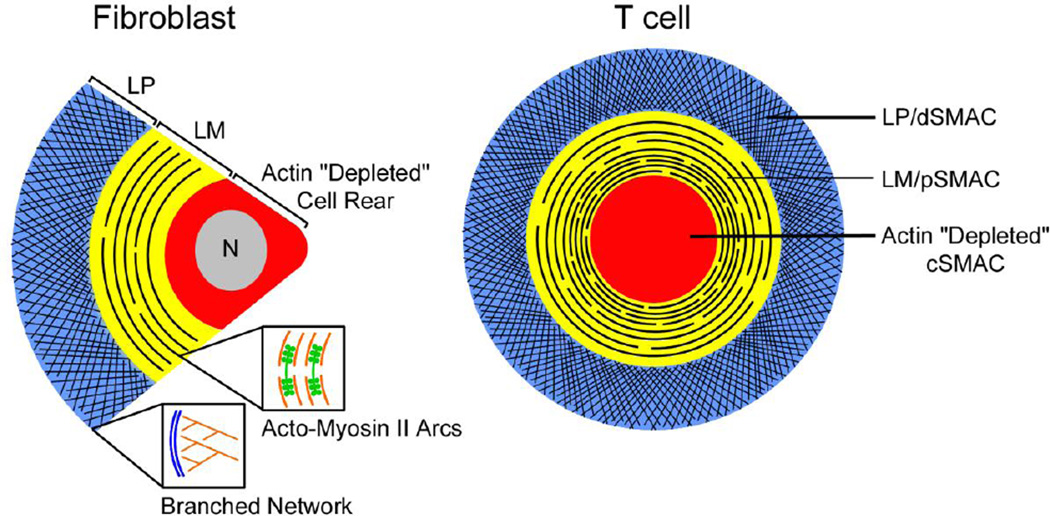
Figure 1. Organization of the actomyosin II network at the IS.
The cartoon highlights the close relationship between the organization of the actin cytoskeleton in migrating mesenchymal cells (right) and in the radially symmetric IS of T cells (left). Specifically, the T cell’s dSMAC and pSMAC correspond to lamellipodial (LP) and lamellar (LM) actin structures seen in mesenchymal cells, respectively. The insets show the distinct organization of F-actin in these two zones, with branched filament arrays dominating the dSMAC/LP and actomyosin II arcs/fibers dominating the pSMAC/LM (red, F-actin; green, myosin II filaments). These latter structures may be difficult to discern using GFP-actin because GFP-actin is a poor substrate for formin-dependent actin nucleation [53], which probably plays an important role in the assembly of LM (i.e. pSMAC) actin [13]. While F-actin reporters like F-Tractin that highlight these actin arcs could in principle augment their formation (although see [27]), the fact that these structures are seen in untransfected, phalloidin-stained cells supports their existence.
Organization of the actomyosin II network
Several labs have reported strong staining for endogenous myosin II1 at the IS usually concentrated along with LM markers like tropomyosin in the pSMAC [27–31]. Moreover, time lapse images of Jurkat T cells expressing fluorescently-tagged myosin II reveal concentric, myosin II-rich “arcs” that first become prominent at the dSMAC/pSMAC boundary and then move inward, eventually dissipating at the pSMAC/cSMAC boundary [27,28]. While previous studies employing GFP-actin did not report corresponding actin arcs in the pSMAC [3,20–22,32,33], these structures are seen in phalloidin-stained Jurkat T cells without myosin II overexpression [27]. Moreover, they become much more obvious in live cells using an indirect reporter for F-actin like F-Tractin [27,28]. Importantly, these actin arcs exactly overlap the GFP-myosin II arcs in the pSMAC [25,26]. Together, these results support the existence of actomyosin II arcs in the pSMAC region of the maturing IS. The presence of these structures within the integrin-rich pSMAC is consistent with the fact that the pSMAC’s counterpart in fibroblasts, the actomyosin II-rich LM, is the site of pronounced, integrin-dependent attachment to the substrate [34–36].
In many mesenchymal cell types, the speed of centripetal actin flow is faster in the LP than in the LM [10,11,37]. Consistent with this, in two studies of Jurkat T cells where flow speed was measured under conditions where cytoskeletal organization in the pSMAC was clear [27,28], the speed of actin retrograde flow in the LP/dSMAC was several fold faster than the speed of inward actomyosin II arc movement in the LM/pSMAC (although the transition in speed was reported to be rather abrupt in one study, and more gradual in the other). Yi et al. simultaneously measured the speeds of TCR MC movements and actin flow in T cells responding to activating planar lipid bilayers and found that the two rates were statistically indistinguishable [27]. In other words, centripetal TCR MC transport was fast in the LP/dSMAC and slow in the LM/pSMAC, and there appeared to be little if any uncoupling of MC movement from actin flow. This observation is at odds with the report of Kaizuka et al. [20] that TCR MCs move inward at ~half the speed of actin, a result that has been cited widely in support of the frictional coupling model of TCR MC/actin cytoskeletal interaction [38–40]. While studies employing physical barriers to TCR MC movement have provided strong support for this frictional coupling model, it may be that under unrestricted conditions the coupling between actin flow and TCR MC movement is fairly tight. Finally, it’s worth noting that not all signaling MCs are coupled to actin flow; MCs containing the adapter protein SLP-76 actually move faster than actin flow in the pSMAC/LM region [28], pointing to the existence of another transport mechanism for some molecules [28,41].
Myosin II function in MC transport and IS formation
While there is consensus that myosin II is enriched in the pSMAC region of the IS, efforts to define its functional significance for MC transport and IS formation have yielded widely disparate results (Table 1). On one end of the spectrum is a study by Ilani et al. [42], who reached the conclusion that myosin II is essential for formation of a mature IS. These authors used blebbistatin (BB) to inhibit myosin II motor activity, as well as inhibitors of myosin regulatory light chain phosphorylation and siRNA-mediated myosin II knockdown to probe myosin II function in bilayer-engaged Jurkat and human primary CD4+ T cells. They presented evidence that myosin II plays major roles in the growth and centripetal transport of TCR MCs, cSMAC formation and maintenance of sustained T cell-APC contacts. Myosin II was also found to be required for key TCR signaling events, including intracellular Ca++ elevation and tyrosine phosphorylation downstream of Lck.
Summary of Findings Regarding Myosin II Function
| Paper | Cells | Stimulation | Myosin II perturbation |
molecules Signaling at the IS |
cSMAC formation | pSMAC formation | TCR MC translocation |
Actin dynamics | Ca++ signaling | Kinase signaling | Additional Relevant Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jacobelli 2004 [31] | D10 (murine CD4+ T cell clone) | Ag pulsed B cells | Blebbistatin | Yes | Yes – fixed at late time point | Yes – fixed at late time point | – | – | – | – | T cell migration is inhibited by BB. TCR signals induce phosphorylation of myosin II HC. |
| Ilani 2009 [42] | Jurkat (human CD4+ T lymphoma) | OKT3/ICAM1 on bilayers | Blebbistatin | Yes | Inhibited | – | inhibited | – | Inhibited | pSrc OK, pLAT and pZAP70 reduced | BB destabilizes T cell-APC contacts. TCR signals induce phosphorylation of myosin II light chain. |
| Human primary CD4+ T cell blasts | OKT3/ICAM1 on bilayers | siRNA | Yes | Inhibited | – | inhibited | – | – | pSrc OK, pLAT and pZAP70 reduced | ||
| Yi 2012 [27] | Jurkat | OKT3/ICAM1 on bilayers | Blebbistatin | Yes | Delayed | Poorly focused | slowed, meandering increased | Slowed, arcs buckle | – | – | Rates of centripetal actin flow and TCR MC transport match in the dSMAC/LP and pSMAC/LM. |
| Yu 2012 [29] | Murine CD4+ AND T cell blasts | pMHC/ICAM1 on bilayers | ML-7 | Yes | Slightly delayed | Unaffected | Eliminates early fast phase; later phase unaffected | Eliminates early fast phase; later phase unaffected | Inhibited | pZAP70 reduced | BB and ML-7 inhibit phosphorylation of the mechano-sensing protein CasL. |
| Blebbistatin | Yes | Slightly delayed | Unaffected | Eliminates early fast phase; later phase unaffected | – | – | pZAP70 reduced | ||||
| Babich 2012 [28] | Jurkat | OKT3 on glass | Y-27632 | Yes | – | – | TCR MCs not measured, but SLP76 MCs unaffected | Unaffected | Unaffected | pZAP70 and pPLCγ1 OK | Actin dynamics are required for sustained signaling through PLCγ1. |
| Blebbistatin | – | – | – | – | Unaffected | – | – | ||||
| siRNA | – | – | – | – | Unaffected | – | – | ||||
| Human primary CD4+ T cell blasts | OKT3 on glass | Y-27632 | – | – | – | – | Unaffected | – | – | ||
| Kumari 2012 [45] | Murine CD4+ AND T cell blasts | pMHC/ICAM1 on bilayers | siRNA | Yes | Inhibited | Poorly focused | TCR slowed slightly, path length unaffected, meandering increased | – | Blunted | pSrc inhibited | Myosin II suppression inhibits TCR-induced phosphorylation of CasL. |
| Beemiller 2012 [44] | Murine CD8+ OTI T cell blasts | pMHC/ICAM1 on bilayers | Blebbistatin | Yes | Unaffected | – | Unaffected (track length, speed, straightness) | – | – | – | The central actin depleted zone and the cSMAC are offset in migrating T cells. |
| Ex vivo gene deletion | Yes | Unaffected | – | Unaffected (track length, speed, straightness) | – | – | – |
On the other end of the spectrum are two papers from the Krummel lab, both of which argue that myosin II plays no measurable role in MC transport or IS formation. Using the D10 murine T cell clone and murine primary T cell blasts, Jacobelli et al. [31] reported that while inhibition of myosin II with BB inhibited cell polarity and motility prior to APC contact (see also [43]), it had no effect on conjugate formation with antigen-specific APCs, or on segregation of TCR and LFA-1 into prototypical cSMAC and pSMAC structures. On the basis of these findings, the authors argued that myosin II activity is dispensable for formation of the mature IS. While this first study addressed IS formation using only end-point assays, Beemiller et al. [44] subsequently reported that the speed, degree of centralization, and directional persistence of TCR MC transport were all normal in bilayer-engaged mouse T cells pretreated with BB and in mouse T cells in which the myosin IIA heavy chain gene was genetically deleted ex vivo (of note, these latter T cells contain ~20–30% of the normal amount of myosin IIA). Based on experiments using low dose Jasplakinolide to inhibit F-actin depolymerization, the authors argued that actin retrograde flow, driven by polymerization at the IS periphery and depolymerization near the center, is what drives inward TCR MC transport and cSMAC formation. Interestingly, this study showed that while the central actin-poor zone created by actin depolymerization directly overlaps the cSMAC in stationary T cells, in migrating T cells the cSMAC trails slightly behind the actin-poor zone, and TCR MCs move toward the actin-poor zone rather than the cSMAC.
Finally, bracketed by these studies are four reports that can be loosely grouped as arguing for a significant albeit non-essential role for myosin II in MC transport and IS formation. We say loosely because the magnitude of myosin II’s contribution, as well as the specific function attributed to it, varies widely within this group of papers. On the “high” end of the spectrum is work by Yi et al. [27]. In addition to showing the existence of actomyosin II arcs within the pSMAC, these authors showed using BB that myosin II contributes significantly to the centripetal flow of actin and TCR MCs in bilayer-engaged Jurkat T cells. Moreover, simultaneous inhibition of both myosin II and actin assembly resulted in a complete cessation of actin flow and centripetal MC transport. Based on these and other observations, Yi and colleagues concluded that the relatively fast pushing force of polymerization-driven actin retrograde flow in the dSMAC and the slower pulling force of actomyosin II arc contraction in the pSMAC cooperate in an interdependent fashion to drive centripetal TCR MC transport at the IS at two distinct rates. While this work indicates that myosin II is not absolutely required for TCR MC transport (MCs still move inward in the presence of BB, albeit slowly and haphazardly due to the disorganization of actin arcs in the pSMAC), it argues that the myosin II contributes significantly to the kinetics of MC transport and SMAC coalescence.
On the “low” end of the spectrum is work by Babich et al. [28]. Like Yi et al., this group found clear evidence for the existence of actomyosin II arcs in the pSMAC and quantitated actin flow rates across the IS. However, using coverslip-engaged Jurkat and human primary T cells, Babich and colleagues concluded that actin polymerization is the primary driver of actin retrograde flow, since flow rate was not affected by various myosin II inhibitors or by suppression of myosin II. While the authors did not measure TCR MC mobility, they showed that inhibition of myosin II contraction using a Rho kinase inhibitor had no effect on the centripetal transport of SLP-76 MCs (although further addition of Jasplakinolide led to a complete cessation of both actin flow and SLP-76 MC transport). This raises the important point that individual MC components may utilize distinct mechanisms for centripetal movement. Although this study points to a minimal role for myosin II function, these authors did present evidence that myosin II contributes to the maintenance of radial symmetry at the IS.
In the third paper in this middle group, Kumari et al. [45] showed that suppressing myosin IIA in bilayer-engaged primary mouse CD4+ T cells had a profound inhibitory effect on the formation of the typical bull’s eye-patterned IS. Somewhat surprisingly, however, this effect was not due to a defect in centripetal TCR MC transport, which slowed only slightly, but rather to enhanced cell spreading (resulting in larger distances to traverse) and reduced directionality of movement (formation of an organized pSMAC was also inhibited, albeit to a lesser extent). Finally, Yu et al. [29] showed that inhibition of myosin II in bilayer-engaged mouse primary T cells completely suppressed a transient (0–2 min) acceleration of in inward actin flow and TCR MC movement that occurred immediately after contact with the activating bilayer. After this early phase, however, myosin II played no obvious role in retrograde actin flow or centripetal TCR MC movement. While myosin II inhibition caused a slight delay in coalescence of the cSMAC, the authors concluded that polymerization-driven actin retrograde flow acting across the entire IS provides the long-lasting driving force for the centripetal transport of TCR MCs to the cSMAC.
We (and we assume the reader as well!) are struck by the wide variation in results regarding the functional significance of myosin II in TCR MC transport and IS formation (Table 1). Some of the discrepancies could be due to significant differences in experimental parameters, which include differences in cell type (Jurkat versus primary mouse T cells), method of engagement (glass, bilayer, conjugate, anti-CD3 versus antigen-specific stimulation), methods of analysis (end-stage assays versus dynamic data), type and degree of myosin II inhibition (small amounts of myosin II in knockdown and knockout cells may be functionally sufficient), off-target effects of chemical inhibitors, and differences in visualization probes (GFP-actin versus F-Tractin).
Fortunately, there are several important points of consensus. First, it is now clear that the dSMAC and pSMAC regions of the IS are analogous to LP and LM regions of other cells, a useful insight since knowledge about other cell types can be brought to bear on T cell biology. Second, researchers agree that myosin II is recruited to the IS, and that it is organized into arcs within the LM/pSMAC region. Finally, there is wide agreement that actin polymerization in the LP region is a major driver of MC assembly and centripetal movement. The debate is really over the degree to which myosin II contractility augments this process, and the role that myosin II plays in TCR signaling (discussed below). The preponderance of evidence indicates that while myosin II is not essential for IS formation, it plays a supporting role. Most studies show that perturbation of myosin II leads to slowed mobility and increased meandering of TCR MCs, and to delayed or defective formation of compact cSMAC and/or pSMAC regions within the IS. The one notable exception to this is the study by Beemiller et al., which showed no effects of BB treatment or partial myosin IIA depletion on TCR MC speed, track length or straightness. Interestingly, this is the only study to use CD8+ T cells, raising the interesting possibility that differences in actomyosin II regulatory mechanisms contribute to the known differences in CD4+ and CD8+ T cell triggering.
Myosin II function in T cell signaling
Importantly, four of the seven papers reviewed in detail above addressed not only the role of myosin II in the dynamic architecture of the IS, but also its role in promoting TCR signaling. Here again, results are divergent. Ilani et al. showed that treatment with BB led to an immediate drop in intracellular Ca++ and reduced tyrosine phosphorylation of LAT and Zap70 downstream of Lck. Yu et al. showed similar effects using ML-7 to inhibit MLCK activity. Using myosin IIA siRNA, Kumari et al. showed inhibition of Src kinase phosphorylation, but reported only blunting of Ca++ mobilization. Finally, Babich et al. showed no effect of a Rho-kinase inhibitor on either Ca++ mobilization or tyrosine phosphorylation, although addition of both Rho kinase inhibitor and Jasplakinolide, which immobilizes the actin cytoskeleton, inhibited Ca++ signaling at the level of PLCγ1 phosphorylation.
What sense can be made of these disparate findings? With the exception of the work by Babich et al, there is agreement that myosin II function is needed for optimal phosphorylation of early signaling intermediates, beginning at least with ZAP-70. Discrepancies about the involvement of Lck may be due to the stimulatory conditions used, since Lck phosphorylation is affected by myosin II perturbation when T cells are stimulated with pMHC [29,45], but not with anti-TCR [42], and pMHC engages CD4, which signals strongly through Lck. In the case of Ca++ mobilization, it is important to consider possible off-target effects of the inhibitors used. Of note, the Ca++ data in the Ilani paper are based on treatment of cells with BB, which has known cytotoxic effects at the wavelengths used for Ca++ measurements [46]. Similar concerns apply for the Yu et al. paper, since ML-7 is structurally similar to ML-9, an inhibitor that was recently shown to inhibit Ca++ influx in a myosin-independent manner [47]. Thus, if we consider only studies where siRNA or other inhibitors are used, it seems that Ca++ responses are either unaffected or blunted. Given that Ca++ signaling depends on tyrosine phosphorylation events, it makes sense that the response should be blunted under conditions where phosphorylation is diminished. Finally, the lack of signaling defects in the Babich et al. paper may be attributable to the use of stimulatory ligands immobilized on glass surfaces vs. the mobile ligands used in all other studies. Evidently, the requirement for myosin II activity is context dependent – an effect that could reflect either differences in tension-based signaling mechanisms or the ability to continuously form new contacts with TCR ligands.
Conclusions and future directions
Recent studies clearly show that actomyosin II arc-like structures exist in the pSMAC, but controversy remains about their functional significance. While it seems reasonable to assume that the disruption of these structures would have some measurable effect on IS formation and T cell signaling, this effect could be relatively small and context dependent. Perhaps the field needs to look for additional readouts. One obvious area would be effects on integrin dynamics and function, given the tight structural and functional association between myosin II and integrin-based adhesions in other cell types [48,49]. Indeed, several groups have already shown a reduction in the phosphorylation of the mechanosensing protein CasL in myosin II-inhibited cells [29,45], consistent with significant myosin II-dependent force generation at the IS. Interestingly, Yi et al. [27] showed that an accumulation of integrin clusters at the inner aspect of the LM/pSMAC depends on actomyosin II contraction. Moreover, loss of myosin II has been linked to defects in de-adhesion from ICAM1 [50], and treatment with BB was recently shown to decrease mechanosensitivity in mouse T cells [51]. Importantly, any myosin II-dependent mechanotransduction in T cells would involve the ability of actomyosin II structures to generate tension through adhesive contacts between T cell and APC. Therefore, future efforts to define the role of myosin II in mechanotransduction will require detailed studies in cell conjugates where, unlike in bilayer-engaged T cells, there is a significant resistance to the movement of integrin clusters in the plane of the membrane. Moreover, the force generated by the physical coupling of myosin II contractile arrays to integrin clusters may be highly variable as the T cell adapts to differences in substrate stiffness, ligand mobility and other parameters. If so, understanding such adaptation may ultimately resolve current controversies regarding the role of myosin II at the IS [52].
Highlights.
F-actin and myosin II form arcs at the immunological synapse.
IS domains correspond to the lamellipodium and lamellum of other cells.
Researchers disagree about the magnitude of myosin II’s role in IS formation and T cell signaling.
The magnitude of myosin II’s contribution may be context dependent.
Acknowledgements
We apologize to colleagues whose works were not cited due to space constraints. We thank Alexander Babich and Jason Yi for thoughtful insights and assistance with preparation of the manuscript. This work was supported by National Institutes of Health Grant R01AI065644 and P01CA093615 to JKB and funding from the Division of Intramural Research, National Heart, Lung and Blood Institute to JAH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Mice and humans possess three type II myosin heavy chains, myosin IIA (MyH9), myosin IIB (MyH10) and myosin IIC (MyH14). Murine primary T cells express only myosin IIA [31], while human Jurkat T cells express both myosin IIA and myosin IIB [28]. Both molecules are inhibited by treatment with blebbistatin [52]. siRNA experiments performed to date target only myosin IIA; myosin IIB function has not be explicitly tested. For simplicity, we will use the general term “myosin II” throughout this review.
References and recommended reading
Papers of particular interest, published within the period of the review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Dustin ML, Chakraborty AK, Shaw AS. Understanding the structure and function of the immunological synapse. Cold Spring Harb Perspect Biol. 2010;2:a002311. doi: 10.1101/cshperspect.a002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 3.Bunnell SC, Kapoor V, Trible RP, Zhang W, Samelson LE. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt JK, Carrizosa E, Shaffer MH. The actin cytoskeleton in T cell activation. Annu Rev Immunol. 2008;26:233–259. doi: 10.1146/annurev.immunol.26.021607.090347. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML, Groves JT. Receptor signaling clusters in the immune synapse. Annu Rev Biophys. 2012;41:543–556. doi: 10.1146/annurev-biophys-042910-155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokosuka T, Saito T. The Immunological Synapse, TCR Microclusters, and T Cell Activation. Curr Top Microbiol Immunol. 2010;340:81–107. doi: 10.1007/978-3-642-03858-7_5. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 8.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 9.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305:1782–1786. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- 11.Yam PT, Wilson CA, Ji L, Hebert B, Barnhart EL, Dye NA, Wiseman PW, Danuser G, Theriot JA. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178:1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson CA, Tsuchida MA, Allen GM, Barnhart EL, Applegate KT, Yam PT, Ji L, Keren K, Danuser G, Theriot JA. Myosin II contributes to cell-scale actin network treadmilling through network disassembly. Nature. 2010;465:373–377. doi: 10.1038/nature08994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tojkander S, Gateva G, Schevzov G, Hotulainen P, Naumanen P, Martin C, Gunning PW, Lappalainen P. A molecular pathway for myosin II recruitment to stress fibers. Curr Biol. 2011;21:539–550. doi: 10.1016/j.cub.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Higgs HN, Pollard TD. Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem. 1999;274:32531–32534. doi: 10.1074/jbc.274.46.32531. [DOI] [PubMed] [Google Scholar]
- 15.Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112:453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 16.Lim JI, Sabouri-Ghomi M, Machacek M, Waterman CM, Danuser G. Protrusion and actin assembly are coupled to the organization of lamellar contractile structures. Exp Cell Res. 2010;316:2027–2041. doi: 10.1016/j.yexcr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson TW, Vaughan AN, Cramer LP. Retrograde flow and myosin II activity within the leading cell edge deliver F-actin to the lamella to seed the formation of graded polarity actomyosin II filament bundles in migrating fibroblasts. Mol Biol Cell. 2008;19:5006–5018. doi: 10.1091/mbc.E08-01-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers--assembly, dynamics and biological roles. J Cell Sci. 2012;125:1855–1864. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 19.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci U S A. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez TS, McCarney SD, Carrizosa E, Labno CM, Comiskey EO, Nolz JC, Zhu P, Freedman BD, Clark MR, Rawlings DJ, et al. HS1 Functions as an Essential Actin- Regulatory Adaptor Protein at the Immune Synapse. Immunity. 2006;24:741–752. doi: 10.1016/j.immuni.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nolz JC, Gomez TS, Zhu P, Li S, Medeiros RB, Shimizu Y, Burkhardt JK, Freedman BD, Billadeau DD. The WAVE2 Complex Regulates Actin Cytoskeletal Reorganization and CRAC-Mediated Calcium Entry during T Cell Activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dustin ML. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Current opinion in cell biology. 2007;19:529–533. doi: 10.1016/j.ceb.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dustin ML. The cellular context of T cell signaling. Immunity. 2009;30:482–492. doi: 10.1016/j.immuni.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS biology. 2011;9:e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown AC, Oddos S, Dobbie IM, Alakoskela JM, Parton RM, Eissmann P, Neil MA, Dunsby C, French PM, Davis I, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS biology. 2011;9:e1001152. doi: 10.1371/journal.pbio.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yi J, Wu XS, Crites T, Hammer JA., 3rd Actin Retrograde Flow and Acto-Myosin II Arc Contraction Drive Receptor Cluster Dynamics at the Immunological Synapse in Jurkat T-Cells. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-08-0731. ** This paper describes actomyosin II arcs in the pSMAC/lamellum, and shows that TCR MC movement and pSMAC formation are slowed, but not abrogated, by myosin II inhibition. Simultaneous inhibition of myosin II and actin polymerization freezes TCR MC transport.
- 28. Babich A, Li S, O'Connor RS, Milone MC, Freedman BD, Burkhardt JK. F-actin polymerization and retrograde flow drive sustained PLCgamma1 signaling during T cell activation. J Cell Biol. 2012;197:775–787. doi: 10.1083/jcb.201201018. ** This paper describes an actomyosin-rich region corresponding to the lamellum. Perturbing myosin II has no effect on centripetal flow of actin or SLP-76-MCs, and arrest of the actin network, but not myosin inhibition, perturbs Ca++ signaling and PLC1 phosphorylation.
- 29. Yu Y, Fay NC, Smoligovets AA, Wu HJ, Groves JT. Myosin IIA Modulates T Cell Receptor Transport and CasL Phosphorylation during Early Immunological Synapse Formation. PLoS One. 2012;7:e30704. doi: 10.1371/journal.pone.0030704. ** This paper is unique in addressing early vs late phases of T cell spreading. During the early phase, but not the late phase, myosin II inhibition affects TCR MC translocation. Signaling studies show that myosin activity is needed for calcium influx and tyrosine phosphorylation.
- 30.Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 31. Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. ** This is the first paper to show myosin II accumulation at the IS. Myosin II is dispensable for formation of a mature IS, although needed for cell motility.
- 32.Barda-Saad M, Braiman A, Titerence R, Bunnell SC, Barr VA, Samelson LE. Dynamic molecular interactions linking the T cell antigen receptor to the actin cytoskeleton. Nat Immunol. 2005;6:80–89. doi: 10.1038/ni1143. [DOI] [PubMed] [Google Scholar]
- 33.Bunnell SC, Hong DI, Kardon JR, Yamazaki T, McGlade CJ, Barr VA, Samelson LE. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J Cell Biol. 2002;158:1263–1275. doi: 10.1083/jcb.200203043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91:3907–3920. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, Sheetz MP. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. doi: 10.1016/s0092-8674(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 36.Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-IIresponsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nature cell biology. 2011;13:383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henson JH, Svitkina TM, Burns AR, Hughes HE, MacPartland KJ, Nazarian R, Borisy GG. Two components of actin-based retrograde flow in sea urchin coelomocytes. Mol Biol Cell. 1999;10:4075–4090. doi: 10.1091/mbc.10.12.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:12729–12734. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–3292. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu CH, Wu HJ, Kaizuka Y, Vale RD, Groves JT. Altered actin centripetal retrograde flow in physically restricted immunological synapses. PLoS One. 2010;5:e11878. doi: 10.1371/journal.pone.0011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto-Tane A, Yokosuka T, Sakata-Sogawa K, Sakuma M, Ishihara C, Tokunaga M, Saito T. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity. 2011;34:919–931. doi: 10.1016/j.immuni.2011.05.012. * This paper shows that microtubule motors may also contribute to TCR MC translocation, particularly within the actin-poor cSMAC region.
- 42. Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–539. doi: 10.1038/ni.1723. ** This paper shows that myosin inhibition or suppression inhibits TCR MC translocation and IS formation, as well as Ca++ signaling and tyrosine phosphorylation downstream of Lck.
- 43.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 44. Beemiller P, Jacobelli J, Krummel MF. Integration of the movement of signaling microclusters with cellular motility in immunological synapses. Nat Immunol. 2012 doi: 10.1038/ni.2364. ** This study is unique in testing myosin II function in CD8+ T cells. Myosin inhibition or ex vivo deletion has no effect on TCR MC translocation.
- 45. Kumari S, Vardhana S, Cammer M, Curado S, Santos L, Sheetz MP, Dustin ML. T Lymphocyte Myosin IIA is Required for Maturation of the Immunological Synapse. Front Immunol. 2012;3:230. doi: 10.3389/fimmu.2012.00230. ** This paper shows that myosin suppression inhibits formation of well focused cSMAC and pSMAC regions, even though it has relatively subtle effects on TCR MC translocation. Ca++ and tyrosine phosphorylation responses are blunted.
- 46.Kolega J. Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem Biophys Res Commun. 2004;320:1020–1025. doi: 10.1016/j.bbrc.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 47.Smyth JT, Dehaven WI, Bird GS, Putney JW., Jr Ca2+-store-dependent and - independent reversal of Stim1 localization and function. J Cell Sci. 2008;121:762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dobereiner HG, Dubin-Thaler BJ, Giannone G, Sheetz MP. Force sensing and generation in cell phases: analyses of complex functions. J Appl Physiol. 2005;98:1542–1546. doi: 10.1152/japplphysiol.01181.2004. [DOI] [PubMed] [Google Scholar]
- 49.Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nature reviews. Molecular cell biology. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morin NA, Oakes PW, Hyun YM, Lee D, Chin YE, King MR, Springer TA, Shimaoka M, Tang JX, Reichner JS, et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J Exp Med. 2008;205:195–205. doi: 10.1084/jem.20071543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Judokusumo E, Tabdanov E, Kumari S, Dustin ML, Kam LC. Mechanosensing in T lymphocyte activation. Biophys J. 2012;102:L5–L7. doi: 10.1016/j.bpj.2011.12.011. * Using stimulatory ligands on polyacrylamide gels, this paper shows that T cells respond differentially to substrate stiffness, supporting the view that mechanotransduction is an important aspect of T cell activation.
- 52.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 53.Chen Q, Nag S, Pollard TD. Formins filter modified actin subunits during processive elongation. Journal of structural biology. 2012;177:32–39. doi: 10.1016/j.jsb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]