Abstract
Purpose
Cancer-related-fatigue (CRF) is common in advanced cancer. The primary objective of the study was to compare the effects of methylphenidate (MP) with those of placebo (PL) on CRF as measured using the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) fatigue subscale. The effect of a combined intervention including MP plus a nursing telephone intervention (NTI) was also assessed.
Patients and Methods
Patients with advanced cancer with a fatigue score of ≥ 4 out of 10 on the Edmonton Symptom Assessment Scale (ESAS) were randomly assigned to one of the following four groups: MP+NTI, PL+NTI, MP + control telephone intervention (CTI), and PL+CTI. Methylphenidate dose was 5 mg every 2 hours as needed up to 20 mg per day. The primary end point was the median difference in FACIT-F fatigue at day 15. Secondary outcomes included anxiety, depression, and sleep.
Results
One hundred forty-one patients were evaluable. Median FACIT-F fatigue scores improved from baseline to day 15 in all groups: MP+NTI (median score, 4.5; P = .005), PL+NTI (median score, 8.0; P < .001), MP+CTI (median score, 7.0; P = .004), and PL+CTI (median score, 5.0; P = .03). However, there were no significant differences in the median improvement in FACIT-F fatigue between the MP and PL groups (5.5 v 6.0, respectively; P = .69) and among all four groups (P = .16). Fatigue (P < .001), nausea (P = .01), depression (P = .02), anxiety (P = .01), drowsiness (P < .001), appetite (P = .009), sleep (P < .001), and feeling of well-being (P < .001), as measured by the ESAS, significantly improved in patients who received NTI. Grade ≥ 3 adverse events did not differ between MP and PL (40 of 93 patients v 29 of 97 patients, respectively; P = .06).
Conclusion
MP and NTI alone or combined were not superior to placebo in improving CRF.
INTRODUCTION
Cancer-related fatigue (CRF) is the most common symptom in advanced cancer.1,2 Methylphenidate (MP) is the most often studied pharmacologic agent for treating CRF.3–13 Previous studies conducted by our team and others have yielded mixed results.4,5,9 A recent systematic review of five studies of psychostimulants (n = 426 patients; four studies using MP9–12 and one study using dexamphetamine13) revealed a standardized mean difference of −0.28 in CRF between the psychostimulant and placebo (PL) groups in most of the studies despite the absence of a significant benefit.5 Hence, further studies are needed to determine the effect of MP on CRF in patients with advanced cancer.
A prior study by our team suggested that patients receiving MP or PL responded favorably to a nursing telephone intervention (NTI) in addition to MP treatment.9 Recent reports suggest that telephone counseling by a nurse can improve fatigue, depression, and quality of life.14–20 In a 6-week telephone intervention study of 48 patients with breast cancer, Badger et al21 found improvement in fatigue and depression. In 109 patients with early cancer, Yates et al22 found that a telephone intervention resulted in improved ability to cope with fatigue over a period of 1 to 2 weeks. Given et al23 found that nursing intervention improved pain, fatigue, and physical and social functioning significantly better than conventional care alone.
The rationale for the current study was based on our preliminary data supporting the use of MP and an NTI for CRF in patients with advanced cancer.8,9 The primary objective of this study was to determine whether MP taken as needed for CRF is superior to PL as measured by the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) fatigue subscale.24 The secondary objective was to investigate the effects of combined interventions including MP plus an NTI for CRF.
We hypothesized that CRF would improve in patients receiving MP compared with patients receiving PL and that patients receiving an NTI would experience lower symptom intensity and higher quality of life than those participating in a control telephone intervention (CTI; nontherapeutic call from a nonprofessional).
PATIENTS AND METHODS
The Institutional Review Board of The University of Texas MD Anderson Cancer Center and the Committee for the University of Texas Health Science Center at Houston approved this protocol, and all participants provided written informed consent.
Patients
Patients were recruited from outpatient palliative care and oncology clinics at MD Anderson Cancer Center or from outpatient clinics at Lyndon B. Johnson General Hospital in Houston, Texas. Inclusion criteria included a diagnosis of advanced cancer; a fatigue score of ≥ 4 on the Edmonton Symptom Assessment Scale25 (ESAS; a 0 to 10 scale); a normal score (≥ 24 of 30) on the Mini-Mental State Examination; hemoglobin level of ≥ 8 g/dL within 2 weeks of enrollment; no history of tachycardia, arrhythmia, uncontrolled hypertension, glaucoma, severe anxiety disorders, major depression, or substance abuse; and no current treatment with monoamine oxidase inhibitors, tricyclic antidepressants, clonidine, warfarin, or erythropoietin. Pregnant and lactating women were excluded from the study.
Intervention
Patients enrolled onto the study were randomly assigned to receive one of the following four treatments: one 5-mg MP capsule administered orally every 2 hours as needed up to 20 mg per day for 14 days and NTI phone calls four to six times over the 2 weeks; one PL capsule administered orally every 2 hours as needed up to four capsules per day for 14 days and NTI phone calls four to six times over the 2 weeks; one 5-mg MP capsule administered orally every 2 hours as needed up to 20 mg per day for 14 days and CTI phone calls four to six times over the 2 weeks; or one PL capsule administered orally every 2 hours as needed up to four capsules per day for 14 days and CTI phone calls four to six times over the 2 weeks.
MP and PL
All patients received a 14-day supply of MP or PL. Aside from those in the Department of Investigational Pharmacy Services, all of the members of the research team were blinded to treatment assignment.
NTI and CTI
All patients randomly assigned to the NTI were contacted by phone by a research nurse with training in palliative care for a total of four to six sessions during the study period. Patients who were randomly assigned to the CTI were telephoned by a person who was not a nurse or other professional four to six times during the study period, and the CTI was controlled for time. The NTI phone calls were standardized to ensure consistency in their content and duration. Briefly, the NTI call started with a 1-minute introduction and a statement of the purpose of the phone call. The introduction was followed by three components. One component was symptom assessment using the MD Anderson Symptom Inventory (MDASI).26 The research nurse addressed the symptoms in the order of their severity as recorded on the MDASI. A second component was a review of the types and dosages of medications and adverse effects. The third component was psychosocial support and patient education. Nurse counseling was conducted according to the Methylphenidate and Nursing Intervention for Cancer-Related Fatigue Study Manual. The manual includes Research Nurse Training Curriculum, Weekly Research Nurse Refresher Curriculum, Information on Methylphenidate, Nursing Telephone Intervention Information, and Symptom Assessment and Management. During each call, the research nurse asked open-ended questions regarding general well-being of the patient and family. The research nurse listened empathetically, answered the patient's questions, provided supportive statements, and then ended the telephone call.
The nontherapeutic calls were conducted by a nonprofessional. She assessed the symptoms using the MDASI and asked about medications. No psychosocial support or education was provided. If patients raised concerns, they were directed to discuss them with their physician. The research nurses conducting the NTI underwent a training program conducted by the principal investigator of the study, a research manager, and a nurse specialist. All calls were transcribed and reviewed by a nurse researcher, who periodically discussed the calls with the nurses and nonprofessionals conducting the calls to ensure intervention fidelity was maintained.
Outcome Measures
Patient demographic data were recorded at the time of random assignment. Patients completed the following assessments at baseline, day 8, and day 15: the FACIT-F fatigue subscale, the ESAS, the Hospital Anxiety Depression Scale (HADS), and the Pittsburg Sleep Quality Index (PSQI).
FACIT-F.
The FACIT-F consists of 27 questions about the patient's general quality of life categorized into four domains (physical, social, emotional, and functional) and a 13-item fatigue subscale.24 Patients rate the intensity of their fatigue and its related symptoms on a scale of 0 to 4 (0 = not at all, 4 = very much). The FACIT-F fatigue subscale is the primary outcome measure because it has been widely used in CRF treatment trials.5,6,17,27 This scale has been shown to have strong internal consistency (α = .93 to .95), a sensitivity of 0.92, and a specificity of 0.69.28
ESAS.
The ESAS was used to assess the following nine symptoms commonly experienced by cancer patients: pain, fatigue, nausea, depression, anxiety, drowsiness, dyspnea, anorexia, and well-being.25 The ESAS is both valid and reliable for assessing the intensity of symptoms of patients with cancer.29,30
HADS.
Depression and anxiety were assessed using the HADS, a 14-item questionnaire that has been validated in a number of clinical situations and is widely used to assess medically ill patients.31
PSQI.
Sleep disorders were evaluated using the PSQI, an effective instrument for determining the quality and patterns of sleep. Numerous studies using the PSQI have confirmed its high level of validity and reliability.32
Statistical Analysis
Our primary objective was to determine whether the improvement in CRF from baseline to day 15 was greater in patients who received MP than in patients who received PL as measured using the FACIT-F fatigue subscale. The primary end point was the difference in the FACIT-F fatigue subscale scores from baseline to day 15. Because the data were non-normally distributed we compared the median differences between main effects of treatment and NTI from baseline to day 15 and from baseline to day 8 using Wilcoxon two-sample tests. We also compared the median differences in the FACIT-F fatigue subscale scores from baseline to day 15 (and from baseline to day 8) among the four groups (MP+NTI, MP+CTI, PL+NTI, and PL+CTI) using Kruskal-Wallis tests. In addition, we analyzed differences over time within subgroups for the FACIT-F fatigue subscale and ESAS fatigue and within NTI groups for all ESAS symptoms. At baseline, we compared subgroups using Kruskal-Wallis tests.
A sample size of 212 was chosen for this study to have adequate power to be able to detect significant differences in change scores as large as or larger than 33% from baseline to day 15 between MP and PL (primary outcome). The proposed sample size was also be able to detect significant differences in change scores ≥ 33% from baseline to day 15 between NTI and CTI and ≥ 50% from baseline to day 15 between any of the four arms of the study. The sample size was calculated based on a previous pilot study8 that showed that the baseline mean value was 17.5 (standard deviation, 11.3) and had improved to 34.7 (standard deviation, 10.0) at day 7.
The median differences in the changes in HADS and PSQI scores from baseline to day 15 were analyzed using the Wilcoxon two-sample test to compare the MP and PL groups and the Kruskal-Wallis test to compare all four treatment groups. The signed ranked test was used to analyze the improvement in HADS scores at day 15 in patients treated with either MP or NTI.
We compared the numbers of patients experiencing grade ≥ 3 adverse events using the χ2 test. Last, we documented the types of adverse events in the MP and PL groups.
All results reported in this study are based on two-sided tests. P ≤ .05 was considered statistically significant. Analyses were performed using SAS software (version 9.2, windows 5.1; SAS Institute, Cary, NC).
RESULTS
One hundred forty-one of 190 patients who enrolled were evaluable (Fig 1). Patient demographics, diagnosis, and baseline scores are listed in Table 1. The median patient age was 58 years, 67% of patients (n = 128) were women, 72% of patients (n = 136) were white, and GI cancers (22%, n = 41) were the most common. Baseline patient characteristics, ESAS fatigue (P = .47), FACIT-F (P = .87), HADS anxiety (P = .67), HADS depression (P = .47), and PSQI scores (P = .78) did not differ among the four groups.
Fig 1.
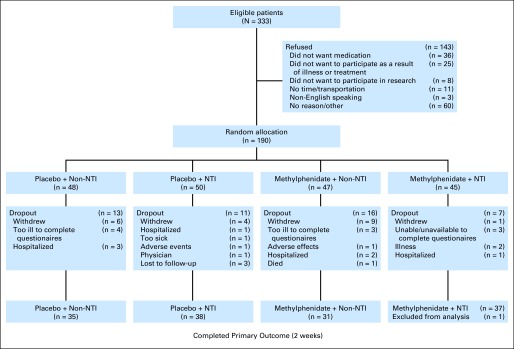
CONSORT diagram. NTI, nursing telephone intervention.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Methylphenidate |
Placebo |
Total (N = 190) | P* | ||
|---|---|---|---|---|---|---|
| NTI (n = 45) | CTI (n = 47) | NTI (n = 50) | CTI (n = 48) | |||
| Age, years | .67 | |||||
| Median | 58.0 | 56.0 | 59.0 | 58.0 | 57.5 | |
| Range | 29-83 | 32-83 | 32-84 | 52-83 | 25-84 | |
| Sex, No. | .86 | |||||
| Male | 16 | 13 | 17 | 16 | 62 | |
| Female | 29 | 34 | 33 | 32 | 128 | |
| Race, No. | .89 | |||||
| White non-Hispanic | 34 | 34 | 36 | 32 | 136 | |
| Hispanic | 8 | 6 | 10 | 9 | 33 | |
| Black non-Hispanic | 3 | 5 | 4 | 7 | 19 | |
| Asian/other | 2 | 2 | ||||
| Diagnosis, No. | .13 | |||||
| GI | 9 | 7 | 15 | 10 | 41 | |
| Lung | 9 | 7 | 9 | 14 | 39 | |
| Breast | 7 | 8 | 12 | 5 | 32 | |
| Genitourinary | 9 | 8 | 12 | 5 | 32 | |
| Melanoma | 5 | 9 | 6 | 5 | 25 | |
| Hematologic | 0 | 2 | 3 | 4 | 9 | |
| Other | 6 | 6 | 1 | 4 | 17 | |
| Baseline FACIT-F fatigue subscale score | .87 | |||||
| Median | 21.50 | 20.00 | 20.00 | 21.50 | 20.00 | |
| IQR | 15.00-27.00 | 15.00-29.00 | 12.00-25.00 | 13.25-27.75 | 14.0-27.00 | |
| Baseline ESAS fatigue score | .56 | |||||
| Median | 7.00 | 6.00 | 6.00 | 6.00 | 6.00 | |
| IQR | 5.00-8.00 | 5.00-7.25 | 5.00-7.25 | 5.00-7.00 | 5.00-8.00 | |
| Baseline HADS depression score | .47 | |||||
| Median | 8.00 | 6.00 | 6.00 | 7.00 | 7.00 | |
| IQR | 4.50-10.00 | 4.00-10.00 | 4.00-9.00 | 4.00-10.00 | 4.00-10.00 | |
| Baseline HADS anxiety score | .67 | |||||
| Median | 6.00 | 7.00 | 6.00 | 7.00 | 7.00 | |
| IQR | 3.00-9.00 | 5.00-9.00 | 2.75-10.25 | 4.00-10.00 | 4.00-9.00 | |
| Baseline ESAS physical distress score | .90 | |||||
| Median | 21.00 | 20.50 | 21.00 | 20.00 | 20.00 | |
| IQR | 15.00-29.00 | 13.25-27.00 | 16.00-26.00 | 15.25-27.00 | 15.00-27.00 | |
| Baseline ESAS psychological distress score | .44 | |||||
| Median | 3.00 | 4.50 | 3.50 | 2.50 | 4.00 | |
| IQR | 0-8.00 | 1.75-8.000 | 0-9.25 | 0-6.00 | 0-8.00 | |
| Baseline ESAS symptom distress score | .50 | |||||
| Median | 25.00 | 25.50 | 25.50 | 23.00 | 25.00 | |
| IQR | 20.50-34.50 | 16.00-32.25 | 17.00-32.25 | 15.25-30.75 | 17.00-32.00 | |
| Baseline FACIT-F score | .79 | |||||
| Median | 88.00 | 87.50 | 94.00 | 92.00 | 91.00 | |
| IQR | 73.00-100.00 | 75.75-108.25 | 76.50-105.00 | 77.00-107.75 | 75.75-105.00 | |
| Baseline PSQI score | .78 | |||||
| Median | 10.00 | 9.00 | 9.00 | 10.00 | 10.00 | |
| IQR | 6.50-14.00 | 6.00-12.00 | 5.00-13.00 | 6.00-13.00 | 6.00-13.00 | |
Abbreviations: CTI, control telephone intervention; ESAS, Edmonton Symptom Assessment Scale; FACIT-F, Functional Assessment of Cancer Therapy–Fatigue; HADS, Hospital Anxiety Depression Scale; IQR, interquartile range; NTI, nursing telephone intervention; PSQI, Pittsburg Sleep Quality Index.
Kruskal-Wallis test was used to compare subgroups (methylphenidate + NTI, methylphenidate + CTI, placebo + NTI, and placebo + CTI).
The median FACIT-F fatigue subscale and ESAS fatigue scores significantly improved between baseline and day 15 in all four groups (Table 2). The difference in the median improvement in FACIT-F fatigue subscale and ESAS fatigue scores from baseline to day 15 between the MP and PL groups was not statistically significant. Likewise, the differences in the median improvement in the FACIT-F fatigue subscale and ESAS fatigue scores between the NTI and CTI groups and among the MP+NTI, MP+CTI, PL+NTI, and PL+CTI groups were not statistically significant (Table 3).
Table 2.
FACIT-F and ESAS Fatigue Scores at Baseline, Day 8. and Day 15 in the Four Subgroups
| Treatment | FACIT-F Fatigue Subscale Scores |
ESAS Fatigue Scores |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Day 8 |
Day 15 |
P* | Baseline |
Day 8 |
Day 15 |
P* | |||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| MP+NTI | 21.5 | 15.0-27.0 | 28.0 | 21.0-35.0 | 25.0 | 21.0-35.5 | .005 | 7.0 | 5.0-8.0 | 5.0 | 3.0-5.75 | 4.0 | 2.0-6.0 | < .001 |
| MP+CTI | 20.0 | 15.0-29.0 | 26.5 | 21.2-37.75 | 27.0 | 19.0-33.0 | .004 | 6.0 | 5.0-8.0 | 5.0 | 4.0-7.0 | 5.0 | 4.0-7.0 | .003 |
| PL+NTI | 20.0 | 12.0-25.0 | 32.0 | 24.0-38.0 | 28.5 | 22.5-33.2 | < .001 | 6.0 | 5.0-7.25 | 4.0 | 3.0-6.0 | 4.0 | 1.5-6.0 | < .001 |
| PL+CTI | 21.5 | 13.25-27.75 | 31.0 | 17.0-36.5 | 28.0 | 13.0-36.0 | .03 | 6.0 | 5.0-7.0 | 5.0 | 3.0-7.5 | 4.0 | 2.0-7.0 | .001 |
Abbreviations: CTI, control telephone intervention; ESAS, Edmonton Symptom Assessment Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; IQR, interquartile range; MP, methylphenidate; NTI, nursing telephone intervention; PL, placebo.
Kruskal-Wallis test was used to analyze the differences in the FACIT-F and ESAS fatigue scores over time.
Table 3.
Change in the Fatigue Scores at Day 8 and Day 15 by Treatment/Intervention
| Treatment/ Intervention | Change in FACIT-F (Fatigue Subscale) Scores |
Change in ESAS Fatigue Scores |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 8-Baseline |
Day 15-Baseline |
Day 8-Baseline |
Day 15-Baseline |
|||||||||||||
| No. of Patients | Median | IQR | P | No. of Patients | Median | IQR | P* | No. of Patients | Median | IQR | P | No. of Patients | Median | IQR | P* | |
| Treatment | .87 | .69 | .98 | .86 | ||||||||||||
| MP | 71 | 6.00 | 0 to 16.00 | 68 | 5.50 | −1.00 to 11.00 | 71 | −2.00 | −3.00 to 0 | 66 | −2.00 | −4.00 to 0 | ||||
| Placebo | 76 | 7.00 | 0.50 to 12.00 | 73 | 6.00 | 2.00 to 11.00 | 76 | −2.00 | −3.00 to 0 | 71 | −2.00 | −5.00 to 0 | ||||
| Intervention | .22 | .27 | < .01 | .14 | ||||||||||||
| NTI | 78 | 6.50 | 1.00 to 15.58 | 75 | 6.00 | 0 to 14.00 | 79 | −2.00 | −4.00 to −1.00 | 74 | −2.50 | −5.00 to 0 | ||||
| CTI | 69 | 6.00 | −1.00 to 12.00 | 66 | 5.50 | 1.00 to 10.00 | 68 | −1.00 | −2.50 to 1.00 | 63 | −2.00 | −4.00 to 0 | ||||
| All groups | .19 | .16 | .02 | .45 | ||||||||||||
| MP+NTI | 39 | 4.00 | 0 to 16.00 | 37 | 4.00 | −2.00 to 11.0 | 40 | −2.00 | −3.00 to −1.00 | 37 | −3.00 | −4.00 to −1.00 | ||||
| MP+CTI | 32 | 6.50 | 0.50 to 16.00 | 31 | 7.00 | 2.00 to 11.00 | 31 | −1.00 | −2.00 to 0 | 29 | −1.00 | −3.00 to 0 | ||||
| PL+NTI | 39 | 10.00 | 4.00 to 15.00 | 38 | 8.50 | 3.00 to 17.00 | 39 | −2.00 | −4.00 to −1.00 | 37 | −2.00 | −5.00 to 0 | ||||
| PL+CTI | 37 | 6.00 | −3.00 to 10.00 | 35 | 5.00 | 0 to 6.00 | 37 | −1.00 | −3.00 to 1.00 | 34 | −2.00 | −4.00 to 0 | ||||
Abbreviations: CTI, control telephone intervention; ESAS, Edmonton Symptom Assessment Scale; FACIT-F, Functional Assessment of Chronic Illness Therapy–Fatigue; IQR, interquartile range; MP, methylphenidate; NTI, nursing telephone intervention; PL, placebo.
Wilcoxon two-sample test and Kruskal-Wallis test.
Table 4 lists the change in ESAS symptoms at day 8 and day 15 by telephone intervention. Fatigue (P < .001), nausea (P = .01), depression (P = .02), anxiety (P = .01), drowsiness (P < .001), appetite (P = .009), sleep (P < .001), and feeling of well-being (P < .001) as measured by the ESAS significantly improved in patients who received NTI. In the CTI group, however, only ESAS fatigue (P = .001), depression (P = .03), and shortness of breath (P = .05) improved significantly.
Table 4.
Change in Symptoms at Day 8 and Day 15 by Telephone Intervention
| ESAS Symptom | CTI Group |
NTI Group |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline |
Day 8 |
Day 15 |
P* | Baseline |
Day 8 |
Day 15 |
P* | |||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Pain | 3.0 | 0-6.0 | 3.0 | 0-6.5 | 2.0 | 0-4.0 | .30 | 3.0 | 1.0-5.0 | 2.0 | 0-5.0 | 3.0 | 1.0-5.0 | .90 |
| Fatigue | 6.0 | 5.0-8.0 | 5.0 | 4.0-7.0 | 4.5 | 3.0-6.0 | .001 | 6.0 | 5.0-8.0 | 4.0 | 3.0-6.0 | 4.0 | 2.0-6.0 | < .001 |
| Nausea | 0 | 0-3.0 | 0 | 0-2.0 | 0 | 0-1.5 | .17 | 1.0 | 0-3.0 | 0 | 0-1.0 | 0 | 0-2.0 | .01 |
| Depression | 1.0 | 0-3.25 | 0 | 0-2.0 | 0 | 0-2.0 | .03 | 1.0 | 0-4.0 | 0 | 0-3.0 | 0 | 0-2.0 | .02 |
| Anxiety | 2.0 | 0-4.0 | 0 | 0-3.0 | 0 | 0-3.0 | .059 | 2.0 | 0-5.0 | 0 | 0-3.0 | 0 | 0-3.0 | .01 |
| Drowsiness | 4.0 | 0-7.0 | 3.0 | 0-5.0 | 1.0 | 0-5.0 | .21 | 4.0 | 2.0-7.0 | 3.0 | 0-4.0 | 2.0 | 0-3.0 | < .001 |
| Shortness of breath | 1.0 | 0-4.0 | 0 | 0-3.0 | 0 | 0-3.0 | .050 | 1.0 | 0-4.0 | 0 | 0-3.0 | 0 | 0-3.0 | .06 |
| Appetite | 4.0 | 1.0-5.0 | 3.0 | 0-5.75 | 2.0 | 0-6.0 | .07 | 3.0 | 1.0-5.0 | 2.0 | 0-4.0 | 2.0 | 0-5.0 | .009 |
| Sleep | 4.0 | 2.0-6.5 | 2.0 | 0-5.0 | 3.0 | 1.0-5.0 | .10 | 3.0 | 2.0-6.0 | 2.0 | 0-4.0 | 2.0 | 0-4.0 | < .001 |
| Feeling of well-being | 4.0 | 2.0-5.0 | 3.0 | 2.0-5.0 | 3.0 | 2.0-5.0 | .35 | 4.0 | 3.0-5.0 | 2.0 | 0-5.0 | 3.0 | 1.0-5.0 | < .001 |
Abbreviations: CTI, control telephone intervention; ESAS, Edmonton Symptom Assessment Scale; IQR, interquartile range; NTI, nursing telephone intervention.
Kruskal-Wallis test was used to analyze the differences in the ESAS item scores over time.
In the MP versus PL groups, the differences in the changes in HADS anxiety (median, 0.5 [interquartile range {IQR}, −3 to 1] v −1 [IQR, −3 to 1], respectively; P = .32), HADS depression (median, 0 [IQR, −1 to 2] v −1 [IQR, −2.5 to 1], respectively; P = .08), and PSQI scores (median, 0 [IQR, −3 to 1] v −2 [IQR, −3 to 1], respectively; P = .31) at day 15 were not statistically significant. Similarly, the differences in the changes in HADS anxiety and HADS depression scores at day 15 among the four subgroups (MP+NTI v MP+CTI v PL+NTI v PL+CTI) were not statistically significant (median, 0 [IQR, −2 to 2] v −1 [IQR, −4 to 0] v −1 [IQR, −3 to 0] v −1 [IQR, −3 to 1.5], respectively; P = .12; and median, 0 [IQR, −1 to 1] v 0 [IQR, −1 to 2] v −1 [IQR, −2 to 1] v −1 [IQR, −3 to 2], respectively; P = .29).
The signed ranked test was used to analyze the improvement in HADS anxiety and HADS depression scores at day 15 in patients treated with either MP or NTI. This analysis revealed no significant improvement in HADS anxiety (P = .058) or HADS depression (P = .6) scores at day 15 in patients treated with MP. In the NTI group, there was significant improvement in HADS anxiety (P = .02) but not in HADS depression (P = .1). There was no significant difference in the median number of phone calls between the MP group and the PL group (median, four calls [IQR, three to five calls] and four calls [IQR, four to five calls], respectively; P = .71). Also, there was no significant difference in the median number of capsules actually taken daily between the MP group and the PL group (median, 18 capsules [IQR, 11 to 33 capsules] and 20 capsules [IQR, 12 to 33 capsules], respectively; P = .71).
In addition, there was no significant differences in the median number of phone calls between the MP+NTI (five calls; IQR, four to six calls), MP+CTI (four calls; IQR, three to five calls), PL+NTI (five calls; IQR, four to six calls), and PL+CTI (four calls; IQR, four to five calls) groups (P = .14) and median number of capsules taken over the 14-day study period between the MP+NTI (18 capsules; IQR, 11 to 30 capsules), MP+CTI (18 capsules; IQR, 10 to 36 capsules), PL+NTI (21 capsules; IQR, 12 to 33 capsules), and PL+CTI (19 capsules; IQR, 13 to 34 capsules) groups (P = .80).
The difference in the number of grade ≥ 3 adverse events between the MP and PL groups was not statistically significant (40 of 93 patients v 29 of 97 patients, respectively; P = .06). Table 5 lists various types of grade ≥ 3 adverse events related to MP and PL.
Table 5.
Summary of Types of Adverse Events (grade ≥ 3) Experienced by Patients in the Methylphenidate and Placebo Groups
| Event | No. of Events | Methylphenidate (n = 11) | Placebo (n = 12) |
|---|---|---|---|
| Pain | 6 | 3 | 3 |
| Insomnia | 6 | 2 | 4 |
| Mood alteration (depression or anxiety) | 3 | 2 | 1 |
| Nausea | 1 | 0 | 1 |
| Hypertension | 1 | 1 | 0 |
| Anorexia | 1 | 1 | 0 |
| Syncope | 1 | 0 | 1 |
| Flu-like symptom | 2 | 1 | 1 |
| Tachycardia | 1 | 0 | 1 |
| Slurred speech | 1 | 1 | 0 |
NOTE. Only grade ≥ 3 adverse events related to the study treatment were summarized. No significant differences were found in the incidence of grade ≥ 3 toxicities between patients who received methylphenidate and those who received placebo (P = .06).
DISCUSSION
In this study, MP alone, NTI alone, or combination of MP and NTI did not prove to be significantly better than PL for CRF. These results confirm the findings of our previous PL-controlled study and other studies that have found no significant benefit of MP over PL for CRF in patients with advanced cancer.9,33,34Our results also showed that there was no significant difference in efficacy between NTI and CTI for CRF. However, several cancer-related symptoms were significantly improved in the NTI group (Table 4).
MP is a piperidine derivative that is structurally related to amphetamines. Previous studies by our group revealed that MP can improve opioid-induced sedation and cognitive dysfunction.34,35 In patients with opioid sedation, MP was also found to improve fatigue as a secondary outcome.35
These results led to pilot and PL-controlled randomized controlled studies.7,8 An open study by our team using as-needed MP showed significant improvement in CRF with a dose of 5 mg every 2 hours as needed, with a maximum of four doses a day. In a subsequent randomized controlled study with the same dose regimen comparing MP with PL, MP significantly improved CRF, but it was not better than PL. The authors hypothesized that this lack of significant difference was a result of the nurse telephone counseling that both groups received. Other longer duration studies were conducted in survivors of cancer in whom the mechanism of CRF is different compared with patients with advanced cancer.11,12
Patients and families welcome NTI as a way to maintain contact with nurses and other health care professionals.14–16 NTIs were perceived by patients with ovarian cancer as helpful for symptom management, assessment of adverse effects, and promotion of self-care.36 Poncia et al37 found that follow-up calls in elderly patients after discharge resulted in a significant number of interventions in the form of advice or action to ensure patients' well-being. The preliminary studies of MP and donepezil by our team found major improvements in fatigue in both the treatment and PL arms.8,9,27 One common denominator was the nursing phone calls. Patients frequently expressed satisfaction with participation in phone calls. These results led to us hypothesize that NTI could provide a benefit in the management of CRF and other cancer-related symptoms. However, in the present study, we found no differences in the patients receiving NTI and CTI. Nonetheless, the NTI group showed significant improvement in multiple cancer-related symptoms at day 15 (Table 4). Further research on the role of NTI on CRF and related symptoms in patients with advanced cancer is needed.
This study also suggests that participation in the clinical trial resulted in clinically relevant improvement in CRF in all four groups over a considerable period of time in this considerably ill patient population (Table 2). More research is necessary to better characterize the subjective benefits that derive from participation of clinical trials of CRF.
Our findings support the use of PLs and attention control for CRF intervention trials. The expectation of improvement in fatigue as a result of participation in a clinical trial may have been an important factor that contributed to the improvement of symptoms in all four arms. This finding may have resulted from PL/nocebo effects.38 More research is necessary to determine the role of the expectation of improvement as a result of clinical trial participation on reduction of symptoms.
In this study, adverse events were similar between the MP and PL arms. These results are consistent with our previous findings9 and contradict the findings of a recent study of 148 patients with cancer who received 54 mg per day of long-acting MP orally for 4 weeks.33 That study showed significantly higher levels of nervousness and appetite loss in the MP group than the PL group. The low frequency of adverse effects of short-acting MP may be a result of the dose-duration effect of MP used in the present study.
This study has several limitations. There is a possibility that the study was underpowered for the reported outcomes because of lower than planned accrual (190 patients, instead of the planned 215). Additional limitations include the fact that there was no statistical control for multiple comparisons, so some of the significant findings might be a result of type I error.
We conclude that MP alone, NTI alone, or MP and NTI combined was not superior to PL for improving CRF. Several cancer-related symptoms were significantly improved in the NTI group. Further research on NTI in the advanced cancer setting is needed.
Supplementary Material
Footnotes
Supported by National Institutes of Health National Institute of Nursing Research Grant No. R01 NR010162-01A1. S.Y. is supported in part by an American Cancer Society grant entitled Research Scholar Grant for Independent Investigators/Multimodal Study for Cancer Related Fatigue (Grant No. RSG-11-170-01-PCM).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00424099.
See accompanying editorial on page 2372
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Eduardo Bruera, J. Lynn Palmer, Marlene Z. Cohen
Financial support: Eduardo Bruera
Administrative support: Eduardo Bruera, Sriram Yennurajalingam
Provision of study materials or patients: Eduardo Bruera, Sriram Yennurajalingam
Collection and assembly of data: All authors
Data analysis and interpretation: Eduardo Bruera, Sriram Yennurajalingam, J. Lynn Palmer, Pedro E. Perez-Cruz, Marlene Z. Cohen
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Yennurajalingam S, Bruera E. Palliative management of fatigue at the close of life: “It feels like my body is just worn out.”. JAMA. 2007;297:295–304. doi: 10.1001/jama.297.3.295. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence DP, Kupelnick B, Miller K, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;2004:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 3.Piper BF, Cella D. Cancer-related fatigue: Definitions and clinical subtypes. J Natl Compr Canc Netw. 2010;8:958–966. doi: 10.6004/jnccn.2010.0070. [DOI] [PubMed] [Google Scholar]
- 4.Minton O, Richardson A, Sharpe M, et al. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;7:CD006704. doi: 10.1002/14651858.CD006704.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minton O, Richardson A, Sharpe M, et al. Psychostimulants for the management of cancer-related fatigue: A systematic review and meta-analysis. J Pain Symptom Manage. 2011;41:761–767. doi: 10.1016/j.jpainsymman.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Peuckmann V, Elsner F, Krumm N, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2010;11:CD006788. doi: 10.1002/14651858.CD006788.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-related fatigue. J Natl Compr Canc Netw. 2010;8:904–931. doi: 10.6004/jnccn.2010.0067. [DOI] [PubMed] [Google Scholar]
- 8.Bruera E, Driver L, Barnes EA, et al. Patient-controlled methylphenidate for the management of fatigue in patients with advanced cancer: A preliminary report. J Clin Oncol. 2003;21:4439–4443. doi: 10.1200/JCO.2003.06.156. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: A double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073–2078. doi: 10.1200/JCO.2005.02.8506. [DOI] [PubMed] [Google Scholar]
- 10.Butler JM, Jr, Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007;69:1496–1501. doi: 10.1016/j.ijrobp.2007.05.076. [DOI] [PubMed] [Google Scholar]
- 11.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: A randomized clinical trial. J Pain Symptom Manage. 2009;38:650–662. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16:577–583. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 13.Auret KA, Schug SA, Bremner AP, et al. A randomized, double-blind, placebo-controlled trial assessing the impact of dexamphetamine on fatigue in patients with advanced cancer. J Pain Symptom Manage. 2009;37:613–621. doi: 10.1016/j.jpainsymman.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Cusack M, Taylor C. A literature review of the potential of telephone follow-up in colorectal cancer. J Clin Nurs. 2010;19:2394–2405. doi: 10.1111/j.1365-2702.2010.03253.x. [DOI] [PubMed] [Google Scholar]
- 15.Beney J, Devine EB, Chow V, et al. Effect of telephone follow-up on the physical well-being dimension of quality of life in patients with cancer. Pharmacotherapy. 2002;22:1301–1311. doi: 10.1592/phco.22.15.1301.33480. [DOI] [PubMed] [Google Scholar]
- 16.Cox K, Wilson E. Follow-up for people with cancer: Nurse-led services and telephone interventions. J Adv Nurs. 2003;43:51–61. doi: 10.1046/j.1365-2648.2003.02672.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JE, Wong FK, You LM, et al. A qualitative study exploring the nurse telephone follow-up of patients returning home with a colostomy. J Clin Nurs. 2012;21:1407–1415. doi: 10.1111/j.1365-2702.2011.03824.x. [DOI] [PubMed] [Google Scholar]
- 18.Salonen P, Tarkka MT, Kellokumpu-Lehtinen PL, et al. Telephone intervention and quality of life in patients with breast cancer. Cancer Nurs. 2009;32:177–190. doi: 10.1097/NCC.0b013e31819b5b65. [DOI] [PubMed] [Google Scholar]
- 19.Yesilbalkan OU, Karadakovan A, Göker E. The effectiveness of nursing education as an intervention to decrease fatigue in Turkish patients receiving chemotherapy. Oncol Nurs Forum. 2009;36:E215–E222. doi: 10.1188/09.ONF.E215-E222. [DOI] [PubMed] [Google Scholar]
- 20.Godino C, Jodar L, Durán A, et al. Nursing education as an intervention to decrease fatigue perception in oncology patients. Eur J Oncol Nurs. 2006;10:150–155. doi: 10.1016/j.ejon.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Badger T, Segrin C, Meek P, et al. Telephone interpersonal counseling with women with breast cancer: Symptom management and quality of life. Oncol Nurs Forum. 2005;32:273–279. doi: 10.1188/05.ONF.273-279. [DOI] [PubMed] [Google Scholar]
- 22.Yates P, Aranda S, Hargraves M, et al. Randomized controlled trial of an educational intervention for managing fatigue in women receiving adjuvant chemotherapy for early-stage breast cancer. J Clin Oncol. 2005;23:6027–6036. doi: 10.1200/JCO.2005.01.271. [DOI] [PubMed] [Google Scholar]
- 23.Given B, Given CW, McCorkle R, et al. Pain and fatigue management: Results of a nursing randomized clinical trial. Oncol Nurs Forum. 2002;29:949–956. doi: 10.1188/02.ONF.949-956. [DOI] [PubMed] [Google Scholar]
- 24.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 25.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 26.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 27.Bruera E, El Osta B, Valero V, et al. Donepezil for cancer fatigue: A double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2007;25:3475–3481. doi: 10.1200/JCO.2007.10.9231. [DOI] [PubMed] [Google Scholar]
- 28.Cella D, Eton DT, Lai JS, et al. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 29.Carvajal A, Centeno C, Watson R, et al. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47:1863–1872. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Johnston M, Pollard B, Hennessey P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res. 2000;48:579–584. doi: 10.1016/s0022-3999(00)00102-1. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28:3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruera E, Chadwick S, Brenneis C, et al. Methylphenidate associated with narcotics for the treatment of cancer pain. Cancer Treat Rep. 1987;71:67–70. [PubMed] [Google Scholar]
- 35.Bruera E, Miller MJ, Macmillan K, et al. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain. 1992;48:163–166. doi: 10.1016/0304-3959(92)90053-E. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DF, Faught WJ, Holmes LA. Ovarian cancer treatment: The benefit of patient telephone follow-up post-chemotherapy. Can Oncol Nurs J. 1999;9:175–178. doi: 10.5737/1181912x94175178. [DOI] [PubMed] [Google Scholar]
- 37.Poncia HD, Ryan J, Carver M. Next day telephone follow up of the elderly: A needs assessment and critical incident monitoring tool for the accident and emergency department. J Accid Emerg Med. 2000;17:337–340. doi: 10.1136/emj.17.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de la Cruz M, Hui D, Parsons HA, et al. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116:766–774. doi: 10.1002/cncr.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


