Abstract
Community engagement is an innovative and required component for Clinical and Translational Science Awards (CTSAs) funded by the National Institutes of Health (NIH). However, the extent of community engagement in NIH‐funded research has not been previously examined. This study assessed baseline prevalence of community engagement activities among NIH‐funded studies at a large Midwestern university with a CTSA. An online survey was e‐mailed to principal investigators of recent NIH‐funded studies (N = 480). Investigators were asked to identify what types of community engagement activities had occurred for each study. Responses were received for 40.4% (194/480) of studies. Overall, 42.6% reported any community engagement activities. More collaborative types of engagement (e.g., community advisory board) were less common than activities requiring less engagement (e.g., sharing study results with community members). Studies with more collaborative community engagement were less likely to be described as basic or preclinical research compared to all other studies. Given NIH's inclusive call for community engagement in research, relatively few NIH‐funded studies reported community engagement activities, although this study used a broad definition of community and a wide range of types of engagement. These findings may be used to inform the goals of CTSA community engagement programs. Clin Trans Sci 2010; Volume *: 1–4
Keywords: community engagement, practice‐ and community‐based research, translational research, CTSA
Introduction
Federal health agencies, including the National Institutes of Health (NIH), are increasingly recognizing, supporting, and in many cases requiring community engagement in health‐related research. 1 , 2 , 3 , 4 , 5 Community involvement in the research process can increase the relevance and quality of health research, even in clinical studies. 6 , 7 For example, increased public participation in all stages of the research process has been identified as critical to achieving diverse representation and sufficient enrollment of participants for clinical studies. 5 Community representatives and patient advocates can contribute substantially to identifying important research questions, designing effective informed consent processes and research protocols, and disseminating research results. 4 , 8 Engaging community‐based primary care clinicians in research can improve the effectiveness and efficacy of interventions. 1
However, it can be challenging to operationalize community engagement in research practice. Community‐based participatory research (CBPR) is on one end of the “engagement” spectrum. 9 CBPR is defined as equal participation of community partners and researchers throughout the research process with shared decision making. On the other end of the spectrum, communities are simply informed about current research and results. In the middle of the spectrum, communities are engaged in important but limited aspects of research studies, such as recruiting and retaining research participants. 10 It is important to recognize that more participation is not always better; some community partners may not have the time, interest, or expertise to participate as equal partners in every stage of the research process. 7
In 2006, the NIH National Center for Research Resources launched the Center for Clinical and Translational Science Award (CTSA) program that included a community engagement component for each Center. 11 A recent summary of best practices emerging from CTSAs across the country identified two major goals for community engagement activities: (1) engage communities as valued members of the research team, and (2) build stable infrastructure in communities for participating in research and implementing new knowledge resulting from research to improve community health outcomes. 12 The best practices document also described numerous principles for developing community‐academic partnerships, such as recognizing that community members are part of multiple communities and including community partners early in research planning.
Although much has been written about the importance of engaging communities in the research process and the principles for doing so, to date, there is a paucity of research about the prevalence of community engagement in research, especially among clinical and translational research studies traditionally funded by NIH. The current study assessed baseline prevalence of community engagement in research among NIH‐funded studies at a large Midwestern university with a CTSA funded in 2008.
Methods
Sample and setting
The units of observation were individual research studies funded by NIH during a specified time frame. A list of all studies that met eligibility criteria was obtained from the university's online, searchable database. To be eligible, a study had to be active in the past 5 years and completed by December 31, 2008. For each study in the sample, the following information was obtained from the database: study title, principal investigator, co‐investigator(s), start date, end date, and department.
Measures
A data collection tool was developed for this study because no applicable instruments were identified in the literature. Respondents were first asked to report the study title, the principal investigator's name, the type of research, and an eligibility screening question ( Figure 1 ). The remainder of the survey was designed to assess two “levels” of community engagement. Level 1 included activities consistent with principles of CBPR such as having a community advisory group or other process for meaningfully involving community representatives in various steps throughout the research process. 13 Community representatives were denned for survey respondents as individuals or organizations outside of traditional university research teams, including community residents, organizations, public health agencies, social service providers, healthcare providers, or healthcare payers. Respondents reporting Level 1 engagement activities were also asked to identify the research steps in which community representatives were involved (e.g., identifying community health concerns, collecting data, interpreting findings, etc.) and whether community representatives received training or payment for their involvement.
Figure 1.
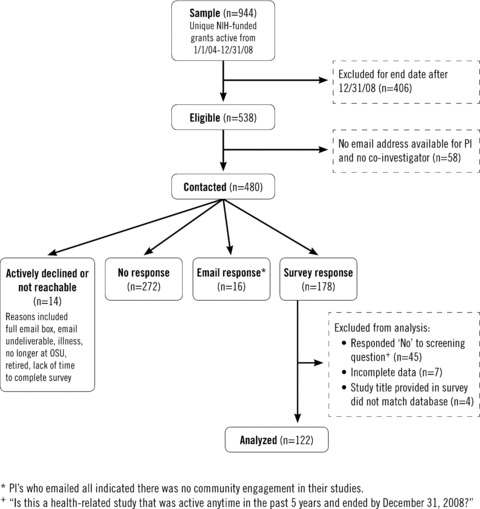
Sample selection process and response rates.
Level 2 activities did not necessarily require significant collaboration between research and community partners but may still be important elements of community engagement, especially for the types of clinical and translational research studies most commonly funded by NIH. These activities are listed in Table 1 . Many Level 2 activities were derived from conferences and communications directed toward CTSA community engagement programs and therefore reflect ways that community engagement is being operationalized across the country. Investigators could also describe additional examples of community engagement not assessed in close‐ended survey questions.
Table 1.
Number and percent of studies reporting each type of community engagement activity (n= 122).*
| Yes Number (%) | No Number (%) | Not sure Number (%) | |
|---|---|---|---|
| Level 1 activities | |||
| Advisory board, group, or committee of community representatives | 13 (10.7) | 103 (84.4) | 6 (4.9) |
| [If no] Input from community representatives at any time during study | 8 (6.6) | 99 (81.1) | 1 (0.8) |
| Level 2 activities | |||
| Focus groups or other methods to get input from community representatives | 10 (8.2) | 107(87.7) | 3 (2.5) |
| Memorandum of understanding/agreement with nonuniversity affiliated organization | 13 (10.7) | 101 (82.8) | 5(4.1) |
| Data collection activities somewhere other than main medical center or university campus | 30 (24.6) | 90 (73.8) | 1 (0.8) |
| Special events or recognition for study participants | 11 (9.0) | 104(85.2) | 4 (3.3) |
| Study findings shared with community representatives* | 26(21.3) | 78 (63.9) | 9 (7.4) |
*All categories do not sum to 100% due to skip patterns or missing data.
*Five respondents selected “not applicable—there have not been any findings to date.”
Procedure
Investigators were invited to complete a separate online survey for each of their studies in the sample. A hyperlink to the survey was distributed through e‐mail messages to each principal investigator (or co‐investigator if contact information was not available for the principal investigator). E‐mail addresses were obtained manually from the university’ s online directory. E‐mails were personalized with the investigator’ s name and the title of the study included in the sample. For investigators with multiple studies, the e‐mail invitation listed all studies and requested a separate survey be completed for each one. Respondent tracking was conducted manually by linking the study title and principal investigator's name from each survey to the original sample. Two reminder messages with another link to the survey were e‐mailed to nonrespondents after the initial invitation. All analyses were conducted using Microsoft Excel 2003.
Results
Respondents
A total of 480 studies led by 308 unique principal investigators were contacted to participate in the study ( Figure 1 ). Responses were received for 40.4% (194/480) of the studies, including submitted surveys and responses e‐mailed directly to research staff. These responses represented about half (50.6%, 156/308) of the unique principal investigators.
The final number of complete, eligible survey responses was 122 ( Figure 1 ). Almost all (95.9%) surveys were completed by the principal investigator. A large proportion of studies (48.4%) were described as basic or preclinical research. Fewer were classified as clinical or clinical trials (n= 9), survey research (n= 9), intervention research (n= 9), or cohort studies (n= 7). The remaining studies (n= 25) were described using less frequent terms provided in the survey (e.g., dissemination research, case control study) or in response to the open‐ended “other” option (e.g., multidisciplinary research, observational study).
Community engagement activities
Overall, 42.6% (52/122) of studies reported any community engagement. Of these, 17.2% (21/122) reported Level 1 community engagement activities and 39.3% (48/122) reported Level 2 activities ( Table 1 ). For Level 1,13 studies had an advisory board, group, or committee of community representatives for the study. Of the studies without a community board, an additional eight reported obtaining input from community representatives at some time during the study. The most commonly reported Level 2 activities were collecting data in a location other than the main university center or campus (24.8%), and sharing study findings with community representatives (23.0%).
Of the studies with Level 1 engagement, the most common research step in which community representatives were involved was “recruiting and retaining study participants” (n= 10) and the least common was “translating findings into practice or policy” (n= 1) ( Figure 2 ). Eight studies provided research‐related training for community representatives, five required the online Collaborative IRB Training Initiative (CITI) course, and four provided a stipend or other form of payment for community representatives.
Figure 2.
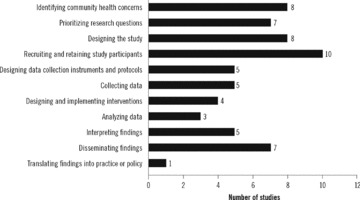
Number of studies reporting community involvement in steps of the research process.
Studies with Level 1 engagement were significantly less likely to be described as basic or preclinical research compared to all other studies (19.0% vs. 54.5%, χ2= 8.728, p= 0.003). However, these studies were distributed across a range of investigators and colleges. Only three investigators had two studies with Level 1 engagement; the rest were unique investigators. There was not a dominant college or departmental affiliation for studies with Level 1 engagement; for example, affiliations included the College of Public Health, Human Development and Family Science, Nursing, School of Communication, Neuroscience, Obstetrics and Gynecology, Ophthalmology, and Psychiatry, among others.
Discussion
Overall, less than half of NIH‐funded studies reported any type of community engagement activities. Fewer than one‐fifth reported Level 1 community engagement activities, defined as having a community advisory group or obtaining input from community representatives at some point during the study (not including focus groups). Of those, few involved community representatives in multiple steps of the research process.
Level 2 engagement activities that did not necessarily involve community representatives directly in the research process were also relatively uncommon in this study. Data were collected somewhere other than the main university campus in about one‐fourth of all studies; however, data were collected at another university as opposed to a community‐based setting in about half of those. Findings were shared with community representatives in only about one‐fifth of studies. Sharing study findings with communities involves a minimum level of community engagement and should be relevant to most health‐related studies.
A recent study found that a majority of studies funded by the National Heart, Lung, and Blood Institute in 2006 addressed earlier phases of the translational continuum. 14 It is possible that community engagement activities listed in the survey were not appropriate for basic research studies or those in early phases of the translational continuum. In the current study, fewer than 20% of studies with Level 1 engagement were described as basic research, compared to more than half of other studies.
However, in its recent best practices document, 12 NIH adhered to a fairly traditional definition of community engagement, describing the need for “strong collaborative partnerships, based on mutual understanding and trust, between communities and local academic institutions” (p. 4). This document did not differentiate types of community engagement that may be more or less appropriate for different types of research. A broader definition of community‐engaged scholarship that includes community‐oriented teaching (e.g., service‐learning) and service (e.g., outreach, advocacy) in addition to community‐based research 15 maybe necessary for achieving some level of community engagement in a wider range of NIH‐funded studies.
Strengths of this study included a response rate from principal investigators that was over 50% and a focus on NIH‐funded research, which makes the findings maximally applicable for CTSA community engagement programs. The survey also provides baseline data about the prevalence of community engagement in NIH‐funded research that may contribute to the evaluation of the CTSA.
This study had several limitations. First, inclusion criteria involved only studies completed by December 31, 2008. Because NIH funding for CBPR has increased in recent years, some current studies with strong community engagement were likely excluded. Second, the survey did not include all possible definitions of “community engagement in research.” It focused on relatively traditional definitions of community participation based on CBPR principles and strategies 9 despite broad definitions of “community” and “engagement.” Other institutional‐level strategies to support community engagement were also not assessed in this study, such as community advisory boards for specific diseases (e.g., cancer, AIDS) or initiatives to make faculty tenure and promotion procedures more supportive for community‐engaged research. A final limitation was that the quality of reported community engagement activities was not assessed. The distinctions made in this article between levels of engagement were based on the types of activities in each category rather than confirmed evidence from the studies themselves.
Conclusion
Although much has been written about principles of community‐based participatory research 9 ’ 13 ’ 16 and other scholarly community engagement activities, 15 it is challenging to define and assess community engagement in NIH‐funded research. This study provides a baseline measurement of community engagement in NIH‐funded research at a large Midwestern university with an NIH‐funded CTSA. The prevalence of any type of community engagement in research was relatively low given that NIH has not specified community engagement as more appropriate or important for some types of research compared to others. CTSAs should consider clarifying whether community engagement programs should strive to increase the number of authentic CBPR studies, increase less intensive community engagement activities in all NIH‐funded research, or both. If increasing the number of CBPR studies is a primary goal, the proportion of funds allocated to CBPR studies may need to be increased as well. However, if some type of community outreach or engagement is desired for all NIH‐funded studies, a broader definition of community‐engaged scholarship that includes teaching and service as well as research may be required.
Acknowledgements
The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources, funded by the Office of the Director, National Institutes of Health (OD) and supported by the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1. Westfall JM, Mold J, Fagnan L. Practice‐based research—”blue highways” on the NIH roadmap. JAMA. 2007; 297(4): 403–406. [DOI] [PubMed] [Google Scholar]
- 2. Seifer SD, Shore N, Holmes SL. Developing and Sustaining Community‐University Partnerships for Health Research: Infrastructure Requirements. Seattle , WA : Community Campus Partnerships for Health; 2003. [Google Scholar]
- 3. Viswanathan M, Ammerman A, Eng E, Garlehner G, Lohr KN, Griffith D, Rhodes S, Samuel‐Hodge C, Maty S, Lux L, Webb L, Sutton SF, Swinson T, Jackman A, Whitener L. Community‐based participatory research: assessing the evidence. Evid Rep Technol Assess (Summ). 2004; 99: 1–8. [PMC free article] [PubMed] [Google Scholar]
- 4. Education Network to Advance Cancer Clinical Trials (ENACCT), Community‐Campus Partnerships for Health (CCPH) . Communities as Partners in Cancer Clinical Trials: Changing Research, Practice, and Policy. Silver Spring , MD : Education Network to Advance Cancer Clinical Trials (ENACCT), Community‐Campus Partnerships for Health (CCPH); 2008. [Google Scholar]
- 5. Sung NS, Crowley WF, Genel M, Salber P, Sandy L, Sherwood LM, Johnson SB, Catanese V, Tilson H, Getz K, Larson EL, Scheinberg D, Reece EA, Slavkin H, Dobs A, Grebb J, Martinez RA, Kom A, Rimoin D. Central challenges facing the national clinical research enterprise. JAMA. 2003; 289(10): 1278–1287. [DOI] [PubMed] [Google Scholar]
- 6. Hubbard G, Kidd L, Donaghy E. Involving people affected by cancer in research: a review of literature. Eur J Cancer Care. 2008; 17(3): 233–244. [DOI] [PubMed] [Google Scholar]
- 7. Green LW. Community‐based participatory research perspective on partnerships In: Community‐university Partnerships: Translating Evidence into Action. Proceedings of a National Symposium Jointly Sponsored by Community‐Campus Partnerships for Health and HUD's Office of University Partnerships. San Diego , CA ; 2003. [Google Scholar]
- 8. Lindenmeyer A, Heamshaw H, Sturt J, Ormerod R, Aitchison G. Assessment of the benefits of user involvement in health research from the Warwick diabetes care research user group: a qualitative case study. Health Expect. 2007; 10(3): 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Israel BA, Eng E, Schulz AJ, Parker EA. Introduction to methods in community‐based participatory research for health In: Israel BA, Eng E, Schulz AJ, Parker EA, eds. Methods in Community‐Based Participatory Research for Health. San Francisco , CA : Jossey‐Bass; 2005: 2–26. [Google Scholar]
- 10. Paskett ED, Katz ML, DeGraffinreid CR, Tatum CM. Participation in cancer trials: recruitment of underserved populations. Clin Adv Hematol Oncol. 2003; 1 (10): 607–613. [PubMed] [Google Scholar]
- 11. National Center for Research Resources . Clinical and Trans/ational Science Awards. Advancing Scientific Discoveries Nationwide to improve Health. Progress report 2006–2008. Bethesda , MD : National Institutes of Health; NIH Publication No. 09‐7404; 2009. [Google Scholar]
- 12. The Clinical and Translational Science Award (CTSA) Consortium's Community Engagement Key Function Committee, CTSA Community Engagement Workshop Planning Committee . Researchers and Their Communities: The Challenge of Meaningful Community Engagement. Bethesda , MD : National Center for Research Resources; 2009. [Google Scholar]
- 13. Israel BA, Schulz AJ, Parker EA, Becker AB, Allen AJ III, Guzman JR. Critical issues in developing and following CBPR principles In: Minkler M, Wallerstein N, eds. Community‐Based Participatory Research for Health: From Process to Outcomes. 2nd ed San Frandsco , CA : Jossey‐Bass; 2008: 47–66. [Google Scholar]
- 14. Kleinman MS, Mold JW. Defining the components of the research pipeline. Clin Transl Sci. 2009; 2(4): 312–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calleson DC, Jordan C, Seifer SD. Community‐engaged scholarship: is faculty work in communities a true academic enterprise? Acad Med. 2005; 80(4): 317–321. [DOI] [PubMed] [Google Scholar]
- 16. Wallerstein N, Duran B, Minkler M, Foley K. Developing and maintaining partnerships with communities In: Israel BA, Eng E, Schulz AJ, Parker EA. eds. Methods in Community‐Based Participatory Research for Health. San Francisco , CA : Jossey‐Bass; 2005: 31–51. [Google Scholar]


