Abstract
In humans, genetic variation occurs through different types of alleles that vary in frequency and severity of effect. Mendelian mutations, such as those in the low-density lipoprotein (LDL) receptor (LDLR) that result in familial hypercholesterolemia, are rare and have powerful phenotypic effects. Conversely, alleles that are common in the population (such that homozygotes for the minor allele are present even in modest sample sizes) typically have very modest phenotypic effects. In the middle of the spectrum are Goldilocks alleles such as mutations in the gene for proprotein convertase subtilisin/kexin type 9 (PCSK9). Loss-of-function mutations in PCSK9 result in significantly decreased LDL-cholesterol (LDL-C) levels, and a disproportionately large reduction in coronary heart disease risk by reducing the exposure to LDL-C throughout life. Several agents to inhibit PCSK9 are currently in development demonstrating the potential utility of translating genetics into clinical therapeutics. To date, most investigations aimed at identifying the genes responsible for hypercholesterolemia have used linkage analysis, which requires samples collected from multiple families with defects in the same gene, or common variant analysis which requires thousands of samples from the population. However, case studies have shown that with advances in whole genome sequencing or exome sequencing (targeted exome capture), the process of discovering causal genetic mutations can be significantly streamlined. Astute clinical observation of individual patients and their families with atypical lipid profiles, followed by sequencing of the affected individual, has the potential to lead to important findings regarding the genetic mutations that cause lipid abnormalities.
Keywords: familial hypercholesterolemia, LDL cholesterol, PCSK9, genetics, sequencing
Introduction
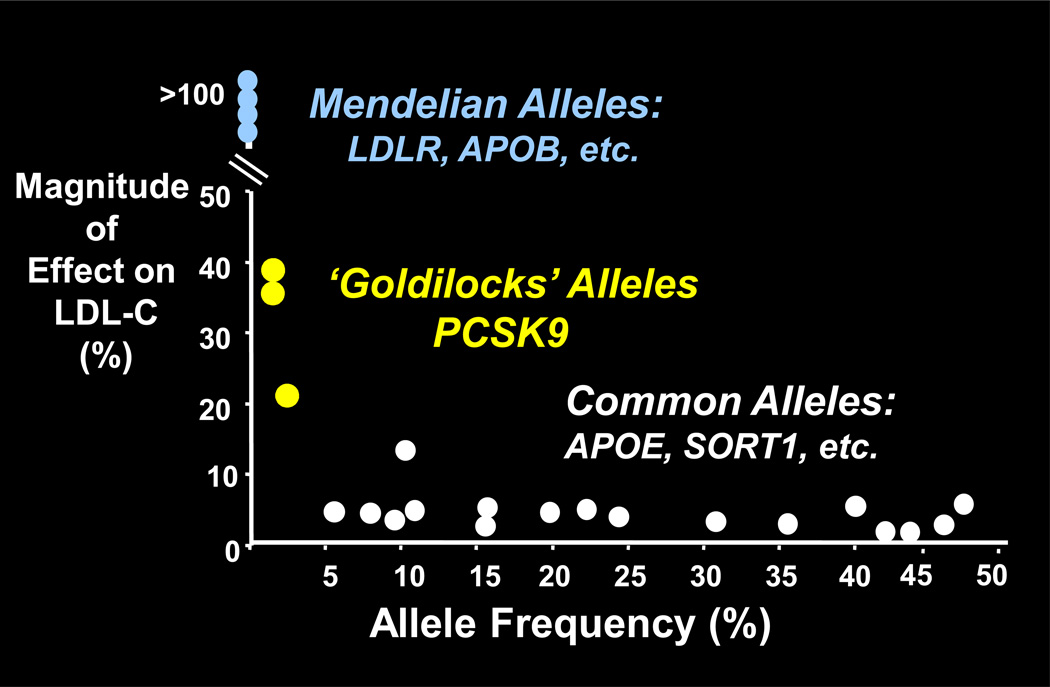
The study of genetics is primarily an examination of genetic variation, which in humans occurs through diverse alleles with varying frequencies and effect sizes (Figure 1). At one extreme are mutations that cause Mendelian diseases such as familial hypercholesterolemia (FH) arising from mutations in the low-density lipoprotein (LDL) receptor gene (LDLR) and familial defective Apo B arising from defects in the apolipoprotein (Apo) B gene (APOB).1,2 These mutations are inevitably rare and have powerful effects on phenotype. At the other end of the mutation spectrum are common alleles, e.g., the Apo E (APOE) allele and variants in the sortilin 1 gene (SORT1).3, 4 These variations are characterized by small phenotypic effects. In the middle of the spectrum is a class of alleles termed “Goldilocks alleles.” The quintessential example of a Goldilocks allele is provided by the proprotein convertase subtilisin/kexin type 9 gene (PCSK9),5 which has mutations that are common enough to be useful in epidemiological analyses, in contrast to Mendelian alleles, but still produce readily detectable effects in biochemical assays.
Figure 1.
Schematic of the relationship between allele frequency and effect size
Abbreviations: APOB = gene for apolipoprotein B, APOE = gene for apolipoprotein E, LDL-C = low-density lipoprotein cholesterol, LDLR = gene for low-density lipoprotein receptor, PCSK9 = gene for proprotein convertase subtilisin/kexin type 9, SORT1 = gene for sortilin 1
Mendelian Alleles
Mendelian alleles have traditionally been identified by linkage studies in families. The classic example of FH is shown in a pedigree in which two individuals with unusually high LDL cholesterol (-C) levels and mutations in the LDLR married and produced two heterozygote children and one homozygote with severe hyperlipidemia. Studies indicate that ~5% of patients who have had a myocardial infarction (MI) before 60 years of age have heterozygous FH, and ~50% of untreated FH heterozygotes will have an MI by age 60.6, 7 These results suggest that an isolated high LDL-C level is sufficient to produce coronary heart disease (CHD).
The genetics of FH were initially investigated in Lebanon in the 1970s, prior to the era of molecular genetics, when Khachadurian recognized that there was more than one type of FH.8 The dominant form, where at least one parent was affected, was well known, but another type of FH was suspected in a family in which two parents with normal LDL-C levels had four children with extremely high LDL-C levels. To date, there are at least five known dominant and recessive disorders of LDL metabolism. Dominant disorders include FH caused by a mutation of LDLR; familial defective Apo B caused by a defect in APOB,9 which diminishes the ability of LDL to bind to the LDL receptor; and FH type 3 caused by mutations in PCSK9 which increase degradation of the LDL receptor.10
There are two well-defined recessive disorders of hypercholesterolemia: autosomal recessive hypercholesterolemia and sitosterolemia.11, 12 Autosomal recessive hypercholesterolemia (ARH) is caused by a defect in the ARH gene, also known as the LDL receptor adaptor protein 1 gene (LDLRAP1), which encodes an adaptor protein that is required for localization of the LDL receptor to clathrin-coated pits.12 In the absence of functioning ARH, LDL receptors sit on the surface of the cell where they can bind to LDL but are unable to internalize it. Sitosterolemia is a disorder of neutral sterol excretion characterized by an accumulation of plant sterols. It is caused by mutations in two pumps, adenosine triphosphate-binding cassette sub-family G member 5 (ABCG5) and member 8 (ABCG8) which together form a complex that pumps neutral sterols out of enterocytes and hepatocytes.11 Individuals with sitosterolemia have varying degrees of hypercholesterolemia. All five of these dominant and recessive disorders are significantly associated with premature severe CHD, demonstrating that, irrespective of cause, elevated LDL-C is a risk factor for CHD.
Common Alleles
There is a great deal of interest in finding common alleles that explain the differences in LDL-C levels in the general population. Genome wide association studies (GWAS) examine many genetic variants to determine if any are associated with a trait, typically major diseases or conditions like hypercholesterolemia. Results from a monumental meta-analysis of GWAS evaluating more than 100,000 individuals indicated that there are at least 95 different loci in the human genome that are systematically associated with plasma lipid and lipoprotein levels.4 However, essentially all of these effects are small individually, and cumulatively these sequence variations explain only a small fraction of the variation in LDL-C or high-density lipoprotein (HDL)-C levels.
There are pros and cons of GWAS.13, 14 In many cases the effects of the variants identified are small and difficult to interpret biologically. On the other hand, they may reveal genes that are not amenable to Mendelian genetics. .For example, it’s unlikely that a Mendelian defect in 3- hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate limiting enzyme in cholesterol biosynthesis, will ever be detected, because no cell can live without HMG-CoA reductase. Yet more subtle mutations or variations HMG CoA reductase have been captured by a GWAS in the general population.
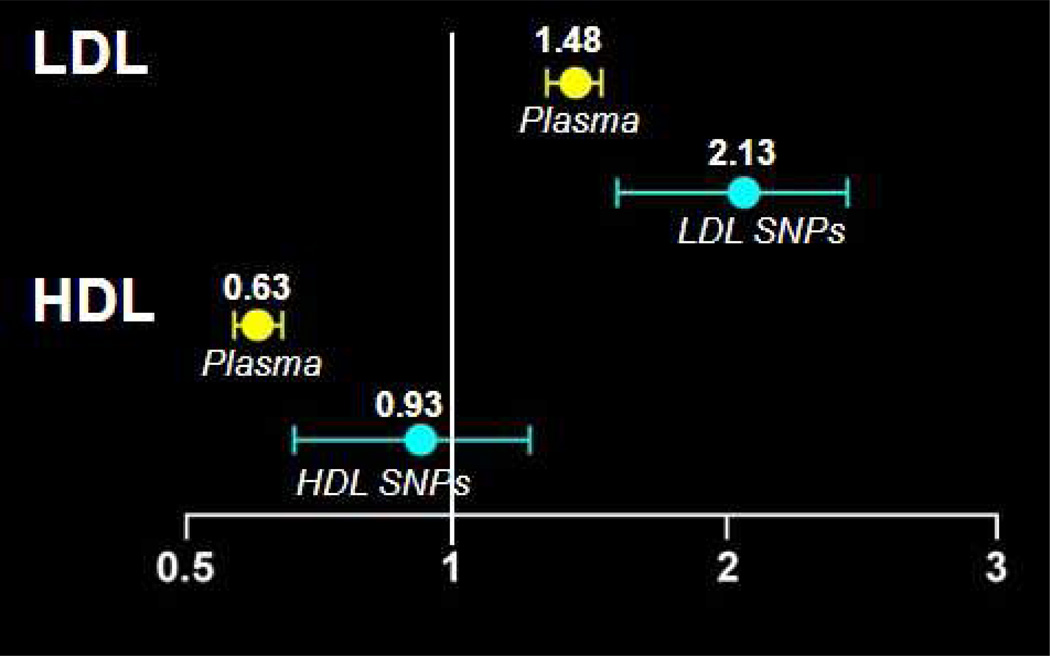
Epidemiological investigations have demonstrated a strong positive correlation between increased LDL-C and increased risk for CHD, and a negative correlation between HDL-C and CHD risk.13 However, this correlation, although highly consistent, does not prove that low HDL-C causes CHD. While there is a significant body of evidence pointing to the causality of high LDL-C and CHD, whether or not a high HDL-C concentration per se protects from CHD is unclear.15 To demonstrate causality, investigations are needed that assess whether sequence variations that systematically confer either a high HDL-C or a low HDL-C level, absent of any other change in the lipoprotein profile, are associated with CHD. In aMendelian randomization analysis of data from prospective studies, Kathiresan and colleagues analyzed multiple variants affecting HDL-C levels, and their correlation with CHD.17 Using a genetic score of common polymorphisms associated with LDL-C as a positive control, a genetic score based on gene sequence variations that increased HDL-C levels was not significantly associated with reduced risk of MI [odds ratio (OR) per standard deviation increase in HDL-C due to HDL-C genetic score 0.93, 95% confidence interval (CI) 0.68–1.26, p = 0.63] (Figure 2).17 These data support the argument that HDL-C is not causally related to CHD. Although it is clearly associated, HDL-C is more likely a marker for the disease process than a direct cause thereof. Notably the estimate from observational epidemiology that a one standard deviation increase in LDL-C was associated with increased risk for MI was concordant with that determined from the LDL-C genetic score [OR 2.13, 95% CI 1.69–2.69, p = 2x10(−10)], supporting the theory that genetic mechanisms that raise LDL-C translate directly into increased MI.17
Figure 2.
Hazard ratios of myocardial infarction in prospective studies (n = 25,000) per standard deviation increase in lipid level. Plasma denotes predicted hazard ratios for the change in plasma levels of each lipoprotein that would be caused by the SNPs.
Abbreviations: HDL = high-density lipoprotein, LDL = low-density lipoprotein, SNPs = single nucleotide polymorphisms
17Voight BF, et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomization study. Lancet. 2012; 380:572–580.
Goldilocks Alleles – PCSK9
PCSK9 was initially implicated in lipoprotein metabolism when PCSK9 mutations were found in two families with autosomal dominant hypercholesterolemia, but normal LDLR alleles. Studies in mice revealed that these mutations were, in fact, gain-of-function. An examination of the low end of the LDL-C distribution in the Dallas Heart Study found that a significant fraction of individuals had mutations in PCSK9.5, 18, 19 These were loss-of-function mutations. About 2% of the African Americans evaluated had one loss-of-function allele in PCSK9 that lowered LDL-C by 40%. Among European Americans, ~3% had a less severe mutation that resulted in 21% lower LDL-C levels.
An examination of the Atherosclerosis Risk in Communities (ARIC) population showed that among African Americans, the average LDL-C reduction in carriers of the PCSK9 mutation (heterozygotes) was 28%. This was associated with an 88% reduction in incident CHD over a period of 15 years.5 In European Americans, the LDL-C reduction was 15%, leading to a 46% reduction in CHD. Of note, more than half of the African Americans studied were hypertensive, one-third smoked, and almost 20% had diabetes (risk factors which occurred with the same frequency in mutation carriers and non-carriers). Nonetheless, the PCSK9 mutation conferred marked protection from CHD up to ~age 70, the upper age limit of the study participants at the time of screening.
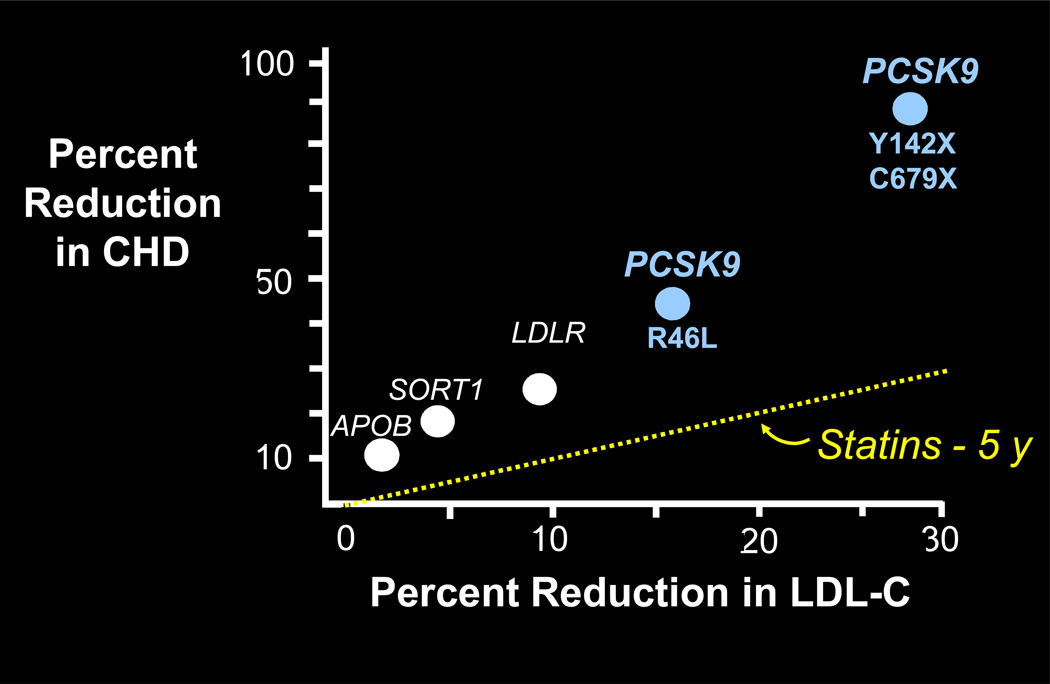
The magnitude of protection against CHD provided by the lower LDL-C level observed in those with PCSK9 loss-of-function mutations in the Dallas Heart and ARIC studies is greater than might be expected based on results from statin trials.20, 21 This discrepancy holds true in other populations, and is not peculiar to PCSK9. Variants such as APOB, SORT1, and LDLR also confer a disproportionate increase in CHD risk protection relative to the level of LDL-C, based on predictions from statin trials (Figure 3).1, 22 The reason for this may be that genotypes capture another dimension – time. The phenotype of LDL-C concentration gives a snapshot of the cholesterol concentration at the present time, but genotypes integrate the “tendency” of an individual’s LDL-C concentration since birth.21
Figure 3.
Reduction in coronary heart disease associated with LDL-C lowering according to gene variants or statin use
Abbreviations: APOB = gene for apolipoprotein B, CHD = coronary heart disease, LDL-C = low-density lipoprotein cholesterol, LDLR = gene for low-density lipoprotein receptor, PCSK9 = gene for proprotein convertase subtilisin/kexin type 9, SORT1 = gene for sortilin 1
1Linsel-Nitschke P, et al. Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease – a Mendelian Randomisation study. PLoS One. 2008;3:e2986.
22Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169.
Pragmatically, it appears that complex diseases, particularly CHD, may not actually be so complex. In the case of CHD it is clear there is a single predominant risk factor. While the value of other risk factors should not be ignored, the cardinal risk factor is high LDL-C concentration combined with the amount of time the patient has been exposed to increased LDL-C (or atherogenic LDL particles). A measure of cumulative exposure, analogous to pack-years for smokers, would provide a more accurate prediction of risk of CHD. Currently, therapeutic steps are not taken until an individual has an increased 10-year risk beyond some threshold; typically this involves a treatment delay until patients are at least 50 years of age.15 Genetic data suggest that starting treatment earlier might produce “more bang for the therapeutic buck” whether that therapy is diet or statins.
Early experiments raised considerable interest in the utility of PCSK9 for LDL-C-lowering and CHD prevention, but questions remained regarding the safety of manipulating PCSK9, particularly because it is expressed in several tissues in addition to the liver (e.g., kidneys, brain). In order to investigate whether the absence of PCSK9 would be compatible with good health, family members of individuals in the Dallas Heart Study with a single mutation in PCSK9 were identified. In one of these families, the proband was a 53-year-old woman with an LDL-C level of 49 mg/dL. Her 32-year-old daughter had an unusually low LDL-C level (14 mg/dL).23 The daughter had inherited her mother’s mutation in PCSK9, Y142X, which is a premature stop code in the protein transcript, and another mutation from her father which deleted an arginine at codon 97. For practical purposes, these mutations rendered both copies of the gene non-functional resulting in total deficiency of PCSK9. The deficiency in PCSK9 did not appear to have any adverse effects. Her intelligence was normal (she was a college graduate); she had normal kidney and liver functions and normal blood pressure. PCSK9 is expressed at fairly high levels in the cerebellum, particularly during development, but neurological examination and magnetic resonance imaging results, plus her vocation as an aerobics instructor, confirmed that the PCSK9 deficiency did not adversely affect her motor control. A follow-up six years later verified that after nearly 40 years of total absence of PCSK9 and extremely low LDL-C (14–29 mg/dL), this individual continued to be in good health. Subsequently, a 21-year-old pregnant Zimbabwean woman was identified as a compound heterozygote with two PCSK9 mutations.24 Little is known about this patient, other than she had an extremely low LDL-C level (16 mg/dL) when she visited an antenatal clinic.
The culmination of these genetic efficacy and safety studies has been the development of agents designed to inhibit PCSK9. It has proved difficult to target PCSK9 with small molecules, but several companies have developed monoclonal antibodies that inhibit PCSK9. The results of the first trial demonstrated that inhibition of PCSK9 caused a sharp, prompt, and relatively isolated reduction in LDL-C, without obvious side effects, exactly as would be predicted from the genetics.25 The rapid course of this work illustrates the utility of genetics in the development of therapeutic agents. From the initial discovery to clinical trials has taken seven years: the initial discovery of PCSK9 in 2003, to the discovery of LDL-C-lowering variants in the gene and target validation, and completion of phase one trials four years later. Several trials of PCSK9 inhibitors are now in progress.
Whole Genome Sequencing
New developments in sequencing technology have made it possible to screen the entire genome (or exome), potentially allowing the identification of disease-causing mutations in novel genes in individual patients. The power of this approach for patients with lipid disorders was illustrated by the discovery of a family with atypical hypobetalipoproteinemia in which four of the offspring had very low levels of LDL-C, HDL-C, and triglycerides. These individuals were distinguished from carriers of typical APOB mutations that prevent secretion of very low-density lipoprotein because they did not have steatosis, which almost invariably occurs in individuals with conventional familial hypobetalipoproteinemia.26 Exome sequencing revealed that the affected offspring were homozygous for a mutation in ANGPTL3, a gene which encodes angiopoietin-like 3 – an inhibitor of lipoprotein lipase and endothelial lipase.27 This finding demonstrates that astute clinical observation of individual patients and their families with atypical and unexplained lipid profiles, followed by sequencing of the affected individuals, has the potential to provide major new insights into the molecular basis of lipid diseases.
Acknowledgements
The author acknowledges his collaborators at the University of Texas Southwestern Medical Center, primarily his scientific partner, Helen Hobbs, MD, and others who worked on the projects described herein: Stefano Romeo, PhD, Julia Kozlitina, PhD, Shaoqing He, PhD, Dawei Zhang, PhD candidate, and Jay Horton, MD; also Eric Boerwinkle, PhD, University of Texas Houston, Len Pennacchio, PhD, Lawrence Berkeley National Lab, and David Cox formerly of Perlegen Sciences, Inc.
List of Abbreviations
- ABCG5
adenosine triphosphate-binding cassette sub-family G member 5
- ABCG8
adenosine triphosphate-binding cassette sub-family G member 8
- ANGPTL3
angiopoietin-like 3 gene
- Apo
apolipoprotein
- APOB
apolipoprotein B gene
- APOE
apolipoprotein E gene
- ARH
autosomal recessive hypercholesterolemia
- CHD
coronary heart disease
- CI
confidence interval
- FH
familial hypercholesterolemia
- GWAS
genome wide association studies
- HDL-C
high-density lipoprotein cholesterol
- HMG CoA Reductase
3-hyroxy-3-methylglutaryl coenzyme A reductase
- LDL-C
low-density lipoprotein cholesterol
- LDLR
low-density lipoprotein receptor gene
- MI
myocardial infarction
- OR
odds ratio
- PCSK9
proprotein convertase subtilisin/kexin type 9
- SORT1
sortilin 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Linsel-Nitschke P, Götz A, Erdmann J, Braenne I, Braund P, Hengstenberg C, Stark K, Fischer M, Schreiber S, El Mokhtari NE, Schaefer A, Schrezenmeir J, Rubin D, Hinney A, Reinehr T, Roth C, Ortlepp J, Hanrath P, Hall AS, Mangino M, Lieb W, Lamina C, Heid IM, Doering A, Gieger C, Peters A, Meitinger T, Wichmann HE, König IR, Ziegler A, Kronenberg F, Samani NJ, Schunkert H, Wellcome Trust Case Control Consortium (WTCCC); Cardiogenics Consortium Lifelong reduction of LDL-cholesterol related to a common variant in the LDL-receptor gene decreases the risk of coronary artery disease – a Mendelian Randomisation study. PLoS One. 2008;3:e2986. doi: 10.1371/journal.pone.0002986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varret M, Abifadel M, Rabes JP, Boileau C. Genetic heterogeneity of autosomal dominant hypercholesterolemia. Clin Genet. 2008;73:1–13. doi: 10.1111/j.1399-0004.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 3.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: a HuGE review. Am J Epidemiol. 2002;155:487–495. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 4.Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, Johansen CT, Fouchier SW, Isaacs A, Peloso GM, Barbalic M, Ricketts SL, Bis JC, Aulchenko YS, Thorleifsson G, Feitosa MF, Chambers J, Orho-Melander M, Melander O, Johnson T, Li X, Guo X, Li M, Shin Cho Y, Jin Go M, Jin Kim Y, Lee JY, Park T, Kim K, Sim X, Twee-Hee Ong R, Croteau-Chonka DC, Lange LA, Smith JD, Song K, Hua Zhao J, Yuan X, Luan J, Lamina C, Ziegler A, Zhang W, Zee RY, Wright AF, Witteman JC, Wilson JF, Willemsen G, Wichmann HE, Whitfield JB, Waterworth DM, Wareham NJ, Waeber G, Vollenweider P, Voight BF, Vitart V, Uitterlinden AG, Uda M, Tuomilehto J, Thompson JR, Tanaka T, Surakka I, Stringham HM, Spector TD, Soranzo N, Smit JH, Sinisalo J, Silander K, Sijbrands EJ, Scuteri A, Scott J, Schlessinger D, Sanna S, Salomaa V, Saharinen J, Sabatti C, Ruokonen A, Rudan I, Rose LM, Roberts R, Rieder M, Psaty BM, Pramstaller PP, Pichler I, Perola M, Penninx BW, Pedersen NL, Pattaro C, Parker AN, Pare G, Oostra BA, O'Donnell CJ, Nieminen MS, Nickerson DA, Montgomery GW, Meitinger T, McPherson R, McCarthy MI, McArdle W, Masson D, Martin NG, Marroni F, Mangino M, Magnusson PK, Lucas G, Luben R, Loos RJ, Lokki ML, Lettre G, Langenberg C, Launer LJ, Lakatta EG, Laaksonen R, Kyvik KO, Kronenberg F, König IR, Khaw KT, Kaprio J, Kaplan LM, Johansson A, Jarvelin MR, Janssens AC, Ingelsson E, Igl W, Kees Hovingh G, Hottenga JJ, Hofman A, Hicks AA, Hengstenberg C, Heid IM, Hayward C, Havulinna AS, Hastie ND, Harris TB, Haritunians T, Hall AS, Gyllensten U, Guiducci C, Groop LC, Gonzalez E, Gieger C, Freimer NB, Ferrucci L, Erdmann J, Elliott P, Ejebe KG, Döring A, Dominiczak AF, Demissie S, Deloukas P, de Geus EJ, de Faire U, Crawford G, Collins FS, Chen YD, Caulfield MJ, Campbell H, Burtt NP, Bonnycastle LL, Boomsma DI, Boekholdt SM, Bergman RN, Barroso I, Bandinelli S, Ballantyne CM, Assimes TL, Quertermous T, Altshuler D, Seielstad M, Wong TY, Tai ES, Feranil AB, Kuzawa CW, Adair LS, Taylor HA, Jr, Borecki IB, Gabriel SB, Wilson JG, Holm H, Thorsteinsdottir U, Gudnason V, Krauss RM, Mohlke KL, Ordovas JM, Munroe PB, Kooner JS, Tall AR, Hegele RA, Kastelein JJ, Schadt EE, Rotter JI, Boerwinkle E, Strachan DP, Mooser V, Stefansson K, Reilly MP, Samani NJ, Schunkert H, Cupples LA, Sandhu MS, Ridker PM, Rader DJ, van Duijn CM, Peltonen L, Abecasis GR, Boehnke M, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 6.Marks D, Thorogood M, Neil HA, Humphries SE. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis. 2003;168:1–14. doi: 10.1016/s0021-9150(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins PN, Toth PP, Ballantyne CM, Rader DJ. National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: prevalence, genetics, diagnosis, and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol. 2011;5(3 Suppl):S9–S17. doi: 10.1016/j.jacl.2011.03.452. [DOI] [PubMed] [Google Scholar]
- 8.Khachadurian AK. Prospects for the prenatal diagnosis of familiar hypercholesterolemia. J Med Liban. 1973;26:325–329. [PubMed] [Google Scholar]
- 9.Innerarity TL, Weisgraber KH, Arnold KS, Mahley RW, Krauss RM, Vega GL, Grundy SM. Familial defective apolipoprotein B-100: low-density lipoproteins with abnormal receptor binding. Proc Natl Acad Sci U S A. 1987;84:6919–6923. doi: 10.1073/pnas.84.19.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abifadel M, Varret M, Rabés JP, Allard D, Oufuerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Lu K, Patel SB. Genetic basis of sitosterolemia. Curr Opin Lipidol. 2001;12:141–149. doi: 10.1097/00041433-200104000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen JC, Kimmel M, Polanski A, Hobbs HH. Molecular mechanisms of autosomal recessive hypercholesterolemia. Curr Opin Lipidol. 2003;14:121–127. doi: 10.1097/00041433-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Bauer RC, Stylianou IM, Rader DJ. Functional validation of new pathways in lipoprotein metabolism identified by human genetics. Curr Opin Lipidol. 2011;22:123–128. doi: 10.1097/MOL.0b013e32834469b3. [DOI] [PubMed] [Google Scholar]
- 14.Willer CJ, Mohlke KL. Finding genes and variants for lipid levels after genome-wide association analysis. Curr Opin Lipidol. 2012;23:98–103. doi: 10.1097/MOL.0b013e328350fad2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Third report of the National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 16.Kapur NK, Ashen D, Blumenthal RS. High density lipoprotein cholesterol: an evolving target of therapy in the management of cardiovascular disease. Vasc Health Risk Manag. 2008;4:39–57. doi: 10.2147/vhrm.2008.04.01.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart AF, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomization study. Lancet. 2012;380:572–580. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 19.Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen CL, Hobbs HH. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet. 2006;78:410–422. doi: 10.1086/500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPherson R, Kavaslar N. Statins for primary prevention of coronary artery disease. Lancet. 2007;369:1078–1079. doi: 10.1016/S0140-6736(07)60516-9. [DOI] [PubMed] [Google Scholar]
- 21.Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybaerg-Hansen A. PCSK9 R46L, lowdensity lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analysis. J Am Coll Cardiol. 2010;55:2833–2842. doi: 10.1016/j.jacc.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Health SC, Timpson NJ, Najjar SS, Stringham HM, Strait J, Duren WL, Maschio A, Busonero F, Mulas A, Albai G, Swift AJ, Morken MA, Narisu N, Bennett D, Parish S, Shen H, Galan P, Meneton P, Hercberg S, Zelenika D, Chen WM, Li Y, Scott LJ, Scheet PA, Sundvall J, Watanabe RM, Nagaraja R, Ebrahim S, Lawlor DA, Ben-Shlomo Y, Davey-Smith G, Shuldiner AR, Collins R, Bergman RN, Uda M, Tuomilehto J, Cao A, Collins FS, Lakatta E, Lathrop GM, Boehnke M, Schlessinger D, Mohlke KL, Abecasis GR. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rios J, Stein E, Shendure J, Hobbs HH, Cohen JC. Identification by whole-genome resequencing of gene defect responsible for severe hypercholesterolemia. Hum Mol Genet. 2010;19:4313–4318. doi: 10.1093/hmg/ddq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193:445–448. doi: 10.1016/j.atherosclerosis.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 25.Stein EA, Mellis S, Yancopoulos GD, Stahl N, Logan D, Smith WB, Lisbon E, Gutierrez M, Webb C, Wu R, Du Y, Kranz T, Gasparino E, Swergold GD. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–1118. doi: 10.1056/NEJMoa1105803. [DOI] [PubMed] [Google Scholar]
- 26.Schonfeld G, Lin X, Yue P. Familial hypobetalipoproteinemia: genetics and metabolism. Cell Mol Life Sci. 2005;62:1372–1378. doi: 10.1007/s00018-005-4473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musunuru K, Pirruccello JP, Do R, Peloso GM, Guiducci C, Sougnez C, Garimella KV, Fisher S, Abreu J, Barry AJ, Fennel T, Banks E, Ambrogio L, Cibulskis K, Kernytsky A, Gonzalez E, Rudizicz N, Engert JC, DePristo MA, Daly MJ, Cohen JC, Hobbs HH, Altshuler D, Schonfeld G, Gabriel SB, Yue P, Kathiresan S. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010;363:2220–2227. doi: 10.1056/NEJMoa1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]