Abstract
This review provides an outline for fluorescent labeling of proteins. Fluorescent assays are very diverse providing the most sensitive and robust methods for observing biological processes. Here, different types of labels and methods of attachment are discussed in combination with their fluorescent properties. The advantages and disadvantages of these different methods are highlighted, allowing the careful selection for different applications, ranging from ensemble spectroscopy assays through to single-molecule measurements.
Keywords: Fluorescence, Single molecule, Fluorophores, Fluorescent proteins, Quantum dots
Introduction
Fluorescence-based assays are crucial experimental tools which allow the detailed dissection of molecular mechanisms. The assays can be performed in real-time, in solution, with high-time resolution and sensitivity down to single molecules. This has allowed the measurement of protein interactions, enzymatic activity, conformation changes, localization of proteins, and the ability to see individual proteins moving in real time [2, 8, 20, 42, 51, 59].
Proteins have intrinsic fluorescence due to residues such as tryptophan. However, extrinsic labels provide far greater advantages for observing biological processes. These include more flexibility in choosing the location of the label and enhanced fluorescence properties suitable for a greater number of techniques.
A fluorophore reports upon the protein it is attached to and its environment. In the simplest case, this allows proteins to be visualized or counted through photobleaching. More advanced assays can measure protein–protein interactions or conformation changes, by measuring intensity changes and fluorescence/Förster resonance energy transfer (FRET). With all of these approaches, ensemble bulk or single-molecule measurements are possible. Bulk measurements offer the greater flexibility with the least requirements in terms of fluorescence properties. Single-molecule measurements provide a greater sensitivity because the ensemble averaging can mask rare, or short-lived, states [31].
Before selecting a label, it is important to define the type of experiment to be performed: Single molecule or ensemble spectroscopic measurements? Monitoring the protein of interest or use a fluorescent biosensor? Measure a conformation change, a FRET signal or visualize a protein? Each category has specific requirements for the label (Table 1). However, there are some general features. Ideally, a fluorescent label should be small, bright, and stable, without any perturbation to the biological system. Furthermore, the label should be specific without the tendency to oligomerize and with the possibility to label multiple proteins at the same time. Unfortunately, matching all of these criteria is not always possible and compromises have to be made. There are no perfect labels and new methods are constantly in development.
Table 1.
Phore requirements
| Application | Methodology | Labeling requirements | Example fluorophore | Problems/recommendations |
|---|---|---|---|---|
| Conformation change | Intensity change | Site-specific cysteine | MDCC (Coumarin) | Requires an environmentally sensitive fluorophore. |
| FRET/FLIM | Site-specific cysteine | Cy3/Cy5 | Requires sufficient spectral overlap and fluorescent proteins are best for large rearrangements. | |
| fluorescent protein | GFP/RFP | |||
| Single-molecule FRET | Site-specific cysteine | Cy3/Cy5 | Fluorophores must be photostable and bright. | |
| Protein–protein interactions | Intensity change | Site-specific cysteine | MDCC (Coumarin) | Requires an environmentally sensitive fluorophore. |
| Anisotropy | Site-specific cysteine | Fluorescein | Label must be small and fluorescence lifetime ∼5 nsec. | |
| N-terminal amine | Can be attached away from interaction site. | |||
| Peptide tag | ||||
| FRET/FLIM | Site-specific cysteine | Fluorescein/rhodamine | Fluorophores can be positioned away from the site of interaction to prevent interference. | |
| Fluorescent protein | GFP/RFP | |||
| Single-molecule FRET | Site-specific cysteine | Cy3/Cy5 | ||
| Single-molecule/particle tracking | Single-molecule imaging | Site-specific cysteine | Cy3B | Fluorophores can be spaced away from protein and targeted to inert areas. |
| Peptide tag | ||||
| N-terminal amine | ||||
| Biotinylation | Quantum dot | Large label so may interfere with activity. | ||
| Can be used in live cells with recombinant proteins. | ||||
| Fluorescent protein | eGFP | Use only for rapid processes because photobleaching is fast. | ||
| Fluorescent proteins are easiest to use for live cell measurements. | ||||
| Protein counting | Photobleaching | Fluorescent protein | eGFP | Use monomeric fluorescent proteins. Only fluorescent proteins give a defined stoichiometry. |
| Live cell localization | Live cell imaging | Fluorescent protein | eGFP | Use monomeric fluorescent protein. |
| Peptide tag | FlAsH | Organic fluorophores are brighter and more stable than fluorescent proteins but the labeling is less specific. | ||
| Peptide tag | SNAP-Tag |
Today, a range of approaches are available for labeling proteins, genetically encoded tags, small organic fluorophores, and large, bright quantum dots (Qdots). There is also a variety of attachments to proteins with varying degrees of specificity. In this review, different labeling approaches will be discussed (Fig. 1), so that a majority of the ideal criteria can be met and the best method for the required application can be chosen.
Fig. 1.
Methods for fluorescent labeling of proteins. a A generic scheme for labeling the protein of interest (POI). A fluorophore is attached to reactive group A. The complementary reactive group B is attached to the POI for labeling. b Reaction with a maleimide-conjugated fluorophore and cysteine residue on POI. c Reaction with a succinimidyl-ester conjugated fluorophore and N-terminal amine on POI. d Example reaction of peptide ligation. A thioester on the POI is ligated to a fluorescent peptide through an N-terminal cysteine residue. e Example of a self-labeling tetracysteine tag which binds to the bis-ARSenic fluorescein (FlAsH) probe. f Example of a self-labeling protein tag, the SNAP-Tag. The fluorescent O6-benzylguanine is cleaved by hAGT resulting in the fluorophore being covalently linked to the hAGT and POI. g Biotinylation of the POI at the biotin recognition sequence (BRS) by BirA and conjugation with streptavidin–fluorophore or functionalized quantum dot
Flurophore properties
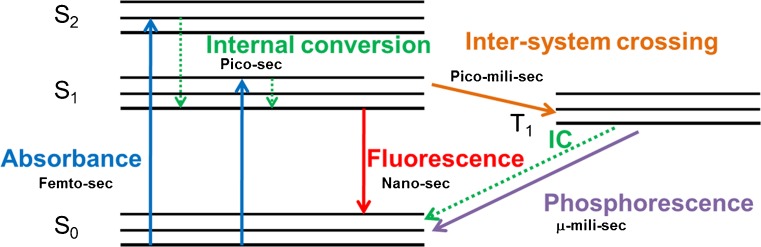
In order to be able to select the most suitable label for a particular application, it is important to be able to compare the fluorescent candidates. Several fluorophore properties are highlighted below and to understand their effect, it is important to consider the excitation and emission cycle of a fluorophore (Fig. 2) [34, 54]. In brief, when a fluorophore absorbs a photon, it is excited to high-energy singlet states (S1 and/or S2). The fluorophore can only emit light as fluorescence from the lowest energy level of S1. Therefore, the molecule must relax to reach the lowest level of S1. This process is termed internal conversion, a nonradiative loss of energy. The molecule can also cross from the singlet state to the triplet state (T1) through intersystem crossing. Relaxation from T1 back to the ground state occurs through nonfluorescent processes or phosphorescence. These states and processes are important parameters associated with the fluorescent properties discussed below.
Fig. 2.
Excitation and emission of a fluorophore. Modified Jablonski diagram representing the excitation and emission cycle of a fluorophore
Fluorophore brightness
A fluorophore’s brightness is defined by two parameters, its extinction coefficient and quantum yield. To compare the brightness of different fluorophores, the relative brightness of fluorophore is taken by multiplying the extinction coefficient with the quantum yield [64]. Comparing eGFP with Cy3B, the relative brightness is 33,000 and 91,000, respectively, clearly showing that Cy3B is threefold brighter.
The extinction coefficient is a measure of the ease in which a fluorophore absorbs light to enter the excited state. Therefore, a high extinction coefficient will lead to a greater amount of light being absorbed. Once a photon has been absorbed, the probability of a photon being emitted through fluorescence is related to the quantum yield. This is a ratio of the number of photons emitted compared to the number absorbed. More precisely, it is derived from a ratio of the rate of fluorescence, over all rates of deactivation that include nonradiative decay and intersystem crossing to the triplet state (Fig. 2) [34, 54]. A molecule with a low rate of deactivation compared to fluorescence will have a high quantum yield. Therefore, a molecule with a high quantum yield and extinction coefficient is the brightest.
The quantum yield and extinction coefficient are frequently affected by the environmental factors such as pH, solvents, and viscosity. Furthermore, they are also affected by structural features of the fluorophore whereby rigid, planar molecules have a higher fluorescence [54]. Using Cy3 as an example, the elongated structure of the two aromatic groups, linked via a polymethylene chain, switches between cis/trans isomers. This isomerization results in a high degree of nonradiative decay which reduces the quantum yield. The isomerization is removed in a similar fluorophore Cy3B, resulting in a brighter molecule. Likewise, when the rotational freedom of Cy3 is reduced, either by a more viscous solvent, or by being confined to a binding site, then the quantum yield will increase.
Fluorescent lifetime
The fluorescent lifetime is the average time the molecule spends in the excited state. This is determined by the rates of fluorescence, internal conversion, and intersystem crossing to the triplet state (Fig. 2). This time is highly variable and dictates how long the molecule has to react with the environment. The lifetime is dependent upon the environment and corresponding changes in lifetime correlate with intensity changes.
Several techniques exist to measure the lifetime of the fluorophore. In one method, the fluorescence is recorded in the time domain, following pulse excitation from a laser source. The lifetime is the time taken for the fluorescence signal to decay to 1/e or to 36.8 % of the original signal. The lifetime can also be measured in the frequency domain. In this instance, the sample is excited by a modulated light source. The fluorescence is modulated and phase-shifted from the original waveform based upon the lifetime of the fluorophore [34, 40, 54].
Stoke’s shift
The loss of energy due to nonradiative decay before release of a photon leads to emission maximum at a lower energy (higher wavelength) than the excitation. The difference between the excitation and emission wavelength is the Stoke’s shift, and the degree of shift is related to the molecular structure of the fluorophore [34, 54]. Cy3 and Cy3B are again good examples, whereby molecules with a greater internal flexibility (Cy3) lose a high amount of energy to nonradiative decay before release of a photon. Therefore, the Stoke’s shift of Cy3 is larger than that of Cy3B, 14 and 11 nm, respectively [33, 35, 43]. In general, larger Stoke’s shifts are useful because the emission peak is further from the excitation source.
Fluorophore size
With organic fluorophores, those in the UV/short visible range are very small, single or two-ring structures, such as coumarin-based fluorophores. As wavelength increases, so does the size of the fluorophore. The larger fluorophores such as Cy3B, which are 5× larger than tryptophan, are more likely to perturb the activity of the labeled protein. Fluorescent proteins are very large labels (30 kDa/4 nm) and some have a tendency to oligomerize into tetramers [6]. And Qdots are even larger (15–50 nm). In both of these cases, the label may be larger than the protein of interest. Therefore, controls must be performed to determine the effects of the labeling on the structure and biological function of the protein.
Photostability
Fluorophores will go through many cycles of excitation and emission but all will eventually be irreversibly deactivated or photobleached. During these cycles, approximately 1:1,000 excitations leads to a transition from the singlet state (S1/S2) to the triplet state (T1; Fig. 2) [26]. This state is long-lived (in millisecond) and the fluorophore will not emit again until it reaches the ground state and is re-excited. These “dark” periods give rise to the phenomena of fluorophore blinking. It is during the long-lived triplet state that the fluorophore can react with oxygen and free radicals leading to irreversible deactivation. Established methods exist to remove oxygen from solution using scavenger enzymes such as glucose-oxidase and catalase. Triplet state quenchers, such as trolox and redox systems such as methyviologen and ascorbic acid can further reduce the photobleaching rate by quenching the highly reactive state [26, 49, 63].
Fluorophore longevity may range from desired to required depending upon the experiment being performed. In the latter case, the fluorophore has to be chosen carefully to ensure low blinking and bleaching rates. Also, optimization of the scavenger system and triplet state quencher has to be performed specifically for the fluorophore chosen.
Types of fluorescent labels
Genetically encoded labels
Green fluorescent protein (GFP) from Aequorea victoria was first used as a fluorescent marker in the early 1990s [13, 55]. Soon after, individual GFPs were imaged, opening up the prospect of single-molecule assays [16, 47]. Since then, a collection of fluorescent proteins have been developed and more are continually available providing additional wavelengths, increased stability, and higher brightness [52, 57]. More recent developments include photoactivatable fluorescent proteins such as mCherry and mEOS [37].
One should keep in mind that certain fluorescent proteins, such as pZsGreen and dsRed, even GFP, do have a tendency to oligomerize, and therefore the appropriate controls should be performed. Recently, mutant variants have been developed to reduce this effect [6, 12, 52].
Aside from the typical fluorescent proteins, a system has been developed to combine fluorescent microscopy with electron microscopy (EM). The mini-Singlet Oxygen Generator system is a small fluorescent flavoprotein based upon Arabidopsis phototropin 2 [56]. When illuminated with blue light, singlet oxygen is created which polymerizes diaminobenzidine, which in turn is stainable for EM imaging. This provides a specific labeling for EM without the need of large antibodies and gold particles.
The target protein can be easily fused through recombinant cloning to the fluorescent protein at the N or C terminus with a fixed stoichiometry (Fig. 1a) [15, 53, 62]. This is also the most specific approach for labeling proteins that results in no background signal from free fluorophore. However, protein degradation can generate a background signal.
Organic fluorophores
Organic fluorophores have superior properties over fluorescent proteins such as a wider spectral range, smaller size, greater photostability, and, in many cases, higher brightness.
Coupling the label to the target protein can be achieved by direct and indirect labeling methods. The greatest problem is controlling the specificity of the labeling and also defining its stoichiometry. In addition, labeling is unlikely to reach 100 % and the presence of free dye is problematic resulting in high background, or nonspecific, signals. Therefore, it is important to be able to purify the labeled from the unlabeled protein. Since labeling is likely to change the electrostatic properties of the protein, ion exchange chromatography can usually be applied. Also, the low molecular weight-free dyes can easily be removed by size-exclusion chromatography. In addition, indirect labeling methods through peptides containing additional purification tags can provide further means of purifying the final construct.
Direct labeling with organic fluorophores
Direct chemical labeling of purified proteins targets cysteine and amine groups (Fig. 1b and c). Cysteines provide the greatest flexibility for choosing the location of the labeling due to their low frequency. Maleimide–fluorophore conjugates are highly specific for the thiol group of cysteine and the reactions are rapid (10 min–2 h) in typical buffers with moderate pH and temperatures. Another conjugate, iodoacetamides, are frequently used to target thiols due to their tolerance to reducing agents such as dithiothreitol [23], which are used before labeling cysteines to ensure that the residues are in their reduced form.
Cysteines can also be introduced at specific sites for labeling using site-directed mutagenesis to convert, for instance, a surface-exposed residue to a cysteine. In general, this should only be performed on proteins with sufficient structural information, allowing defined targeting of fluorophores. However, even under the best circumstances, there will be elements of trial and error.
Complications with labeling cysteines arise from the presence of multiple target residues. Additional residues should be removed by site-directed mutagenesis but care must be taken not to perturb the overall protein structure and/or activity. If the protein has no cysteine in the desired location, then a Cys-free version of the protein can be created before additional mutagenesis to introduce the residue at the chosen location. Other approaches to deal with this problem are based upon measuring the reactivity of each surface-exposed cysteine and then optimizing conditions to selectively label the desired residue. Obviously, this relies upon having differently reactive cysteines which is not always possible [50]. If structural information is available, then it may be possible to selectively label a cysteine following selection of different conformations [4]. A relatively new approach takes advantage of the fact that cysteine also bind to metal ions. This method is based upon the reversible protection of cysteine residues using binding of a group of 12 metal ions (Cd2+ or Zn2+) allowing labeling at a defined site [48]. Additional metal-binding amino acids (histidine) have to be incorporated next to the chosen cysteine residue to favor metal binding at that site. While bound to the metal ion, all other surface exposed cysteines are reacted with non-fluorescent N-ethylmaleimide. The metal ion can then be removed and the chosen site is labeled with the desired fluorophore.
Amine-reactive conjugates, such as succinimidyl-esters or isothiocyanates, can be used to label lysine residues or N-terminal amines. Lysine residues are very frequent in proteins and therefore labeling is likely to result in binding many fluorophores. However, it is possible to specifically target the N-terminal α-amino group due to a lower pKa of 7 compared to the amino groups of lysine (pKa 10–11). This facilitates successful labeling at a specific, but limited location [3, 21].
Peptide tags for organic fluorophores
There are many different types of labeling strategies that can fall into this category. However, there are two main approaches: label a synthetic peptide which is then attached to the target protein through ligation or genetically engineer peptide tags onto the target protein which are then reacted with the corresponding fluorophore conjugates. This approach is useful for proteins sensitive to internal labeling or mutagenesis.
Using C-terminal labeling as an example, the technique of express protein ligation can be used (Fig. 1d) [7]. To achieve this, the target protein has to be produced with a C-terminal thioester. This is created by harnessing the ability of some proteins to remove internal fragments (Inteins) which separate two other fragments (Exteins). Removal of the intein and ligation of the exteins generates the final product. In this case, the protein of interest is expressed in combination with a self-splicing intein with a cysteine residue placed between them. Therefore, in the presence of thiols (2-mercaptoethanesulfonic acid), the intein is spliced generating the protein of interest with a C-terminal thioester. This protein is then ligated through an N-terminal cysteine to a synthetic peptide carrying a fluorophore. Typically, a purification tag (Hexa-His) is included for easy isolation of the final labeled protein.
Short, self-labeling peptides, for example tetracysteine (Fig. 1e) [24] and Hexa-His, react with corresponding fluorescent bis-arsenic fluorescein (FlAsH) probes and Ni2+-NTA [32] functionalized fluorophores, respectively. Several peptide tagging approaches require enzymes to catalyze the reaction, thereby improving the specificity. This includes the Q-tag (PKPQQFM), whereby transglutamase covalently attaches to the tag primary amine-functionalized fluorophores, such as cadavarine conjugates to glutamate residues [36].
A final example of peptide labeling is biotinylation by BirA (Fig. 1g), which covalently attaches biotin to a lysine in a peptide tag containing the biotin recognition sequence (BRS) [14, 29]. This tag can be ligated to the protein or it can be genetically engineered at the N or C terminus. In addition, BirA biotinylation can be performed in vivo and then the final product can be extracted. Alternatively, biotin can also be purchased with reactive groups to target thiol or amine groups [18] but the specificity is lower compared to using BirA. The biotin–streptavidin system provides a diverse method for labeling proteins not just with streptavidin-functionalized organic fluorophores but also with streptavidin-functionalized Qdots or beads.
Protein tags for organic fluorophores
Protein tags such as Halo [38], SNAP [58], and CLIP [22] are self-labeling enzymes that covalently link to fluorescently labeled substrates. These tags are highly specific and many commercial kits are available making the process relatively easy. The SNAP tag was released in 2003 and is one of the most used approaches. This system utilizes the human DNA repair enzyme O6-alkylguanine DNA alkyltransferase (hAGT). hAGT, with a mass of 20 kDa, is genetically linked to the target protein (Fig. 1f). Fluorescently labeled O6-benzylguanine substrates are used to label the protein of interest. hAGT cleaves the benzylguanine and then covalently attaches the fluorophore.
Nanoparticle labels
Qdots are semiconductor nanocrystals made from cadmium and selenium cores with zinc–sulfur outer shells. Qdots have favorable fluorescent characteristics, for instance they are up to 20× brighter and orders of magnitude more photostable than organic fluorophores [1, 41]. This is achieved from a combination of a high quantum yield and high extinction coefficient.
Importantly, Qdots have a very narrow emission peak compared to all other forms of labels. A broad emission is a common problem with organic fluorophores because this increases spectral overlap if more than one fluorophore is used. In addition, Qdots have a very broad excitation, with an increased absorbance in the blue range. This allows a greater freedom for selecting the excitation source and gives rise to an apparently large Stoke’s shift.
Qdots can be purchased with functionalized shells enabling easy, rapid coupling to proteins, typically via biotin, or antibodies, but carboxyl and amine-reactive groups are also available. Functionalized Qdots are rather large by 15–50 nm diameters, significantly larger than fluorescent proteins and potentially larger than many proteins studied. The wavelength is controlled by the size of the Qdot; therefore, the exact size is depended upon wavelength and functionalization used.
As with organic fluorophores, Qdots have to be coupled to the protein of interest. This leads to the same problems which relate to purification of the labeled versus unlabeled protein, and separation from unreacted Qdots. Typically, size exclusion methods do not apply because of the large size of the functionalized Qdots. Therefore, the original purification strategy will have to be performed. However, quick methods may exist that are specific to the protein of interest. Using myosin motors as an example, it is possible to separate the labeled motors from free Qdot by the addition of F actin. The F actin–myosin motor complex is then pelleted by centrifugation. Then, addition of ATP causes the release of myosin motors from the F actin, once the excess Qdots in the supernatant are removed.
Types of measurements
Among the measurements described below, some may directly utilize the fluorescence of the fluorophore, like for instance in the case of measuring intensity changes, visualizing, and tracking a molecule. Other methods such as FRET, fluorescence lifetime imaging microscopy (FLIM), and anisotropy/polarization require specialized approaches, analysis, and interpretation.
FRET is a mechanism of energy transfer between donor and acceptor fluorophores [25]. Energy transfer occurs when two fluorophores with overlapping donor emission and acceptor excitation spectra are in close proximity (<10 nm). Upon exciting the donor molecule, there is nonradiative energy transfer to the acceptor. Therefore, fluorescence from the acceptor is only observed when the fluorophores are in close proximity. The efficiency of energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, meaning that FRET is very sensitive to small distance changes. The fluorescence lifetime of the donor fluorophore is reduced during energy transfer, a process that can be imaged using FLIM [17]. FLIM builds an image based around differences in the exponential decay of fluorescence (the lifetime). This method is particularly useful because it can discriminate fluorescent intensity changes due to the local environment and it is insensitive to the concentration of the fluorophores.
Anisotropy measurements are based upon the rotation (rotation correlation time) of a fluorescent species within its fluorescence lifetime, described in detail [35]. Two parameters are crucial for these measurements: the fluorescence lifetime and the size of the label. If the lifetime is too short, the population will appear highly anisotropic, whereas, if it is too long, the species will have low anisotropy. Fluorescein with a lifetime of 4 ns is useful for this application. Anisotropy measurements are particularly suited when one protein is significantly smaller than the other. When binding to the larger protein, the anisotropy of the smaller unit increases because the larger complex has a slower rotation correlation time. This provides a sensitive measurement of complex formation. However, when a large label is used, as for instance a fluorescent protein, then the rotation is inherently slow giving rise to high anisotropy values, which compromises the sensitivity of the measurements. Therefore, they should be avoided.
The five examples below represent a selection of fluorescence measurements. In each case, the specific requirements of the fluorophores and labeling are discussed.
Conformation changes
Conformation changes occur in proteins for a variety of reasons: change in environment, enzymatic activity, or response to binding events. This diversity is matched by a variety of methods by which to measure the rearrangements. These include monitoring intensity changes and FRET (steady-state/single molecule/FLIM) by using organic fluorophores or fluorescent proteins.
Some experimental strategies take advantage of the environmental sensitivity of certain fluorophores. With respect to organic fluorophores, when labeling sites are more polar than the surrounding solvent, the quantum yield and/or extinction coefficients can increase leading to large enhancements in the fluorescent signal. This has been particularly harnessed in the design of reagent-less biosensors, for example the phosphate-binding protein (MDCC-PBP) [9], widely used to study ATPases and GTPases [46, 60, 61]. In this example, the Escherichia coli PBP was specifically labeled with the coumarin fluorophore, close to the phosphate binding site. Phosphate from ATP cleavage binds rapidly leading to a large conformation change, with the protein closing around the phosphate and the fluorophore. The change in solvent exposure is coupled with a structural confinement, resulting in a planar molecule. The most prominent effect is an eightfold increase in coumarin’s quantum yield. Overall, this leads to a large fluorescent increase of up to 13-fold.
This use of organic fluorophores is versatile and beneficial due to the small size of both the coupling and fluorophore itself. Contrarily, the use of fluorescent proteins, peptide tagging, and the majority of amine labeling reactions, has limited application since labeling occurs at the ends of the protein. Therefore, defined location rearrangements are best observed using cysteine labeling.
Fluorescent proteins are advantageous because there is no free dye in solution which can reduce the overall intensity changes observed with organic fluorophores. But, the large label size and limited choice of labeling sites prevent a targeted approach. Fluorescent proteins can also display environmental sensitivity depending on the conditions. Binding the labeled protein to lipid can provide measureable signals [19] and GFP exhibits a pH sensitivity [5] which can be harnessed as a useful sensor. However, these changes generally refer to global rearrangements in the protein and not local effects. Therefore, fluorescent proteins are best used in FRET assays. For large conformation changes, two fluorescent proteins (e.g., CFP-YFP or GFP-RFP) can be used as a FRET pair. This is particularly useful if the protein unfolds following substrate binding [11, 42]. An advantage of the fluorescent proteins over other fluorophores is the ease at which the same labels can be used in vitro and in vivo, allowing a direct comparison between the assays. In vivo experiments allow FRET to be measured by intensity or lifetime through FLIM [11]. However, FRET measurements, especially at single molecule level can be compromised by poor photophysical properties of the fluorescent proteins. This is especially the case when rapid photobleaching gives rise to false FRET changes.
The specific, targeted labeling by organic fluorophores is the most widely used application for in vitro FRET measurements. Two cysteine labels at specific locations are usually chosen or introduced by mutagenesis. For labeling, bright and stable fluorophores with sufficient spectral overlap, such as Cy3 and Cy5, are chosen for single-molecule measurements. However, these are large fluorophores that may interfere with the conformation change. Therefore, ensemble spectroscopic assays provide greater freedom for choosing smaller fluorophores as the FRET pair.
In general, one should keep in mind that it is possible that the Stoke’s shift of one or both of the fluorophores changes during the conformation change. With FRET measurements, the degree of spectral overlap is directly related to the fluorescent signal; therefore, any changes in Stoke’s shift will give false FRET changes. Likewise, fluorescent intensity changes attributed to environmental sensitivity may complicate the FRET signal, making it difficult to interpret the conformation change from additional local rearrangements in the protein.
Protein–protein interactions
The protein of interest may be regulated by additional proteins, transported by cytoskeletal motors, or be involved in multiprotein signaling pathways. In all cases, monitoring the interaction provides mechanistic details. The methodology to observe these interactions are similar to those for measuring a conformation change.
Firstly, intensity changes can be utilized. This would usually require organic fluorophore labeling of a cysteine positioned close to the site of interaction. Only one of the proteins involved has to be labeled, thereby simplifying the procedure and reducing potential side effects. It is important that the fluorescent species is purified to a high level, so that the signal is not diminished due to a large unlabeled contaminating species.
Similarly to intensity change measurements, anisotropy requires the use of a single organic fluorophore. In this way, the smaller of the two proteins can be labeled either near the binding site (for compatibility with intensity measurements), in an inert area, or even at the N or C terminus. Anisotropy measurements are particularly useful if there are more than one fluorescent intensity change, possibly in response to binding and a conformation change. Anisotropy will only report upon the binding measurements, therefore separating the two processes.
As with measuring conformation changes, FRET is widely used with organic fluorophores and fluorescent proteins. With respect to organic fluorophores, FRET is relatively easy because each protein requires a single label in an inert area of the protein but within 10 nm of the corresponding label when bound in the heterodimer.
Single molecule/particle tracking
Biological systems are highly dynamic environments with molecules diffusing around the cell or undergoing active transport along protein or nucleic acid tracks. It is important to understand the behavior of individual proteins as they move throughout the cell and only single molecule methods can provide these answers.
For single-molecule imaging, the greater the number of photons detected improves the accuracy of localization. Therefore, the fluorescent lifetime is particularly important. A long fluorescence lifetime will reduce the number of excitation and emission cycles, which in turn reduces the overall number of photons detected within a given time frame. For this reason, fluorophores with short lifetimes are preferable (<5 ns) [26]. In addition, smaller-sized fluorophores will reduce interference with the system and therefore organic fluorophores are the best candidates. Currently, there is a large selection of fluorophores to choose from depending on their compatibility with the available laser lines and need for multicolor imaging.
Organic fluorophores can be coupled to the protein of interest directly to a native cysteine, however terminal labeling is likely to reduce any possible side effects of internal labeling. Peptide tagging not only allows terminal labeling of the protein, but also allows multiple fluorophores to be attached to the peptide. Through attaching multiple labels, it is possible to enhance the overall brightness of the protein, making detection easier.
Overall, fluorophore stability is the major requirement for tracking. Therefore, fluorophore must have a low bleaching rate; ideally, this would be slower than the detachment rate of the protein from its track. Intensity fluctuations or fluorophore blinking between bright and dark states can also perturb tracking. This can be overcome with using suitably optimized oxygen scavengers and triplet state quenchers [26]. Alternatively, if multiple labels are present, blinking will not be synchronized and therefore, it will always be possible to visualize the protein.
Fluorescent proteins can be used in vitro but are best applied to tracking proteins in live cells, especially with superresolution microscopy [39]. In comparison with the organic fluorophores, they present the advantage of a fixed stoichiometry which is beneficial for addressing mechanistic questions, like for instance how the movement of vesicles is generated by a known number of molecules. However, their low brightness prevents tracking of movement with high-time resolution. Methods exist to improve the brightness of the target protein by genetically coupling several fluorescent proteins together [10]. However, although the brightness is enhanced by several fold, this approach obviously increases the overall label size. A further problem for single-molecule observations is that fluorescent proteins have the tendency to enter dark states which can last for several hundred miliseconds and provide gaps in single-molecule trajectories [16]. In terms of stability, enhanced fluorescent proteins, such as eGFP, are brighter, but they can frequently be observed only for 10 s before they are photobleached, which poses additional limitations for single-molecule tracking.
Quantum dots are the most stable forms of label, since under most circumstances they do not bleach. The coupling mechanism through biotin–streptavidin is very simple and stable, requiring a single modification to the protein of interest. However, the coupling can easily result with up to 10 proteins bound to one Qdot. Although the number of proteins per Qdot can be control by dilutions, a precise loading is not possible. Therefore, if the number of proteins is an important parameter then this approach is not applicable. Qdots can be introduced into cells by osmotic shock while coupled to a protein of interest and provide a highly sensitive method for single-molecule tracking in live cells [1, 44, 45]. However, it is important to note that this approach uses recombinant purified proteins rather than the natural cellular proteins which are observed with fluorescent proteins.
While providing very bright objects suitable for tracking, Qdots possess a detrimental blinking behavior which means that automated tracking is limited. However, blinking can be controlled by using thiols which significantly decrease the dark states [28]. Moreover, the broad excitation range can be problematic if fluorescent proteins or other organic fluorophores are also used in the assays. Likewise, it prevents Qdots from being used a FRET acceptors. Finally, Qdots are in essence the largest labels available and therefore, unlike organic fluorophores, may impede movement. Overall, a decision has to be made based on whether tracking for extended periods is more important than potential side effects from the size and/or multiple proteins bound to the label.
Protein counting through photobleaching
A protein’s oligomeric state is frequently a controversial point, especially with regards to molecular motors. Biochemical/biophysical characterization using size exclusion chromatography or analytical centrifugation can provide strong evidence for the oligomeric state. But, counting the number of molecules through single-molecule photobleaching provides definitive answers.
The most important parameter for protein counting is to have a known stoichiometry between the label and protein of interest. Fluorescent proteins are the only type of label that ensures a fixed stoichiometry [30]. In addition, the relatively rapid photobleaching is advantageous for performing the measurements. Obviously, it is important to ensure that only monomeric fluorescent proteins are used, such as the monomeric mutant of eGFP which is also bright enough for easy observation.
Using organic fluorophores can provide a brighter signal. The enzyme-coupled labeling with protein tags is highly specific and useful for proteins which contain more than one cysteine. The greatest problem with these forms of labeling is the presence of contaminating unlabeled protein which invalidates attempts to count proteins in a complex. Even the best forms of purification cannot ensure a homogeneous preparation and labeling is unlikely to reach 100 %. While for ensemble measurements, this would not be a great problem, single-molecule sensitivity is too high for such contamination.
Localization in live cells
It is vitally important to be able to visualize proteins in their cellular environment, because in this way it is possible to determine function, regulation, and interactions. Fluorescent proteins are by far the most frequently used labeling methods for in vivo imaging. The fluorescent proteins are easily coupled on to the protein of interest with 100 % specificity. It must be noted that biological breakdown of the fusion proteins may generate background signals leading to diffuse or poor localization. In addition, it is important to use monomeric fluorescent proteins because oligomers could inhibit, or even enhance function. Furthermore, as with all uses of fluorescent proteins, the large size may perturb activity and unless visualizing single molecules for long time scales, the fluorescent properties are perfectly adequate.
Organic fluorophores provide many advantages for in vivo imaging. Currently, indirect labeling techniques, such as FlAsH, are possible for introducing organic fluorophores to their targets [24]. The peptide tag and the FlAsH probe are very small, therefore reducing any complications from the labeling. However, the probe is not always highly specific for the target. Alternatively, protein tags have been successfully used in live cell experiments and superresolution imaging [27]. This approach is very useful because it allows labeling with high specificity. However, in these cases, the labeling procedure still requires the addition of a large protein tag to the protein of interest. Therefore, the enhanced fluorescent properties of this tag must be advantageous for the assay over the defined stoichiometry and ease of using the fluorescent proteins. With both peptide and protein tagging approaches, an amount of unreacted free dye will still remain generating background noise.
Conclusions
Many new strategies exist for labeling proteins providing even more opportunities for different types of experiments. However, no fluorescent label is perfect nor are the methods by which they are attached to proteins. The greatest challenges remain: higher photostability, enhanced brightness, and minimal perturbations due to the labels. New, more stable and small fluorophores are still required to allow a better observation of biological processes.
Acknowledgments
Thanks Dr. Natalia Fili for helpful comments on the manuscript and EMBO for funding.
References
- 1.Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55–76. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 2.Altman D, Goswami D, Hasson T, Spudich JA, Mayor S. Precise positioning of myosin VI on endocytic vesicles in vivo. PLoS Biol. 2007;5(8):e210. doi: 10.1371/journal.pbio.0050210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amitani I, Liu B, Dombrowski CC, Baskin RJ, Kowalczykowski SC. Watching individual proteins acting on single molecules of DNA. Methods Enzymol. 2010;472:261–291. doi: 10.1016/S0076-6879(10)72007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antia M, Islas LD, Boness DA, Baneyx G, Vogel V. Single molecule fluorescence studies of surface-adsorbed fibronectin. Biomaterials. 2006;27(5):679–690. doi: 10.1016/j.biomaterials.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Arosio D, Ricci F, Marchetti L, Gualdani R, Albertazzi L, Beltram F. Simultaneous intracellular chloride and pH measurements using a GFP-based sensor. Nat Methods. 2010;7(7):516–518. doi: 10.1038/nmeth.1471. [DOI] [PubMed] [Google Scholar]
- 6.Baird GS, Zacharias DA, Tsien RY. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proc Natl Acad Sci U S A. 2000;97(22):11984–11989. doi: 10.1073/pnas.97.22.11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker CF, Seidel R, Jahnz M, Bacia K, Niederhausen T, Alexandrov K, Schwille P, Goody RS, Engelhard M. C-terminal fluorescence labeling of proteins for interaction studies on the single-molecule level. ChemBioChem. 2006;7(6):891–895. doi: 10.1002/cbic.200500535. [DOI] [PubMed] [Google Scholar]
- 8.Bianco PR, Brewer LR, Corzett M, Balhorn R, Yeh Y, Kowalczykowski SC, Baskin RJ. Processive translocation and DNA unwinding by individual RecBCD enzyme molecules. Nature. 2001;409(6818):374–378. doi: 10.1038/35053131. [DOI] [PubMed] [Google Scholar]
- 9.Brune M, Hunter JL, Howell SA, Martin SR, Hazlett TL, Corrie JE, Webb MR. Mechanism of inorganic phosphate interaction with phosphate binding protein from Escherichia coli. Biochemistry. 1998;37(29):10370–10380. doi: 10.1021/bi9804277. [DOI] [PubMed] [Google Scholar]
- 10.Cai D, Verhey KJ, Meyhofer E. Tracking single kinesin molecules in the cytoplasm of mammalian cells. Biophys J. 2007;92(12):4137–4144. doi: 10.1529/biophysj.106.100206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calleja V, Alcor D, Laguerre M, Park J, Vojnovic B, Hemmings BA, Downward J, Parker PJ, Larijani B. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5(4):e95. doi: 10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci U S A. 2002;99(12):7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 14.Chen I, Howarth M, Lin W, Ting AY. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods. 2005;2(2):99–104. doi: 10.1038/nmeth735. [DOI] [PubMed] [Google Scholar]
- 15.Davidson MW, Campbell RE. Engineered fluorescent proteins: innovations and applications. Nat Methods. 2009;6(10):713–717. doi: 10.1038/nmeth1009-713. [DOI] [PubMed] [Google Scholar]
- 16.Dickson RM, Cubitt AB, Tsien RY, Moerner WE. On/off blinking and switching behaviour of single molecules of green fluorescent protein. Nature. 1997;388(6640):355–358. doi: 10.1038/41048. [DOI] [PubMed] [Google Scholar]
- 17.Dong CY, French T, So PT, Buehler C, Berland KM, Gratton E. Fluorescence-lifetime imaging techniques for microscopy. Methods Cell Biol. 2003;72:431–464. doi: 10.1016/S0091-679X(03)72021-4. [DOI] [PubMed] [Google Scholar]
- 18.Elia G (2010) Protein biotinylation. Current protocols in protein science/editorial board, John E Coligan et al. (eds). doi:10.1002/0471140864.ps0306s60 [DOI] [PubMed]
- 19.Feeser EA, Ignacio CM, Krendel M, Ostap EM. Myo1e binds anionic phospholipids with high affinity. Biochemistry. 2010;49(43):9353–9360. doi: 10.1021/bi1012657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fili N, Mashanov GI, Toseland CP, Batters C, Wallace MI, Yeeles JT, Dillingham MS, Webb MR, Molloy JE. Visualizing helicases unwinding DNA at the single molecule level. Nucleic Acids Res. 2010;38(13):4448–4457. doi: 10.1093/nar/gkq173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galletto R, Amitani I, Baskin RJ, Kowalczykowski SC. Direct observation of individual RecA filaments assembling on single DNA molecules. Nature. 2006;443(7113):875–878. doi: 10.1038/nature05197. [DOI] [PubMed] [Google Scholar]
- 22.Gautier A, Juillerat A, Heinis C, Correa IR, Jr, Kindermann M, Beaufils F, Johnsson K. An engineered protein tag for multiprotein labeling in living cells. Chem Biol. 2008;15(2):128–136. doi: 10.1016/j.chembiol.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Getz EB, Xiao M, Chakrabarty T, Cooke R, Selvin PR. A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry. Anal Biochem. 1999;273(1):73–80. doi: 10.1006/abio.1999.4203. [DOI] [PubMed] [Google Scholar]
- 24.Griffin BA, Adams SR, Tsien RY. Specific covalent labeling of recombinant protein molecules inside live cells. Science. 1998;281(5374):269–272. doi: 10.1126/science.281.5374.269. [DOI] [PubMed] [Google Scholar]
- 25.Ha T. Single-molecule fluorescence resonance energy transfer. Methods. 2001;25:78–86. doi: 10.1006/meth.2001.1217. [DOI] [PubMed] [Google Scholar]
- 26.Ha T, Tinnefeld P. Photophysics of fluorescent probes for single-molecule biophysics and super-resolution imaging. Annu Rev Phys Chem. 2012;63:595–617. doi: 10.1146/annurev-physchem-032210-103340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hein B, Willig KI, Wurm CA, Westphal V, Jakobs S, Hell SW. Stimulated emission depletion nanoscopy of living cells using SNAP-tag fusion proteins. Biophys J. 2010;98(1):158–163. doi: 10.1016/j.bpj.2009.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohng S, Ha T. Near-complete suppression of quantum dot blinking in ambient conditions. J Am Chem Soc. 2004;126(5):1324–1325. doi: 10.1021/ja039686w. [DOI] [PubMed] [Google Scholar]
- 29.Howarth M, Ting AY. Imaging proteins in live mammalian cells with biotin ligase and monovalent streptavidin. Nat Protoc. 2008;3(3):534–545. doi: 10.1038/nprot.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, Lopez-Casillas F, Wrana JL, Hinck AP. TGF-beta signalling is mediated by two autonomously functioning TbetaRI:TbetaRII pairs. EMBO J. 2011;30(7):1263–1276. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joo C, Balci H, Ishitsuka Y, Buranachai C, Ha T. Advances in single-molecule fluorescence methods for molecular biology. Annu Rev Biochem. 2008;77:51–76. doi: 10.1146/annurev.biochem.77.070606.101543. [DOI] [PubMed] [Google Scholar]
- 32.Kapanidis AN, Ebright YW, Ebright RH. Site-specific incorporation of fluorescent probes into protein: hexahistidine-tag-mediated fluorescent labeling with (Ni(2+):nitrilotriacetic acid (n)-fluorochrome conjugates. J Am Chem Soc. 2001;123(48):12123–12125. doi: 10.1021/ja017074a. [DOI] [PubMed] [Google Scholar]
- 33.Kapanidis AN, Weiss S. Fluorescent probes and bioconjugation chemistries for single-molecule fluorescence analysis of biomolecules. J Chem Phys. 2002;117(24):10953–10964. doi: 10.1063/1.1521158. [DOI] [Google Scholar]
- 34.Lakowicz JR. Principles of fluorescence spectroscopy. 3. Springer: New York; 2006. [Google Scholar]
- 35.LiCata VJ, Wowor AJ. Applications of fluorescence anisotropy to the study of protein–DNA interactions. Methods Cell Biol. 2008;84:243–262. doi: 10.1016/S0091-679X(07)84009-X. [DOI] [PubMed] [Google Scholar]
- 36.Lin CW, Ting AY. Transglutaminase-catalyzed site-specific conjugation of small-molecule probes to proteins in vitro and on the surface of living cells. J Am Chem Soc. 2006;128(14):4542–4543. doi: 10.1021/ja0604111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lippincott-Schwartz J, Patterson GH. Fluorescent proteins for photoactivation experiments. Methods Cell Biol. 2008;85:45–61. doi: 10.1016/S0091-679X(08)85003-0. [DOI] [PubMed] [Google Scholar]
- 38.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF, Wood KV. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem Biol. 2008;3(6):373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 39.Manley S, Gillette JM, Patterson GH, Shroff H, Hess HF, Betzig E, Lippincott-Schwartz J. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat Methods. 2008;5(2):155–157. doi: 10.1038/nmeth.1176. [DOI] [PubMed] [Google Scholar]
- 40.Marcu L. Fluorescence lifetime techniques in medical applications. Ann Biomed Eng. 2012;40(2):304–331. doi: 10.1007/s10439-011-0495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307(5709):538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388(6645):882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- 43.Mujumdar RB, Ernst LA, Mujumdar SR, Lewis CJ, Waggoner AS. Cyanine dye labeling reagents: sulfoindocyanine succinimidyl esters. Bioconjug Chem. 1993;4(2):105–111. doi: 10.1021/bc00020a001. [DOI] [PubMed] [Google Scholar]
- 44.Nelson SR, Ali MY, Trybus KM, Warshaw DM. Random walk of processive, quantum dot-labeled myosin Va molecules within the actin cortex of COS-7 cells. Biophys J. 2009;97(2):509–518. doi: 10.1016/j.bpj.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okada CY, Rechsteiner M. Introduction of macromolecules into cultured mammalian cells by osmotic lysis of pinocytic vesicles. Cell. 1982;29(1):33–41. doi: 10.1016/0092-8674(82)90087-3. [DOI] [PubMed] [Google Scholar]
- 46.Phillips RA, Hunter JL, Eccleston JF, Webb MR. The mechanism of Ras GTPase activation by neurofibromin. Biochemistry. 2003;42(13):3956–3965. doi: 10.1021/bi027316z. [DOI] [PubMed] [Google Scholar]
- 47.Pierce DW, Hom-Booher N, Vale RD. Imaging individual green fluorescent proteins. Nature. 1997;388(6640):338. doi: 10.1038/41009. [DOI] [PubMed] [Google Scholar]
- 48.Puljung MC, Zagotta WN. Labeling of specific cysteines in proteins using reversible metal protection. Biophys J. 2011;100(10):2513–2521. doi: 10.1016/j.bpj.2011.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rasnik I, McKinney SA, Ha T. Nonblinking and long-lasting single-molecule fluorescence imaging. Nat Methods. 2006;3(11):891–893. doi: 10.1038/nmeth934. [DOI] [PubMed] [Google Scholar]
- 50.Ratner V, Kahana E, Eichler M, Haas E. A general strategy for site-specific double labeling of globular proteins for kinetic FRET studies. Bioconjug Chem. 2002;13(5):1163–1170. doi: 10.1021/bc025537b. [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto T, Webb MR, Forgacs E, White HD, Sellers JR. Direct observation of the mechanochemical coupling in myosin Va during processive movement. Nature. 2008;455(7209):128–132. doi: 10.1038/nature07188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. J Cell Sci. 2007;120(Pt 24):4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- 53.Shaner NC, Steinbach PA, Tsien RY. A guide to choosing fluorescent proteins. Nat Methods. 2005;2(12):905–909. doi: 10.1038/nmeth819. [DOI] [PubMed] [Google Scholar]
- 54.Shanker N, Bane SL. Basic aspects of absorption and fluorescence spectroscopy and resonance energy transfer methods. Methods Cell Biol. 2008;84:213–242. doi: 10.1016/S0091-679X(07)84008-8. [DOI] [PubMed] [Google Scholar]
- 55.Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223–239. doi: 10.1002/jcp.1030590302. [DOI] [PubMed] [Google Scholar]
- 56.Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9(4):e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shu X, Shaner NC, Yarbrough CA, Tsien RY, Remington SJ. Novel chromophores and buried charges control color in mFruits. Biochemistry. 2006;45(32):9639–9647. doi: 10.1021/bi060773l. [DOI] [PubMed] [Google Scholar]
- 58.Sun X, Zhang A, Baker B, Sun L, Howard A, Buswell J, Maurel D, Masharina A, Johnsson K, Noren CJ, Xu MQ, Correa IR., Jr Development of SNAP-tag fluorogenic probes for wash-free fluorescence imaging. ChemBioChem. 2011;12(14):2217–2226. doi: 10.1002/cbic.201100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Theissen B, Karow AR, Kohler J, Gubaev A, Klostermeier D. Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proc Natl Acad Sci U S A. 2008;105(2):548–553. doi: 10.1073/pnas.0705488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Toseland CP, Martinez-Senac MM, Slatter AF, Webb MR. The ATPase cycle of PcrA helicase and its coupling to translocation on DNA. J Mol Biol. 2009;392(4):1020–1032. doi: 10.1016/j.jmb.2009.07.071. [DOI] [PubMed] [Google Scholar]
- 61.Toseland CP, Powell B, Webb MR. ATPase cycle and DNA unwinding kinetics of RecG helicase. PLoS One. 2012;7(6):e38270. doi: 10.1371/journal.pone.0038270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsien RY. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 63.Vogelsang J, Kasper R, Steinhauer C, Person B, Heilemann M, Sauer M, Tinnefeld P. A reducing and oxidizing system minimizes photobleaching and blinking of fluorescent dyes. Angew Chem Int Ed Engl. 2008;47(29):5465–5469. doi: 10.1002/anie.200801518. [DOI] [PubMed] [Google Scholar]
- 64.Waggoner A. Covalent labeling of proteins and nucleic acids with fluorophores. Methods Enzymol. 1995;246:362–373. doi: 10.1016/0076-6879(95)46017-9. [DOI] [PubMed] [Google Scholar]