Abstract
Purpose
We report the largest study conducted till date of drug resistant tuberculosis in spine analyzing the drug susceptibility patterns in 111 cases of proven drug resistance.
Methods
An observed cross-sectional study was conducted. Six-hundred and eighty-six patients with positive cultures underwent sensitivity testing to 13 commonly used anti-tubercular drugs using BACTEC MGIT-960 system.
Results
Females (60.3%) outnumbered males (39.6%). Only three patients (2.7%) were found HIV positive, and none of these had AIDS. Forty-four (39.6%) patients had taken AKT in the past for some form of tuberculosis. Eight (7.2%) patients had history of treatment default. The drug sensitivity testing revealed 87 (78.3%) cases of multi drug resistance (resistance to both isoniazid and rifampicin) and 3 (2.7%) cases of XDR-TB spine. Of the individual drugs, widespread resistance was present to both isoniazid (92.7%) and rifampicin (81.9%), followed by streptomycin (69.3%). Least resistance was found to kanamycin, amikacin and capreomycin.
Conclusion
It is recommended to do routine biopsy, culture and drug sensitivity testing in all patients of tuberculosis spine to guide selection of appropriate second-line drugs when required. In cases of non availability of drug susceptibility testing despite repeated attempts, it is suggested to use data from large series such as this to plan best empirical chemotherapy protocol.
Keywords: MDR-TB spine, Second-line drugs, Empiric treatment
Introduction
We present the largest study till date of drug resistant tuberculosis spine comprising 111 culture proven cases. Management of tuberculosis spine is primarily medical with surgical intervention having a supportive role for cord decompression, mechanical instability and stabilization. The last few decades have seen a remarkable increase in incidence of drug resistance in Mycobacterium tuberculosis. From single drug resistance we have reached the era of multidrug resistance and extensive drug resistance. Multidrug resistant (MDR) tuberculosis is defined as tuberculosis caused by M. tuberculosis resistant in vitro to the effects of isoniazid and rifampicin, with or without resistance to other drugs. Extensively drug resistant (XDR) tuberculosis is defined as MDR tuberculosis which is additionally resistant to at least one of the fluroquinolones and to one of the three injectable second-line anti-tubercular drugs: amikacin, kanamycin, and capreomycin [1–6]. Resistance to any one anti-tuberculosis drug is termed as monoresistance whereas resistance to more than one anti-tubercular drug, other than both isoniazid and rifampicin is called polyresistance [2].
The global estimated number of incident MDR-TB cases in 2008 was 440,000. In 2008, MDR-TB caused an estimated 150,000 deaths. Together China and India account for about 50% of all incident cases of MDR-TB [3, 7]. This indicates the quantum of this problem in India and highlights the need to take urgent action.
The main factors attributed to the development of drug resistance include inadequate and incomplete treatment, non-adherence to treatment, and genetic factors [2, 3, 8–10]. Short course chemotherapy with resistant/partially resistant drugs in resistant strains may create even more resistance to the drugs in use, and this has been called as the “amplifier effect” [2]. Ongoing transmission of established drug resistant strains in a population is also a significant source of new drug resistant cases [2]. The routine use of tuberculosis culture sensitivity in a suspected case of tuberculosis spine appears to be a probable solution to this ever increasing problem. There is scarcity of literature about prevalent drug-resistant patterns in tuberculosis spine. This data from a local population can be quite helpful in setting up guidelines for formulating regimens for empiric management of cases with repeated negative cultures in an unresponsive patient or places where standard culture sensitivity facilities are not available.
Objective of the study
To analyze drug sensitivity patterns in cases of drug-resistant spinal tuberculosis, and to help frame guidelines for secondary line chemotherapy in cases where culture isolation is difficult or not possible.
Materials and methods
Study design
An observational cross-sectional study was done from a single lab of an institute involved in tertiary spine care. Patients with tuberculosis of the spine who were culture positive and found drug resistant were included in the study.
Data retrieval
Data retrieval system of the institute was used to retrieve demographic, clinical and drug susceptibility pattern data of the patients. Method of collection of sample, whether open surgical or radiologically guided, was noted. Prior history of any form of tuberculosis, contact history of tuberculosis and drug default history were also noted.
Culture method and drug sensitivity testing
All samples were processed using the BACTEC MGIT (Mycobaterium Growth Indicator Tube) 960 system: a rapid, in vitro, non-radiometric system for rapid detection of mycobacterial growth as per the oxygen consumption [11–16]. Positive cultures could be grown between 10 and 21 days. Negative cultures were declared in case of inactivity for 42 days. The culture colonies isolated were of M. tuberculosis complex which comprises of M. tuberculosis, Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium microti.
The critical concentrations (in µg/ml) used to establish resistance to various drugs were: isoniazid 0.1, rifampicin 1.0, ethambutol 5.0, pyrazinamide 100, streptomycin 1.0, amikacin 1.0, capreomycin 2.5, kanamycin 2.5, ofloxacin 2.0, moxifloxacin 1.0, ethionamide 5.0, para-amino salicylic acid (PAS) 4.0 and clofazamine 0.5.
Results
Out of 686 positive cultures evaluated, 111 were found to have drug-resistant strains to at least one anti-tubercular drug. Females (59.4%) outnumbered males (40.5%) with the ratio of 1:1.5 in this study. Mean age of presentation was 29 years (1.5–78 years) and most of our patients were in the productive years of life. Twelve patients were less than 12 years of age.
Samples were obtained through open surgeries in 72 (64.8%) patients while CT/USG guided biopsies/drainage was used in 39 (35.1%) patients. The dorsal spine was found to be most common site of involvement in 68 (61.2%) patients followed by lumbar spine in 34 (30.6%) patients (Table 1). Forty-two patients (37.8%) had AFB positive smears directly from biopsy samples.
Table 1.
Level of involvement of disease in cases
| Spinal level | No. of cases |
|---|---|
| Dorsal | 68 |
| Lumbar | 34 |
| Dorso-lumbar | 5 |
| Cervical | 2 |
| Cervicodorsal | 1 |
| Cervical + lumbar | 1 |
Sixty-one (54.95%) patients had positive contact history of tuberculosis. Fifty-eight (52.2%) patients had taken anti-tuberculous treatment in the past for some form of tuberculosis at least for a month. Eight (7.2%) patients had history of treatment default. Only three patients (2.7%) were found HIV positive, and none of them suffered from AIDS. Out of 58 patients who had a positive history of past anti-tuberculous treatment, 48 (53.3%) were found to have MDR strains. MDR strains were present in all the eight (100%) patients who had history of treatment default.
Out of the 111 drug-resistant cases, 87 patients had MDR strains (78.37%), 3 had XDR strains (2.7%).
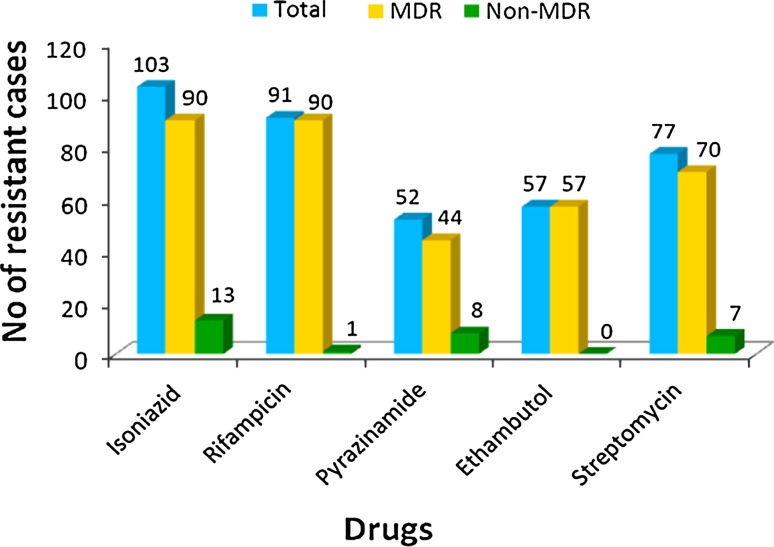
Among the first-line drugs, maximum resistance was found to isoniazid (92.79%) followed by rifampicin (81.98%) and streptomycin (69.36%). Relatively least resistance was found against pyrazinamide (46.8%) among first-line drugs (Table 2; Figs. 1, 2).
Table 2.
Resistance to first-line drugs
| Drug | Number of patients (out of 111) | MDR (out of 90) | Non MDR (out of 21) |
|---|---|---|---|
| Isoniazid | 103 (92.7%) | 90 (100%) | 13 |
| Rifampicin | 91 (81.9%) | 90 (100%) | 1 |
| Pyrazinamide | 52 (46.8%) | 44 (48.8%) | 8 |
| Ethambutol | 57 (51.3%) | 57 (63.3%) | 0 |
| Streptomycin | 77 (69.3%) | 70 (77.7%) | 7 |
Fig. 1.
Proportion of resistance to first-line drugs
Fig. 2.
Proportion of resistance to second-line drugs
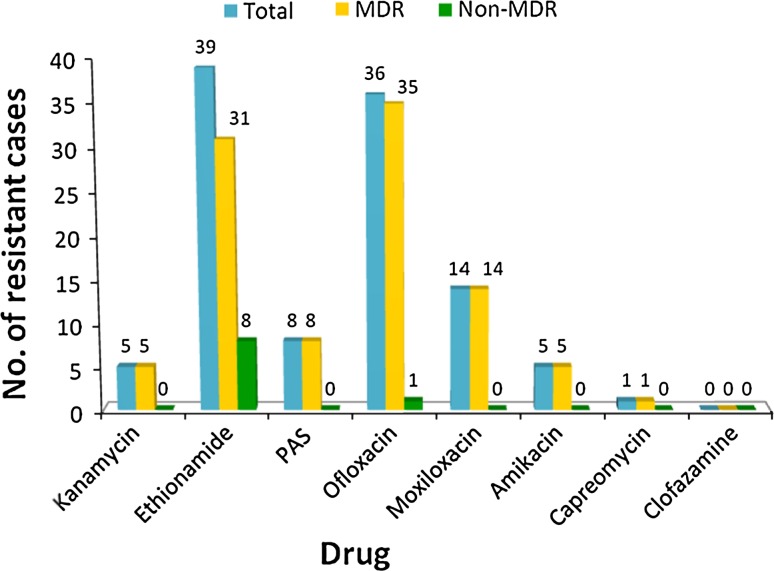
Among the second-line drugs, maximum resistance was found against ethionamide (35.13%) and ofloxacin (32.43%). Least resistance was against kanamycin (4.5%), amikacin (4.5%) and capreomycin (0.9%). No isolate was found to be resistant to clofazamine (Table 3; Fig. 3).
Table 3.
Resistance to second-line drugs
| Drug | Number of patients (out of 111) | MDR (out of 90) | Non MDR (out of 21) |
|---|---|---|---|
| Kanamycin | 5 (4.5%) | 5 (5.55%) | 0 |
| Ethionamide | 39 (35.1%) | 31 (34.4%) | 8 |
| PAS | 8 (7.2%) | 8 (8.8%) | 0 |
| Ofloxacin | 36 (32.4%) | 35 (38.8%) | 1 |
| Moxifloxacin | 14 (12.6%) | 14 (15.5%) | 0 |
| Amikacin | 5 (4.5%) | 5 (5.5%) | 0 |
| Capreomycin | 1 (0.9%) | 1 (1.1%) | 0 |
| Clofazamine | 0 | 0 | 0 |
Fig. 3.
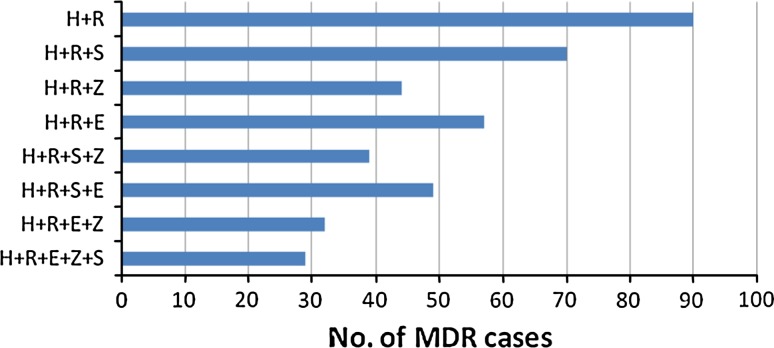
Number of MDR cases showing resistance to combination/s of first-line drugs with/without resistance to other drugs
Among the non-MDR group, maximum resistance was found against isoniazid followed by ethionamide and pyrazinamide. Only one patient showed resistance against rifampicin in this group (Table 4).
Table 4.
Pattern of resistance in non-MDR cases
| Drug | Number of patients (out of 21) | Isolated resistance | Combined resistance |
|---|---|---|---|
| Isoniazid(H) | 13 | 4 | 9* |
| Rifampicin(R) | 1 | 1 | – |
| Streptomycin(S) | 7 | 1 | 6# |
| Pyrazinamide(Z) | 8 | 2 | 6‡ |
| Ethionamide(Eth) | 8 | 1 | 7$ |
| Ofloxacin(O) | 1 | – | 1§ |
The combined resistance was as follows
* H9: 3: H+Eth, 2: H+S+Eth, 1: H+Z, 1: H+S, 1: H+Z+Eth, 1: H+Z+Eth+O
#S6: 3: Z+S, 2: H+S+Eth, 1: H+S
‡Z6: 3: Z+S, 1: H+Z, 1: H+Z+Eth, 1: H+Z+Eth+O
$Eth7: 3: H+Eth, 2: H+S+Eth, 1: H+Z+Eth, 1: H+Z+Eth+O
§O1: 1: H+Z+Eth+O
Discussion
Multi drug resistance is a new dimension to the age old problem of spinal tuberculosis. Its potential to masquerade as other diseases can have devastating implications on the patient. To the best of our knowledge, ours is the first study that attempts to evaluate the drug-sensitivity patterns in drug-resistant tuberculosis spine. We believe that our results will have widely ranging implications on the management of this new dreaded disease.
The most important method of identifying drug resistance is by culture of M. tuberculosis and drug susceptibility testing (DST), both of which require considerable amount of time and expertise [3, 5, 8]. The facilities for the same are also not readily available in many countries, including India [10]. The onus to generate population-based data is limited to tertiary care centres and national laboratories. DST along with strong corroboration by significant contact history, past treatment history, history of drug default, if any, is considered to be the most accurate way of diagnosing drug resistance.
Collection of samples for culture also poses a challenge in TB-spine patients. As a result of these handicaps, DST mainly gets done only in cases where drug resistance is strongly suspected or when there is no improvement with the standard anti-tuberculous treatment. This leads to a diagnostic delay which can significantly affect the final outcome. In a study of MDR pulmonary tuberculosis, a diagnostic delay of 8 months was attributed to not doing a DST in the beginning [17]. In another study from our institute where 25 proven MDR-TB spine patients were evaluated, a diagnostic delay of 7 months was reported [8].
Traditionally, anti-tuberculous drugs are classified as first-line and second-line drugs, with isoniazid, rifampicin, pyrizinamide, ethambutol and streptomycin being the primary first-line drugs. An alternative way of classifying anti-tubercular drugs can be based on efficacy, experience of use and drug class as mentioned in Table 5 [4, 6].
Table 5.
Classification of anti-tubercular drugs
| Groups | Drugs |
|---|---|
| Group-1: first-line oral agents | Isoniazid, rifampicin, ethambutol, pyrazinamide, rifabutin |
| Group-2: injectable agents | Streptomycin, amikacin, kanamycin, capreomycin |
| Group-3: fluroquinolones | Ofloxacin, moxifloxacin, levofloxacin, gatifloxacin |
| Group-4: oral bacteriostatic second-line agents | PAS, cycloserine, terizidone, ethionamide, protionamide |
| Group-5: agents with unclear role in treating drug resistant TB | Clofazamine, linezolid, amoxicillin/clavulunate, thioacetazone, cilastin/imipenem, clarithromycin, high-dose isoniazid |
According to the WHO report on drug resistance (2008), Global population-weighted proportion of resistance to any one drug was found to be 17% in new cases and 35% in previously treated cases. The proportion of MDR was 2.9% in new cases and up to 15.3% in the previously treated cases with total proportion of MDR cases being 5.3%. Data from India reports similar findings of 2.3 and 17.2% of MDR cases from new and previously treated cases, respectively [5]. Out of 686 culture-proven patients evaluated in our study, 111 were found to have drug-resistant strains. Of these 111 cases, 90 had MDR strains (including 3 XDR). The proportion of drug resistance found in our study is falsely high and cannot be used to calculate epidemiological incidence as the study was conducted at a tertiary-level reference centre where most of the cases were those failing standard anti-tubercular therapy and hence the study population had a greater probability of harboring drug-resistant strains to begin with.
Of the 111 patients of drug-resistant tuberculosis spine, highest resistance was found to isoniazid (103 patients: 92.7%) followed by rifampicin (91 patients: 81.9%) and streptomycin (77 patients: 69.3%). The results we got were similar to that published by the WHO for pulmonary tuberculosis in 2008, which showed maximum resistance to isoniazid (13.3%), streptomycin (12.6%) and rifampicin (6.3%) [5]. However, WHO figures are for all patients with TB whereas we have only included the patients with resistant-tuberculosis spine. Significant implication of this distribution is that among the first-line drugs, rifampicin which affects all populations of organism and isoniazid are the only drugs which are bactericidal and hence such a resistance pattern adversely affects the outcome after standard anti-tubercular treatment. Ninety of the 111 patients had MDR strains (including 3 XDR). MDR strains were also resistant to streptomycin (77.7%) followed by ethambutol (63.3%), while pyrazinamide showed the least resistance (48.8%) among the first-line drugs.
Among the MDR group for the second-line drugs, resistance was high to ofloxacin (38.8%) followed closely by ethionamide (34.4%). Moxifloxacin (15.5%) and PAS (8.8%) showed intermediate resistance. Lowest resistance was to aminoglycosides: kanamycin and amikacin (5.55%), capreomycin (1.1%), and no resistance was found to clofazamine. It should be noted that although both ofloxacin and moxifloxacin belong to the same class of drugs, i.e., fluoroquinolones, they have variable cross-resistance, and the presence of resistance to ofloxacin may not indicate resistance to moxifloxacin. However, amikacin and kanamycin show a high-degree of cross-resistance, hence if resistance to one is known, resistance to the other should be assumed [4, 6].
The remaining 21 patients had resistant strains which did not meet the definition of MDR. Isoniazid (13 patients: 61.9%) was the most common resistant drug followed by pyrazinamide (8 patients: 38%), ethionamide (8 patients: 38%) and streptomycin (7 patients: 33.3%). There was one patient who had a strain resistant to ofloxacin. Resistance to rifampicin in the absence of resistance to isoniazid was seen in only one patient. This low incidence of non-MDR rifampicin resistance is not only widely reported but is also important as a measure for reliability of isoniazid susceptibility testing and hence of laboratory accuracy [5]. Isolated rifampicin testing can also be used as an indicator of the presence of MDR [5].
Based on WHO guidelines for the treatment of drug resistance and on findings of this study, the following guidelines can be considered while formulating the second-line regimens in indicated cases:
Individualized treatment is the best for drug-resistant tuberculosis spine patients, but in situations where culture sensitivity facilities are not available or when there is progression of disease inspite of the standard anti-tubercular treatment and in situations of repeated negative culture, an objectively designed empiric second-line regimen can be considered.
Regimen should include at least four new drugs which have not been taken by the patient in the past [4, 6, 18, 19]. It is not recommended to add a single drug to the failing regimen: “Addition Syndrome” [8]. Selection of drugs should be based on their hierarchical order from Groups 1–5, bactericidal activity and adverse effect profile. Total duration of treatment should not be less than 18–24 months [4, 6, 19].
No two drugs of the same pharmacological group or sharing the same drug adverse effect should be clubbed together in the regimen.
Among the Group-1 drugs, only pyrazinamide can be included in the regimen as it was found to be effective in more than 50% MDR strains. This level of resistance, however, makes it undependable. Pharmacologically pyrazinamide has an advantage when used in the initial part of the treatment as it acts well in closed cavities and acidic environments [4, 6].
According to WHO, any second-line regimen should have at least 6 months of initial injectable kanamycin or amikacin or capreomycin. Injectable kanamycin or amikacin can be added, as favorable susceptible patterns were found in most of our cases (resistance in 5.5% each). Pharmacologically also they are bactericidal against tuberculosis bacilli. Aminoglycosides have mainly ototoxic and nephrotoxic adverse effects which should be carefully monitored.
Among the Group-3 (fluroquinolones), moxifloxacin can be added. In this study, resistance to moxifloxacin was 15.5% as compared to ofloxacin (38.8%). In vitro studies have shown decreasing efficacy from moxifloxacin = gatifloxacin > levofloxacin > ofloxacin against M. tuberculosis [4, 6]. Fluroquinolones are bactericidal against tuberculosis bacilli and are also well tolerated; with adverse effect profile including mainly gastrointestinal disturbances. Thus, can be safely included in the regimen.
From Group-4, PAS being resistant in less than 9%, and cycloserine can be included. Ethionamide had more resistance (34.4%) than PAS in our study group. Overall ethionamide is less-toxic than PAS, but with enteric coated PAS, gastrointestinal side effects can be avoided. Giving PAS along with ethionamide should be avoided as their adverse effects are counter productive [4, 6].
An important and useful adjunct can be clofazamine due to favorable susceptibility and toxicity profile.
To adjudge clinical recovery, all drug-resistant tuberculosis patients can be serially monitored with three monthly complete blood count (CBC), Erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and MRI along with drug-toxicity effects so as to modify drug combinations suitably.
In conclusion, our study highlights the growing threat posed by the development of widespread resistance to anti-tuberculous drugs in the management of tuberculosis spine. Complications arise due to delays in diagnosis, obtaining samples and by inappropriate administration of second-line drugs. Based on our findings, we recommend:
Having a high index of suspicion for the presence of drug resistance. Routine biopsy, culture and drug-sensitivity testing of all patients even in the patients proposed for conservative management.
Use of drug susceptibility patterns wherever available to guide selection of appropriate second-line drugs.
In cases of non availability of sensitivity despite the repeated attempts; use of data from large series like this, to plan best empirical chemotherapy protocol.
Consideration to be made to relative drug toxicities, efficacy and compatibility when selecting second-line drugs.
Conflict of interest
None.
References
- 1.WHO (2011) The Global Plan to Stop TB 2011–2015: transforming the fight towards elimination of tuberculosis. Available at: http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf
- 2.Guidelines for the programmatic management of drug-resistant tuberculosis: emergency update 2008. Geneva: WHO; 2008. [Google Scholar]
- 3.WHO (2010) Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response Geneva. who/htm/tb/2010.3
- 4.WHO (2009) Treatment of tuberculosis: guidelines, 4th edn. Geneva. who/htm/tb/2009.420
- 5.WHO (2008) Anti-tuberculosis drug resistance: report no. 4, The WHO/IUALTD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Geneva. who/htm/tb/2008.394:1-142
- 6.WHO (2006) Guidelines for the programmatic management of drug resistant tuberculosis. Geneva. who/htm/tb/2006.361
- 7.Neel Gandhi R, et al. Multidrug-resistant and extensively drug- resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 2010;375(9728):1830–1843. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 8.Pawar UM, Kundnani V, Agashe V, et al. Multidrug-resistant tuberculosis of spine: is it the beginning of the end? Spine. 2009;34(22):E806–E810. doi: 10.1097/BRS.0b013e3181af7797. [DOI] [PubMed] [Google Scholar]
- 9.Prasad Rajendra. Management of multidrug resistant tuberculosis: practitioner’s view point. Indian J Tuberc. 2007;54:3–11. [PubMed] [Google Scholar]
- 10.Udwadia ZF. India’s multi-drug resistant tuberculosis crisis. Ann NY Acad Sci. 2001;953:98–105. doi: 10.1111/j.1749-6632.2001.tb11365.x. [DOI] [PubMed] [Google Scholar]
- 11.Chedore P, Bertucci L, Wolfe J, et al. Potential for erroneous results indicating resistance when using the BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol. 2010;48(1):300–301. doi: 10.1128/JCM.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin SY, Desmond E, Bonato D, et al. Multicentre evaluation of BACTEC MGIT 960 system for second line drug susceptibility testing of Mycobacterium tuberculosis complex. J Clin Microbiol. 2009;47(11):3630–3634. doi: 10.1128/JCM.00803-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rüsch-Gerdes S, Pfyffer GE, Casal M, et al. Multicentre laboratory validation of BACTEC MGIT 960 technique for testing susceptibilities of Mycobacterium tuberculosis to second line drugs and newer antimicrobials. J Clin Microbiol. 2006;44(3):688–692. doi: 10.1128/JCM.44.3.688-692.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morcillo N, Imperiale B, Di Giulio B. Evaluation of MGIT 960 and the colorimetric based method for tuberculosis drug susceptibility testing. Int J Tuberc Lung Dis. 2010;14(9):1169–1175. [PubMed] [Google Scholar]
- 15.Rodrigues C, Jani J, Shenai S, et al. Drug susceptibility testing of Mycobacterium tuberculosis against second line drugs using Bactec MGIT 960 system. Int J Tuberc Lung Dis. 2008;12(12):1449–1455. [PubMed] [Google Scholar]
- 16.Kontos F, Maniati M, Costopoulos C, et al. Evaluation of the fully automated Bactec MGIT 960 system for drug susceptibility testing of Mycobacterium tuberculosis to first line drugs: a multicentre study. J Microbiol Methods. 2004;56(2):291–294. doi: 10.1016/j.mimet.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 17.Schaaf HS, Shean K, Donald PR. Culture confirmed multi-drug resistant tuberculosis: diagnostic delay, clinical features and outcome. Arch Dis Child. 2003;88(12):1106–1111. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caminero JA. Treatment of multidrug resistant tuberculosis: evidence and controversies. Int J Tuberc Lung Dis. 2006;10(8):829–837. [PubMed] [Google Scholar]
- 19.Crofton J, Chaulet P, Maher D (1997) Guidelines for the management of drug resistant tuberculosis. Geneva. WHO/TB/96:210