Abstract
Introduction
Spinal tuberculosis has existed in human beings since the ascent of man on earth. Historically, the management has progressed from regional orthodox therapies to the current, more effective, drugs.
Materials and methods
Historical perspectives regarding the management have been collated by going through the important publications during the past 6 decades. For convenience, the manuscript has been organized as “orthodox traditional” treatment, early “chemotherapy period”, “post chemotherapy treatment”, “middle-path” philosophy, and the current treatment with availability of modern imaging facilities.
Conclusions
Broad conclusions based upon the published data and personal observations (1959–2011) are summarized as follows: If diagnosis is made at predestructive stage and the patient is treated by standard drugs, the infection would heal in about 95 % patients without significant deformities and complications. Neural complications are still encountered in general hospital outpatients. Diagnosis and treatment at early stages would resolve the neurology without operation in about 40 % of cases. Nearly 60 % of patients would require to be operatively decompressed without jeopardizing mechanical stability. However, despite current treatment approximately 8 % of tuberculous paraplegics do not recover functionally. Immunocompromised state and multidrug resistance to standard drugs (8 to 10 %) are the current (and future) challenges to the doctors and the society.
Keywords: Tuberculosis, Spine tuberculosis, Pott’s disease, Historical treatment
Introduction
Tuberculous bacilli have lived in symbiosis with mankind since the ascent of Homo sapiens on Earth. In the western world, the clinical features and communicability of tuberculosis were known before 1,000 BC. Paleopathological evidence of tuberculosis of bones, joints and spine in prehistoric humans has also been recorded [1]. In India, the Rig Veda and the Atharva Veda (3,500–1,800 BC) mention this disease by the name “Yakshama” in all its forms [2, 3]. Tuberculous disease of the spine was described by Percivall Pott in 1799 as “That kind of palsy of lower limbs which is frequently found to accompany a curvature of the spine” [3, 4]. Identification of mycobacterium as the causative organism (1870), use of the Bacilli Calmette Guerin (BCG) vaccination (1945), facilities for radiographic examination, and availability of specific antitubercular drugs (1948–1951) are all important landmarks in the understanding and management of tuberculosis of spine. More recently (since 1987) MRI and CT scans have helped the clinicians to suspect the disease at a predestructive phase especially at difficult and rare sites, such as craniovertebral region, cervicodorsal spine, sacrum and coccyx. According to current estimates of the World Health Organization, tuberculosis will exist in humans so long as there are pockets of malnutrition, poor sanitation, crowded living conditions, exanthematous fevers, repeated pregnancies, immunodeficient status, alcohol and substance abuse, and people living with diabetes and advanced age present in society [5, 6]. Persons infected with ubiquitous mycobacterium would present with clinically manifest disease if there is reduction in the innate immunology and reduction of cell-mediated immunity [7].
Prophylaxis against tuberculosis
Improvement in the socioeconomic and nutritional status of a society is the most important method of reducing the prevalence of tuberculous infection. The BCG vaccination has been available for clinical use since the 1950s for prophylaxis against tuberculosis. In most developing countries, intradermal BCG vaccination has been universally used for all newborns. For other counties, selective immunization of groups at special risk is strongly recommended. The protection afforded by BCG in the control of tuberculosis is about 80 %, probably the severity of disease becomes less.
Evolution of treatment of spinal tuberculosis
The availability of antitubercular drugs (1948–1951), an important milestone, divides the treatment of tuberculosis into three eras [3]: (1) the preantitubercular era, in which the patients were treated either by orthodox (ancient traditional beliefs of various countries), nonoperative regimens or by various “distant surgical procedures”, (2) the postantitubercular era (universal surgical extirpation), in which all patients were treated operatively in conjunction with antitubercular drugs [8–16], and (3) the postantitubercular era (middle-path regimen), in which all patients were treated with antitubercular drugs and surgery was confined to patients failing to respond to drugs or those with complications [17–26].
The preantitubercular era
In ancient India, the Atharvans (1,800–1,000 BC) used to treat cases of skeletal tuberculosis with “Sipudru,” an herbal preparation, and sunshine [2]. Hippocrates (450 BC) and Galen (AD 131–201) tried to correct kyphotic deformity due to tuberculosis of the spine with manual pressure, traction, and mechanical appliances, but failed [3]. The orthodox nonoperative treatment was entirely constitutional, and strongly advocated recumbency and immobilization by means of body casts, plaster beds, and braces. The value of heliotherapy and wind was extolled in specialized hospitals or “sanatoria.” Most of such sanatoria were located in health resorts in various countries. The average time of hospitalization in the sanatoria varied between 1 and 5 years. Rest and adequate food was strongly advised and practiced by John Hilton (1863) and Hugh Owen Thomas (1875). Sir Robert Jones (1923) and Dame Agnes Hunt enhanced these principles and placed sanatoria at the disposal of such patients [27]. This treatment continued up to middle of twentieth century. These dictums still constitute part of the routine treatment of tuberculosis, especially in economically underdeveloped countries, because most of the patients, due to their economic strata, live in subnormal conditions. The natural course of skeletal tuberculosis without chemotherapy passed through three stages spanning 3–5 years. In the “stage of onset”, lasting from 1 month to 1 year, the localized disease developed into a warm tender swelling with marked localized osteoporosis and minimal destruction. In the “stage of destruction”, lasting 1–3 years, the disease progressed until there was gross destruction of the vertebrae with deformity, subluxation, contractures, and abscess formation. The abscesses finally ruptured and drained as ulcers and sinuses, developed frequent secondary pyogenic infection. With superimposed pyogenic infection, the general defense mechanism of the patient became markedly lowered, with severe cachexia, frequent tuberculous dissemination (military tuberculosis, tuberculous meningitis), and death in nearly 1/3 of the patients (it is easy to understand why early writers used the term “consumption”). The survivors entered the “stage of repair and ankylosis,” occurring 2–3 years after the onset of disease. There was improvement in the patients’ general condition. The abscesses resorbed, sinuses underwent healing, and destroyed bones were remineralized. The diseased area generally healed with fusion in a deformed position (kyphus). Bony fusion (generally the outcome with superimposed pyogenic infection) was perceived to minimize recurrence of infection and progress of deformation. When healing took place by fibrous ankylosis, the surgeons considered it unsatisfactory because patients had pain on movement and weight bearing. The reactivation of infection was frequent (with chances of amyloid disease) and deformation aggravated with the passage of time. Primary aim before the availability of chemotherapy was to achieve the “stage of repair and ankylosis” of the diseased joint or spine in the least disabling position by plaster cast immobilization for 2–3 years. The results of orthodox nonoperative treatment were, on the whole, unsatisfactory [3]. Only 30–44 % of treated patients resumed full working capacity [10, 28]. The rest of the patients either died (30–50 %) or were paralyzed and severely crippled. Kyphosis could develop or increase even while the patient was being treated in a plaster bed. Recovery from paraplegia was reported in about 1/3 of the patients, but the failure of the nonoperative treatment in preventing the onset of paraplegia and relapse was also well recognized [21, 28–31].
Prechemotherapy operative treatment and its results
Disappointing results of orthodox nonoperative treatment in the prechemotherapy era induced surgeons to develop approaches for surgical excision of the diseased bones [10, 28, 31, 32]. Most of the earlier operations were for drainage of abscesses or sinuses. Palpable peripheral abscesses were aspirated through “antigravity routes”. All such procedures resulted in persistent serious sinus and ulcer formations, secondary pyogenic infection, and death in many patients. The general outlook regarding surgery was aptly summarized by Calot: the “surgeon who, so far as tuberculosis is concerned, swears to remove the evil from the very root, will only find one result awaiting him—the death of his patient” [33]. As “Direct operations” on the diseased area presented such a gloomy picture, surgeons tended to develop “distant operations” without opening the site of disease. Albee [34] and Hibbs [35] introduced and developed posterior spinal fusion. The aim of these operations was to shorten the period of immobilization in bed and plaster casts, and to provide a permanent internal stability (fusion) to the diseased parts (since no motion was perceived to have least chance of activation of disease). Nothing dramatic, however, happened to the diseased area located anteriorly, where pus, debrise, and necrotic bone remained enmeshed in dense fibrous tissue and the disease persisted, sometimes mildly active or in other cases dormant only to flare up at any provocation. Of the survivors, nearly 50 % were “healthy and fit”, about 30 % were “improved” and 20 % remained “not healed” [31–33]. The posterior spinal surgery had nothing to offer to the paraplegic patients who failed to respond to the standard sanatorium treatment. With the introduction of posterior spinal arthrodesis, the future of spinal surgery was ushered in.
Postantitubercular drugs era
Streptomycin became available for clinical use in 1947, P-aminosalicylic acid (PAS) in 1949, isoniazid in 1952, pyrazinamide in 1952, ethambutol in 1961 and rifampicin in 1965. The period for chemotherapy triumph was ushered in; however, in the wake of enthusiasm (of earlier period) for the surgical treatment of skeletal tuberculosis, the standard treatment practiced and advocated during 1950–1960 was universal excisional surgery in conjunction with antitubercular drugs [11, 13, 16, 36]. One of the arguments offered by the advocates of universal surgical extirpation was that antitubercular drugs do not penetrate into osseous tuberculous lesions. There are, however, many studies showing effective penetration by most of the antitubercular drugs in osseous tuberculous lesions, including cavities, abscesses, and caseous lesions [37–42]. The most spectacular effect of the drugs was the disappearance of sinuses, ulcers, and abscesses, despite extensive surgery, and the elimination of the danger of postoperative dissemination of tuberculous infection. Simultaneously, however, many surgeons reported excellent results by antitubercular drugs alone and confined surgery to patients who failed to respond to drugs or those with complications [4, 17, 18, 20, 22–24, 43]. Over time with development of philosophy of “middle-path regime”, indications for surgery have become universally more selective, less for controlling disease and more for preventing and correcting spinal deformities and neural complications [4, 20, 23, 44–46]. The enthusiasm for direct excisional surgery that started in the prechemotherapy era got further encouragement from the safety provided by antitubercular drugs. “Radical surgical operations” were advocated as if for a malignancy [8–10, 12, 15]. When the potential for remarkable repair and regeneration of the diseased vertebrae (and bones) with multidrug therapy was realized, operative excision and debridement justifiably became less aggressive and were confined (or limited) to removal of sequestrated vertebrae or discs or the offending tissues compressing the dural tube [13, 23, 26, 47, 48]. The non-offending parts of the vertebral bodies well away (anterior) from the dural sheath did not require excision and could undergo healing by drugs and maintain vertebral column stability without compromising the aim of operation. No local treatment, however, is a substitute for prolonged chemotherapy because spinal tuberculosis is a systemic disease (Figs. 1, 2).
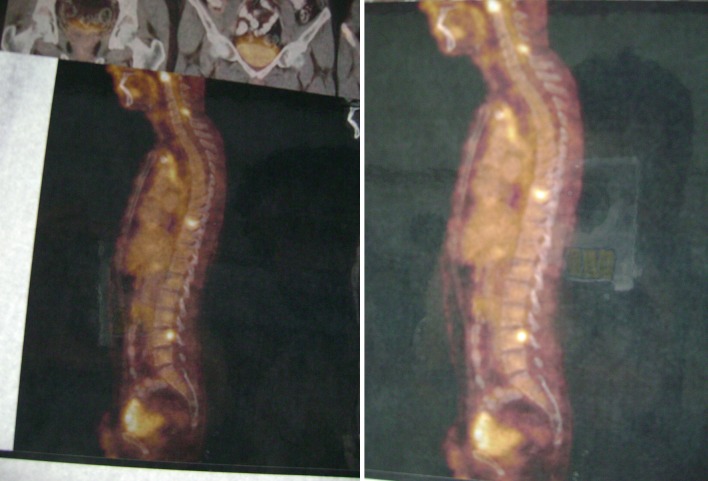
Fig. 1.
Note extensive tuberculous infection of the spine from dorsal 7 to lumbar L1 vertebrae
Fig. 2.
PET scan of a patient who presented with an abscess in the right iliac foss. Note additional active lesions in the vertebral column
Development of surgical approaches for spinal tuberculosis
Most surgical approaches to the spine were originally developed to treat spinal tuberculosis, the commonest location being the dorsal spine. In the prechemotherapeutic era, Hibbs and Albee [34, 35] developed “distant operation” of posterior spinal fusion. The availability of effective antitubercular drugs made direct surgery on the diseased area possible without persistent drainage of operative wounds and without dissemination of tuberculous infection. The effectiveness of chemotherapy, however, has obviated the need for operative treatment in majority of cases. Over the years, a rational philosophy developed that any surgery on the vertebral column must ensure the least disruption of the intact healthy columns. In the classic tuberculous spondylodiscitis where the disease, destruction, and neural compression are in the anterior columns, the operation through the “anterior route” is the rational approach for debulking, debridement, and decompression, thus preserving the only biologic stability the patient has, i.e., the healthy pedicles, posterior arch and ligament complex. Laminectomy for anterior spondylodiscitis is therefore contraindicated. The anterolateral extrapleural approach, probably initiated by Menard (1895) and developed by Griffiths, Roaf, and Seddon, has been used with some modifications by many workers for debridement of the diseased tissues and for mechanical decompression of the cord with or without bone grafting for achieving anterior spinal fusion [15, 21, 22, 26, 30, 31, 47, 49, 50]. Most of the workers consider this approach adequate for dorsal lesions. The transpleural anterior approach has been developed by Hodgson et al. and used by many workers [11, 12, 50, 51] for tuberculous lesions of dorsal spine. The transthoracic transpleural approach, however, does need better infrastructure facilities for surgery, and postoperative care of the patient in the intensive care unit for 2–5 days. For most of the hospitals in the developing countries of the world, which have moderate facilities, the anterolateral approach is practical and safe and offers adequate exposure for tuberculous lesions of the spine from the second dorsal vertebra to the fifth lumbar vertebra. If desired and indicated, one can perform anterior debridement, decompression, bone grafting (anterior or posterior), posterior implant fixation, and kyphus correction. The anterolateral approach offers the surgeon 360° exposure. In patients with extensive pulmonary disease, extrapleural approach is the ideal and safer option. Lumbosacral lesions can also be debrided through a similar approach; one may have to excise the posteromedial 2–3 cm of iliac crest to reach the vertebral bodies of lumbar fifth and sacral first vertebrae.
Indications for surgery
Operative procedure should be limited to tissue diagnosis in case of doubtful diagnosis, drainage of an abscess causing difficulty in deglutition and breathing in cervical spine disease, or evacuation of a large paravertebral abscess showing marked increase in size despite 3–6 months of multidrug therapy, operative debridement and anterior decompression in certain cases of neural complications not recovering with drug therapy, deteriorating neurology despite antitubercular therapy, or those who had recurrence of neural complications. In the growing age (before 11 years), patients at risk of developing severe kyphosis would need fusion of posterior elements to minimize the deformity; care should be taken not to damage the remaining growth plates of the infected vertebral bodies and of adjacent healthy vertebrae while performing anterior debridement and decompression [52]. Any preserved growth potential of vertebral bodies would to some extent negate the deterioration of kyphotic deformity. Unacceptable fixed kyphotic deformities may benefit by careful panvertebral operations [4, 53, 54].
Kyphotic deformity
Severe kyphotic deformity of spine is unacceptable (Fig. 3). Timely operative procedures would prevent a severe deformity. It is wise to identify patients who are at risk to develop severe kyphotic deformity. Following guidelines should help a clinician to identify such patients [4, 53, 54].
Patients younger than 11 year of age
Lesion located between cervical seventh to lumbar one vertebrae
Destruction of vertebral bodies of three or more vertebrae
Loss of vertical height equivalent to or more than 1.5 vertebral bodies.
Deterioration of the kyphotic deformity in successive X-rays.
Fig. 3.
Severe kyphotic deformity is unacceptable. Such deformities generally are a result of extensive vertebral body destruction, during childhood
Deformity may occur during the active stage of disease due to collapse of the destroyed and softened vertebral bodies. The deformity during the growing phase of child deteriorates further due to unrestricted growth of posterior elements in the presence of arrested growth of the vertebral bodies. It is safest to prevent the development of severe kyphotic deformity by timely operative procedures (Fig. 3). Any operative procedure to obtain a worthwhile correction of a fixed (rigid) deformity is a major procedure even in the hands of an experienced surgeon. The cord is grossly deformed and stretched, trapped in a narrow vertebral canal, and physiologically, the neural elements have utilized the maximum of neuronal (reserves) plasticity. Any attempt at deformity correction may lead to permanent neurological damage, if the deformity is 60° or more, and the deformity is above the level of the twelfth dorsal level. Informed consent of the patient and the family is mandatory before operative correction of fixed kyphotic deformities [4, 53, 54].
Instrumentation in spinal tuberculosis
During the last 20 years, there have been a few reports of operative treatment for spinal tuberculosis where the authors have used instrumentation [53, 54]. Posterior instrumentation combined with anterior surgery probably helps the patient (with adequate motor power) for early postoperative ambulation. It may be justified to use instrumentation, when debridement and decompression are indicated in a patient suffering from a panvertebral disease (spondylodiscitis with destruction of posterior elements) or when one is doing anterior loosening of a kyphotic deformity with concomitant wedge resection of posterior elements for attempted correction of fixed kyphotic deformity [53, 55]. The decision to insert anterior implants is fraught with many dangers. Inserting metal implants or cages in the anterior elements with active disease and softened vertebral bodies may result in failures, complications and settling of implants in the osteoporotic bone. The author has observed disengagement of the fixation, breakage of the implants, permanent neural damage and persistent discharging sinuses tracking down to the anterior implants. Insertion of biomaterials in the infected focus appears to be inappropriate. If one is impelled to use implants, posterior instrumentation is safe and simple because the implant is inserted in the healthy parts of vertebrae. Because of tight confines of thoracic spine pedicles and canal, intracanal and extracortical malposition of pedicle screws has been observed [55, 56]. Re-operation to remove the anteriorly placed implants is a very complex operation with higher risks for drastic complications as compared to the original insertion. Catastrophic complications are seldom reported. Anteriorly placed bone graft or implant when required to span 2 or more disc spaces are fraught with more complications of slippage of graft, settling in the osteoporotic vertebral bodies, fracture and resorption (of bone grafts).
Neural complication
The incidence of neural complications in earlier reports has been 10–20 %. Based upon the data from Government general hospitals in India, nearly 20 % of patients suffering from spinal tuberculosis would present with neural complication. Broadly speaking, the pathology of neural complications can be summarized as below [8, 22, 29, 31, 47]:
Inflammatory causes Edema of cord, abscess, granulation tissue.
Mechanical causes Sequestra from bone or disc, internal salient (internal kyphos), canal stenosis.
Intrinsic causes Myelomalacia, syrinx formation, ischemic changes, arachnoiditis, transaction of cord due to pathological dislocation or due to panvertebral disease or iatrogenic.
Spinal tumor syndrome Extradural granuloma or intradural tuberculoma.
In most of the cases, more than one cause may be responsible in the same patient. A practical classification of neural complications and prognostic factors are summarized in Tables 1 and 2. Every patient of neural complications warrants close observation for proper management. No patient should, however, be considered to have too advanced disease for treatment. Many of such patients recover to some extent (providing protective sensation for easier nursing and rehabilitation) after a satisfactory mechanical decompression of the cord with multidrug therapy.
Table 1.
Classification of tuberculous paraplegia/tetraplegia (predominantly based upon motor weakness)
| Stage | Clinical features | |
|---|---|---|
| I | Negligible | Patient unaware of neural deficit, physician detects plantar extensor and/or ankle clonus |
| II | Mild | Patient aware of deficit but manages to walk with support |
| III | Moderate | Nonambulatory because of paralysis (in extension), sensory deficit <50 % |
| IV | Severe | III + Flexor spasms/paralysis in flexion/flaccid/sensory deficit more than 50 %, sphincters involved |
Application to compression of cord and not cauda equine
Table 2.
Clinical factors influencing prognosis in cord involvement
| Cord involvement | Better prognosis | Relatively poor prognosis |
|---|---|---|
| Degree | Partial | Complete |
| Duration | Shorter | Longer (>12 months) |
| Type | Early onset | Late onset |
| Speed of onset | Slow | Rapid |
| Age | Younger | Older |
| General condition | Good | Poor |
| Vertebral disease | Active | Healed |
| Kyphotic deformity | <60 | >60 |
| MRI of cord | Healthy cord | Myelomalacia syringomyelia |
| Operative findings | Wet lesion | Dry lesion |
Role of antitubercular drugs
Pulmonary tuberculous lesions are “open lesions” where a large number of bacilli grow in the walls of cavities. In contrast, the “closed lesions” in skeletal and spinal tuberculosis do not communicate with air and have far smaller bacterial population. A large part of the bacillary population in pulmonary tuberculosis is rapidly multiplying, whereas most of the bacilli in spinal tuberculosis replicate slowly or are almost dormant. Antitubercular drugs are most effective against the rapidly replicating bacterial population. Dormant bacilli tend to retain viability despite chemotherapy, and the need to kill them (when these start replicating) is the reason why skeletal and spinal tuberculosis require a long duration of drug therapy. It took many years before the effect of any regime could be appreciated in skeletal tuberculosis. Konstam aptly wrote “often it seems to me as if surgeons generally did not appreciate the full power of the new antitubercular drugs [19].” Bosworth compared the results before and after the availability of drugs; the mortality prior to the use of drugs was 21.1 % and after the availability 5.8 %, thus mortality was reduced by 72.5 % by antitubercular drugs [57].
Before the advent of antitubercular chemotherapy, sinus formation was regarded as one of the most dreaded complications of spinal tuberculosis, and the surgery for tuberculosis was usually complicated by the formation of sinuses and ulcers. With antitubercular drugs, sinus formation does not occur even after extensive surgery to excise the tubercular focus [17, 18, 22, 23, 43, 57]. Failure of sinuses and ulcers to heal within 4–5 months of multidrug therapy or their appearance while the patient is on drugs suggests infection by resistant organisms or an immunosuppressed state in the patient. Many workers have reported remarkable healing of spinal tuberculosis with drugs alone [17–20, 22, 24, 32, 43, 48]. However, there are always some cases that should be treated surgically. Indications for continuation of drug treatment or for operation can be rationally controlled by careful clinical examination, laboratory investigations, and serial radiography at 3–4 months intervals. Progressive destruction or imperfect or slow healing despite antitubercular drugs is reasonable indication for surgery in early stages [58–63].
Drugs resistance, relapse, or recurrence
The chief limitation of antitubercular drugs is the development of resistant strains of tubercle bacilli. Theoretically, multidrug resistance (MDR) is defined as the resistance of the mycobacterium to isoniazid and rifampicin. If only one drug is used, the chances of the emergence of resistant strains are high; however, it is unusual for bacilli to be resistant to a combination of two drugs. Clinical non-responsiveness or therapeutic resistance to conventional first-line antitubercular drugs is, however, an increasing worldwide problem. MDR at the international level at present seems between 5 and 10 % [4, 64]. A relapse rate of 20 % was observed in patients treated with streptomycin alone between 1946 and 1948. With multidrug therapy, the recurrence rate fell to almost 2 % in a series studied between 1956 and 1962. Low rates are probably due to the effective antitubercular drugs presently available. Yeager [65] observed that the prolonged use of combined antimicrobial therapy has lowered the relapse rate to its lowest point in history.
Under the influence of antitubercular drugs, the incidence of healing with nonoperative therapy varies between 83 and 96.8 % [18, 20, 23, 43]. In series treated with excisional therapy, the incidence of healing has been between 80 and 90 % [9, 11, 16, 60–63]. Skeletal tuberculosis runs an insidious and chronic course with occasional reactivation/relapse many years later. It is obvious that an exact incidence of recurrence can never be calculated accurately. An average figure of recurrence or reactivation at present for patients followed up for more than 5–10 years after the completion of the drugs therapy is 2–5 % [4, 44]. We have observed that the patients with spinal tuberculosis who remained fully active with healed status of disease (treated non-operatively or by operation) for 30–40 years reporting back with reactivation of spinal disease or with an active tuberculous lesion in another site. The probable cause of reactivation was lower immune status caused by diabetes, medical co-morbidities, and aging.
New imaging modalities
Of the newer investigations such as ultrasound, computed tomography scan, and magnetic resonance imaging (MRI) that have become available for general clinical use for spinal tuberculosis, MRI has proved the most useful investigation for diagnosis at predestructive stage, to detect the disease at difficult sites (craniovertebral, cervicodorsal, lumbosacral, sacral, and coccygeal), to visualize the extent of disease, to judge the health of dural tube contents, and to assess the activity of disease and the influence of multidrug therapy.
During the first 5–6 month of effective multidrug chemotherapy, radiographic and MRI-based observation may show an increase in the size of perivertebral abscesses and an increase in the area of osseous destruction and bone edema despite the fact that the patient is showing favorable clinical responses such as absence of pyrexia and improvement in appetite, body weight, and blood picture. This is because the appearance of imaging modalities lags behind the biologic response to repair. The MRI does not differentiate between the inflammatory response of active disease and that of repair [4]. In addition, presumably, there is an immunologic response to dead bacilli and their products. Such an appearance during the first 6 months should not necessarily cause alarm. However, if progressive resolution of osseous edema, abscess shadow and areas of osseous destruction is not observed after 6 months of multidrug therapy, one may need to change the drugs or consider surgery for treatment and tissue diagnosis.
Immunodeficient stage and tuberculous epidemic
People with AIDS virus (or persons with CD 4 + lymphocyte count <100/mm3) are being infected with atypical tuberculous bacilli (which were earlier considered generally nonpathogenic), and many of these strains already show resistance to a large number of antitubercular drugs. HIV-infected persons, due to dysfunction of the host immune system, have a very high risk of getting primary tuberculosis, reactivation of the previous tuberculous lesion in the body and concomitant infection by another strain of tuberculous bacillus (different from that of initial disease—polymycobacterial disease) by exogenous route. The incidence of tuberculosis in patients with AIDS is almost 500 times that in the general population. Patients with HIV and tuberculosis are a potential source for spread of drug-resistant strains of tuberculous bacilli to other members of society. Prolonged use of steroids, methotrexate and immunosuppressive drugs may lead to a clinical picture resembling AIDS without suffering from HIV infection. For multidrug-resistant cases, or therapeutically non-responders or those reporting with recrudesce of infection adjunctive immunomodulation may play a role. Till the availability of better immunopotentiating technologies, we have been using the following routine in our patient with success in approximately 85 % of cases [66, 67]. In brief, 150 mg of levamisole is given by mouth at night for 3 consecutive days at 1-week intervals for a total of 50 tablets. Four injections are administered at 1-month intervals. The first and second are BCG 0.1 ml intradermal, and the third and fourth are diphtheria pertussis and tetanus (DPT) intramuscular. Laboratory studies have shown upgradation of immune profile especially CD4 +T cells in most of the patients treated like this.
The challenges for future
Despite advances in imaging techniques, better understanding of microbiological, histological and immunological biologics, availability of effective antimycobacterial chemotherapies and possibilities of sophisticated and safe operative procedures, there are many grey or unresolved areas that require to be addressed. The status report as observed in the period 1990–2010 is broadly summarized as follows. Lasting healed status rather than “cure” is the best that one can achieve today. MRI pictures in 5–10 % of cases overlap the images of mitotic conditions and osteoporotic fractures, histology of the tissues from the diseased spine gets reported as caseating granuloma/granulomatous pathology in nearly 30 % of cases, and “chronic inflammatory appearance” in 70 % of specimens, and mycobacteria have been harvested in <20 % of tested specimens. Resistance to first-line antitubercular drugs is being observed in 8–12 % of cases; in patients with healed disease followed up for more than 10 years, the recurrence rate is 2–5 %, and despite effective and safe mechanical decompression of cord, nearly 8 % of tuberculous paraplegia patients do not recover. Stem cell biology is still in infancy exploring the possibility of pluripotent cells to migrate, disperse, replicate and metaplacise within the damaged segment of cord for its repair. The incidence of tuberculosis in general is increasing worldwide by 5–10 % per annum because of societal indiscretion, many pockets of deprivation and increasing number of immune-compromised population in the world.
Conflict of interest
None.
References
- 1.Lichtor J, Lichtor A. Paleopathological evidence suggesting pre-Columbian tuberculosis of spine. J Bone Joint Surg Am. 1957;39-A(6):1938–1939. [PubMed] [Google Scholar]
- 2.Duraiswami PK, Orth M, Tuli SM. 5000 years of orthopaedics in India. Clin Orthop Relat Res. 1971;75:269–280. doi: 10.1097/00003086-197103000-00032. [DOI] [PubMed] [Google Scholar]
- 3.Bick KM. Classics of orthopaedics. Philadelphia: JB Lippincott Co.; 1976. [Google Scholar]
- 4.Tuli SM. Tuberculosis of the skeletal system. 4. New Delhi: Jaypee Brothers Medical Publishers; 2010. [Google Scholar]
- 5.Barnes PF, Barrows SA. Tuberculosis in the 1990s. Ann Intern Med. 1993;119:400–410. doi: 10.7326/0003-4819-119-5-199309010-00009. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Collins DA, Bourke BE. Don’t forget tuberculosis. Ann Rheum Dis. 1995;54(3):174–175. doi: 10.1136/ard.54.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott JE, Taor WS. The changing pattern of bone and joint tuberculosis. J Bone Joint Surg Br. 1982;64:250. [Google Scholar]
- 8.Cameron JA, Robinson CL, Robertson DE. The radical treatment of Pott’s disease and Pott’s paraplegia by extirpation of the diseased area and anterior spinal fusion. Am Rev Respir Dis. 1962;86:76–80. doi: 10.1164/arrd.1962.86.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Chahal AS, Jyoti SP. The radical treatment of tuberculosis of the spine. Int Orthop. 1980;4(2):93–99. doi: 10.1007/BF00271091. [DOI] [PubMed] [Google Scholar]
- 10.Fellander M. Radical operation in tuberculosis of the spine. Acta Orthop Scand Suppl. 1955;19:1–117. [PubMed] [Google Scholar]
- 11.Hodgson AR, Stock FE, et al. Anterior spinal fusion. The operative approach and pathological findings in 412 patients with Pott’s disease of the spine. Br J Surg. 1960;48:172–178. doi: 10.1002/bjs.18004820819. [DOI] [PubMed] [Google Scholar]
- 12.Kohli SB. Radical surgical approach to spinal tuberculosis. J Bone Joint Surg Br. 1967;49(4):668–673. [PubMed] [Google Scholar]
- 13.Kondo E, Yamada K. End results of focal debridement in bone and joint tuberculosis and its indications. J Bone Joint Surg Am. 1957;39-A(1):27–31. [PubMed] [Google Scholar]
- 14.Mukopadhaya B, Mishra NK. Tuberculosis of spine. Indian J Surg. 1957;19:59–81. [Google Scholar]
- 15.Risko T, Novasazel T. Experience with radical operations in tuberculosis of the spine. J Bone Joint Surg Am. 1963;45:53–68. [Google Scholar]
- 16.Wilkinson MC. The treatment of tuberculosis of the spine by evacuation of the paravertebral abscess and curettage of the vertebral bodies. J Bone Joint Surg Br. 1955;37-B(3):382–391. doi: 10.1302/0301-620X.37B3.382. [DOI] [PubMed] [Google Scholar]
- 17.Friedman B. Chemotherapy of tuberculosis of the spine. J Bone Joint Surg Am. 1966;48:451–474. [PubMed] [Google Scholar]
- 18.Kaplan CJ. Conservative therapy in skeletal tuberculosis: an appraisal based on experience in South Africa. Tubercle. 1959;40:335–368. doi: 10.1016/S0041-3879(59)80135-5. [DOI] [PubMed] [Google Scholar]
- 19.Konstam PG, Konstam ST. Spinal tuberculosis in Southern Nigeria with special reference to ambulant treatment of thoracolumbar disease. J Bone Joint Surg Br. 1958;40-B(1):26–32. doi: 10.1302/0301-620X.40B1.26. [DOI] [PubMed] [Google Scholar]
- 20.Martin M, editor. Tuberculosis of the bones and joints. Heidelberg: Springer; 1988. [Google Scholar]
- 21.Roaf R. Tuberculosis of the spine. J Bone Joint Surg Br. 1958;40-B(1):3–5. doi: 10.1302/0301-620X.40B1.3. [DOI] [PubMed] [Google Scholar]
- 22.Tuli SM. Treatment of neurological complications in tuberculosis of the spine. J Bone Joint Surg Am. 1969;51(4):680–692. [PubMed] [Google Scholar]
- 23.Tuli SM. Results of treatment of spinal tuberculosis by middle-path regime. J Bone Joint Surg Br. 1975;57(1):13–23. [PubMed] [Google Scholar]
- 24.Tuli SM, Kumar S. Early results of treatment of spinal tuberculosis by triple drug therapy. Clin Orthop Relat Res. 1971;81:56–70. doi: 10.1097/00003086-197111000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Vyaghreswarudu C, Reddy Y. Evaluation of treatment in tuberculosis of spine. Indian J Surg. 1964;26:911–924. [Google Scholar]
- 26.Wilkinson MC. Tuberculosis of the spine treated by chemotherapy and operative debridement: a long-term follow-up study. J Bone Joint Surg Am. 1969;51(7):1331–1342. doi: 10.2106/00004623-196951070-00012. [DOI] [PubMed] [Google Scholar]
- 27.Jones R, Levett RW. Orthopaedic surgery. London: Oxford Medical Publications; 1923. [Google Scholar]
- 28.Dobson J. Tuberculosis of spine: an analysis of the results of conservative treatment and of the factors influencing the prognosis. J Bone Surg Br. 1951;33-B(4):517–531. doi: 10.1302/0301-620X.33B4.517. [DOI] [PubMed] [Google Scholar]
- 29.Editorial (1968) Pott’s paraplegia. Br Med J 2:638–639 [PMC free article] [PubMed]
- 30.Griffiths DL (1952) Tuberculosis of bones and joints: modern practice in tuberculosis, vol 2. Butterworth and Co, London, pp 302–333
- 31.Seddon HJ. Pott’s paraplegia, prognosis and treatment. Br J Surg. 1935;22:769–799. doi: 10.1002/bjs.1800228813. [DOI] [Google Scholar]
- 32.Mercer W. Then and now: the history of skeletal tuberculosis. J R Coll Surg Edinb. 1964;9:243–254. [PubMed] [Google Scholar]
- 33.Calot T. Sur le meilleur traitement local des tuberculoses des os, articulations et ganglions lymphatiques. Acta Chir Scand. 1930;67:206–226. [Google Scholar]
- 34.Albee FH. The bone graft operation for tuberculosis of the spine. JAMA. 1930;94:1467–1471. doi: 10.1001/jama.1930.02710450011004. [DOI] [Google Scholar]
- 35.Hibbs RA, Risser JC. Treatment of vertebral tuberculosis by the spine fusion operation. J Bone Joint Surg. 1928;10:804–814. [Google Scholar]
- 36.Deroy MS, Fisher H. The treatment of tuberculous bone disease by surgical drainage combined with streptomycin. J Bone Joint Surg Am. 1952;34:299–330. [PubMed] [Google Scholar]
- 37.Andre T. Studies on the distribution of tritium-labelled dihydrostreptomycin and tetracycline in the body. Acta Radiol Suppl. 1956;142:1–89. [PubMed] [Google Scholar]
- 38.Barclay WR, Ebert RH, Le Roy GV, Manthei RW, Roth LJ. Distribution and excretion of radioactive Isoniazid in tuberculous patients. JAMA. 1953;151(16):1384–1388. [PubMed] [Google Scholar]
- 39.Hanngren A (1959) Studies on the distribution and fate of C14 and T-labelled p-amino-salicylic acid (PAS) in the body. Acta Radiol (Suppl 175):1–118 [PubMed]
- 40.Wu QQ, Kuan Na XK, Tian WC. The concentrations of four antituberculous drugs in cold abscesses in patients with bone and joint tuberculosis. Chin Med J (Engl) 1987;100:819–822. [PubMed] [Google Scholar]
- 41.Tuli SM, Brighton CT, Morton HE, Clark LW. The experimental induction of localised skeletal tuberculous lesions and their accessibility to streptomycin. J Bone Joint Surg Br. 1974;56(4):551–559. [PubMed] [Google Scholar]
- 42.Tuli SM, Kumar K, Sen PC. Penetration of antitubercular drugs in clinical osteoarticular tubercular lesions. Acta Orthop Scand. 1977;48(4):362–368. doi: 10.3109/17453677708992009. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson FH, Manning CW. Tuberculosis of the spine treated conservatively with chemotherapy: series of 72 patients collected 1949–1954 and followed to 1961. Tubercle. 1962;43:406–411. doi: 10.1016/S0041-3879(62)80011-7. [DOI] [PubMed] [Google Scholar]
- 44.Martin NS. Tuberculosis of the bones and joints. Heidelberg: Springer; 1970. [Google Scholar]
- 45.Moon MS, Kim I, Woo YK, Park YO. Conservative treatment of tuberculosis of the thoracic and lumbar spine in adults and children. Int Orthop. 1987;11(4):315–322. doi: 10.1007/BF00271307. [DOI] [PubMed] [Google Scholar]
- 46.Moon MS, Moon YW, Moon JL, Kim SS, Sun DH. Conservative treatment of tuberculosis of the lumbar and lumbosacral spine. Clin Orthop Relat Res. 2002;398:40–49. doi: 10.1097/00003086-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 47.Langenskiöld A, Riska EB. Pott’s paraplegia treated by antero-lateral decompression in the thoracic and lumbar spine. Acta Orthop Scand. 1967;38(2):181–192. doi: 10.3109/17453676708989632. [DOI] [PubMed] [Google Scholar]
- 48.Medical Research Council (1982) A 10-year assessment of controlled trail comparing debridement and anterior spinal fusion in the management of tuberculosis of the spine in patients on standard chemotherapy in Hong Kong. Eighth report of the Medical Research Council Working Party on Tuberculosis of the Spine. J Bone Joint Surg Br 64:393–398 [DOI] [PubMed]
- 49.Arct W. Operative treatment of tuberculosis of the spine in old people. Bone Joint Surg Am. 1968;50(2):255–267. doi: 10.2106/00004623-196850020-00004. [DOI] [PubMed] [Google Scholar]
- 50.Kirkaldy-Willis WH, Glyn Thomas T. Anterior approaches in the diagnosis and treatment of infections of the vertebral bodies. J Bone Joint Surg Am. 1965;47:87–110. [PubMed] [Google Scholar]
- 51.Kemp HB, Jackson JW, Jeremiah JD, Cook J. Anterior fusion of the spine for infective lesions in adults. J Bone Joint Surg Br. 1973;55(4):715–734. [PubMed] [Google Scholar]
- 52.Girdlestone GR (1965) Tuberculosis of bones and joints. In: Somerville EW, Wilkinson MC (eds), 3rd edn. Oxford University Press, London
- 53.Rajasekaran S. The problem of deformity in spinal tuberculosis. Clin Orthop Relat Res. 2002;398:85–92. doi: 10.1097/00003086-200205000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Jain AK, Maheshwari AV, Jena S. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117–123. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 55.Yilmaz C, Selek HY, Gürkan I, Erdemli B, Korkusuz Z. Anterior instrumentation for the treatment of spinal tuberculosis. J Bone Joint Surg Am. 1999;81(9):1261–1267. doi: 10.2106/00004623-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 56.Oga M, Arizono T, Takasita M, Sugioka Y. Evaluation of the risk of instrumentation or a foreign body in spinal tuberculosis: clinical and biologic study. Spine. 1993;18(13):1890–1984. doi: 10.1097/00007632-199310000-00028. [DOI] [PubMed] [Google Scholar]
- 57.Bosworth DM. Modern concepts of treatment of tuberculosis of bones and joints. Ann N Y Acad Sci. 1963;106:98–105. doi: 10.1111/j.1749-6632.1963.tb16628.x. [DOI] [PubMed] [Google Scholar]
- 58.Cauchoix J, Mechelany EF, Tersen G, Morel G, Cotrel Y. Surgical treatment of Pott’s disease. Rev Chir Orthop Reparatrice Appar Mot. 1961;47:446–469. [PubMed] [Google Scholar]
- 59.Smith GW, Robinson RA. The treatment of certain cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40-A(3):607–624. [PubMed] [Google Scholar]
- 60.Paus B (1964) Treatment for tuberculosis of the spine: anti-tuberculosis drugs in conjunction with radical operation and short hospitalization with no enforced recumbency or immobilization. Acta Orthop Scand (Suppl 72):1–139 [DOI] [PubMed]
- 61.Grewal KS, Singh M. Tuberculosis of spine. Indian J Surg. 1956;18:394–405. [Google Scholar]
- 62.Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39–49. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 63.Rajasekaran S, Shanmugasundaram TK, Prabhakar R, Dheenadhayalan J, Shetty AP, Shetty DK. Tuberculous lesions of the lumbosacral region: a 15-year follow-up of patients treated by ambulant chemotherapy. Spine. 1998;23(10):1163–1167. doi: 10.1097/00007632-199805150-00018. [DOI] [PubMed] [Google Scholar]
- 64.Hobby GL, Johnson PM, Boytar-Papirnyik V (1974) Primary drug resistance: a continuing study of drug resistance in tuberculosis in a veteran population within the United States. X. September 1970 to September 1973. Am Rev Respir Dis 110(1):95–98 [DOI] [PubMed]
- 65.Yeager RL. Opening remarks. Ann N Y Acad Sci. 1963;106:3–4. doi: 10.1111/j.1749-6632.1963.tb16614.x. [DOI] [Google Scholar]
- 66.Tuli SM. Preliminary observations on the effect of immunomodulation in multidrug resistant cases of osteo-articular tuberculosis. Ind J Orthop. 1999;33:83–85. [Google Scholar]
- 67.Arora A, Nadkarni B, Dev G, Chattopadhya D, Jain AK, Tuli SM, Kumar S. The use of immunomodulators as an adjunct to antituberculous chemotherapy in non-responsive patients of osteo-articular tuberculosis. J Bone Joint Surg Br. 2006;88(2):264–269. doi: 10.1302/0301-620X.88B2.17197. [DOI] [PubMed] [Google Scholar]