Abstract
The most dreaded neurological complications in TB spine occur in active stage of disease by mechanical compression, instability and inflammation changes, while in healed disease, these occur due to intrinsic changes in spinal cord secondary to internal salient in long standing kyphotic deformity. A judicious combination of conservative therapy and operative decompression when needed should form a comprehensive integrated course of treatment for TB spine with neurological complications. The patients showing relatively preserved cord with evidence of edema/myelitis with predominantly fluid collection in extradural space on MRI resolve on non-operative treatment, while the patients with extradural compression of mixed or granulomatous nature showing entrapment of spinal cord should be undertaken for early surgical decompression. The disease focus should be debrided with removal of pus caseous tissue and sequestra. The viable bone should only be removed to decompress the spinal cord and resultant gap should be bridged by bone graft. The preserved volume of spinal cord with edema/myelitis and wet lesion on MRI usually would show good neural recovery. The spinal cord showing myelomalacia with reduced cord volume and dry lesion likely to show a poor neural recovery. The internal kyphectomy is indicated for paraplegia with healed disease. These cases are bad risk for surgery and neural recovery. The best form of treatment of late onset paraplegia is the prevention of development of severe kyphosis in initial active stage of disease.
Keywords: Tuberculosis of spine, Neurological complication, Early onset paraplegia, Late onset paraplegia
Introduction
Tuberculosis of spine (TB spine) is the most dreaded neurological complication [1]. The incidence of neurological involvement in Pott’s disease is 10–20 % in highly developed nations and 20–41 % in underdeveloped countries, particularly if thoracic spine is involved. Paraplegia rarely occurs in a tuberculous affection below lumbar one (L1) where the spinal canal is capacious and contains only cauda equina. Dorsal spine is most commonly associated with neurological complications as it has narrow spinal canal and the physiological thoracic kyphosis forces the diseased tissue inside the spinal canal. The abscess tends to remain localized under anterior longitudinal ligament and enters the spinal canal through intervertebral foramina to cause cord compression, unlike in lumbar spine where it trickles down in psoas muscle [1, 2].
Pathology of tuberculosis of spine with neurological complications
The neurological deficit associated with TB spine is divided into two types: (1) paraplegia of early onset (paraplegia with active disease) usually within first 2 years with active disease and (2) paraplegia of late onset (paraplegia with healed disease) usually occurs many years after the apparent quiescence of the disease or could be because of continued grumbling activity in unhealed disease focus [3–6].
Paraplegia with active disease would require active treatment of spinal tuberculosis with or without surgical decompression. The neural complications in active disease can develop secondary to mechanical compression by abscess, granulation tissue, tubercular debris, caseous tissues, and localized pressure of internal salient and/or pathological subluxation/dislocation of the vertebrae. The spinal cord can undergo inflammatory edema and myelomalacia. Hodgson [7] has histologically demonstrated that tuberculosis infection may pass through the barrier of the spinal cord covering and thus be a pathogenic factor in the production of paraplegia. Rarely the infective thrombosis/endarteritis of the spinal vessels could also occur. The paraplegia with healed disease usually develops because of some intrinsic damage to the spinal cord and secondary to localized pressure by transverse ridge of bone anterior to the spinal cord or due to constrictive scarring around dura.
The spinal cord may develop edema, cord atrophy and even syrinx and interstitial gliosis secondary to stretching of spinal cord over internal salient. More than one cause may be acting at the same time. The mechanical compression can be demonstrated objectively on myelography/CT-myelography/MRI [8].
The TB spine is a slowly developing lesion, hence the spinal cord tolerate the gradually developing compression. Hoffman [9] observed 60 % canal occupancy on mid sagittal MRI in cases with paraplegia, while Jain et al. [10] reported that up to 76 % canal occupancy was found compatible with intact neural state. However, with concomitant vascular cause or mechanical instability, the neural deficit may develop at lesser canal compromise. No study so far has conclusively correlated the severity of paraplegia with degree of kyphosis. With the advent of magnetic resonance imaging, the spinal cord and soft tissue could be visualized and the changes occurring in the spinal cord could be observed. Jain et al. [11, 12] reported correlation of clinical course with MRI observations in tuberculous myelopathy. The spinal cord could develop edema, myelomalacia, cord atrophy, syringomyelia, thickening of dura-arachnoid complex and/or arachnoiditis. Extradural compression could be liquid (fluid) compression, caseous tissue or granulation tissue [12].
The patient showing relatively preserved cord size with evidence of myelitis/edema responds well to antitubercular treatment (ATT) with or without surgical decompression [11, 12]. Predominantly, fluid collection in extradural space resolves well with ATT alone, and a conservative trial is likely to be rewarding in cases that along with this finding have apparently preserved normal cord parenchyma at the inception of treatment. In patients with extradural collection of mixed or granulomatous (dry) nature showing entrapment of a normal size cord or a constriction of cord with features suggestive of myelitis shows improvement in neural deficit if taken up for surgical decompression early [12]. Patients having significant cord compression/constriction showing strangulation with evidence of myelomalacia are those in which irreversible changes have already set in and are not likely to show favorable response even after surgical decompression [12].
Mild cord atrophy was observed in all patients who had excellent neural recovery after non-operative/operative treatment. In paraplegia with healed disease, spinal cord showed edema and severe reduction in cord volume in patients with negligible neural deficit; while in a patient with severe neural deficit, cord atrophy was associated with myelomalacia/syringomyelia [1, 11, 12].
Staging of neural deficit
Typically TB spine affects vertebral bodies (VBs) (98 %) and when compression starts anterior to the spinal cord over the anterior column, it shows earliest manifestation as gradual increase in the spasticity which may not be appreciated by the patient but by the clinician, as exaggerated deep tendon reflexes and plantar extensor. As compression increases over the anterior column of cord, the patient starts losing motor power gradually from partial motor weakness to complete motor loss with signs of UMN lesion. By the time, compression is severe enough to cause complete block to the nerve conduction in anterior column, lateral column is also affected partially, thus producing some reduction of sensation (pain, temperature and crude touch). When compression is further increased, even posterior column is also affected leading to complete loss of sensation and disturbances of sphincters. In long standing compression, the spasticity is replaced by flaccidity and flexor spasm. So the neural deficit increases sequentially as cord compression increases. Frankel’s classification was proposed to classify the severity of the neural deficit in cases of acute spinal trauma [13]. The Frankel classification does not classify every type of neural deficit. The Frankel classification does not classify stage 1 neural deficit, where patient does not appreciate motor weakness and the patients only have reflex abnormality, paraplegia with bladder and bowel involvement, paraplegia in flexion/flaccid paraplegia and paraplegia with flexor spasm.
In the ASIA scale, severity of neural deficit as reflected by score depends upon the level of involvement in addition to the severity of cord compression at the involved level. Higher the level of affection of the spinal cord, lower the score.
An ideal classification system should assess the functional status of the tetra/paraplegic patient and should reflect the severity of cord compression. Classification suggested by Tuli and modified by Jain seems most rational which classifies all cases of paraplegia and reflects the severity of cord compression as score for sensory and motor deficit is added [14]. The neurological deficit could be categorized into 5 stages.
Stage I: patient unaware of neural deficit, clinician detects plantar extensor and/or ankle clonus.
Stage II: the patient has spasticity with motor deficit but is a walker. The anticipated motor score in tetraparesis is between 60 and 100. In paraparesis, it is between 80 and 100. The sensory impairment is the lateral column.
Stage III: bedridden spastic patient. Anticipated motor score for quadriplegic is 0–30, and for paraplegic it is 50–80. Sensory scoring is the same as in stage II.
Stage IV: bedridden patient with severe sensory loss, and/or pressure sores. Anticipated motor score in tetraplegia is 0 and in paraplegia it is 50. There is impairment of both lateral and posterior column sensations.
Stage V: same as stage IV and/or bladder and bowel involvement, and/or flexor spasms/flaccid tetraplegia/paraplegia.
Almost in 95 % of all cases, tetraplegia/paraplegia at level of spinal cord in tuberculosis could be classified. However, the lesions around conus and cauda equina presents with sphincter involvement very early in the disease process and also have UMN/LMN mixed paraparesis/plegia with more sensory loss (bizarre neural deficit). Neural deficit association with intraspinal granulomas and atypical locations of the lesions may not always fit in the classification [14].
Clinical presentations of TB spine with neural deficit
Generally TB spine cases have clinical localization of disease; however, in a significant percentage in developing countries, the appearance of weakness brings the patients to the clinician. The patients walk with cautious and clumsy gait. When cervical spine is affected, the patient has weakness of all four limbs (quadriparesis or quadriplegia). In thoracic spine disease, usually both lower limbs are affected (paraparesis or paraplegia). The paralysis of the limbs may be with or without sphincter involvement. In lumbar spine affection, the paraplegia is of lower motor neuron (LMN) type. Tuli [15] has reported a series of 100 cases of TB spine with neurological complications. Thirty-three percent cases reported within 4 weeks, 40 % reported between 4 weeks and 3 months, while 27 % cases reported more than 3 months after appearance of neural deficit. Intraspinal tuberculous granuloma usually presents with compressive myelopathy or cauda equina lesion with sphincter involvement in their first attendance. On the examination, they have no clinical spinal deformity, thus labelled as “spinal tumor syndrome” [16, 17] and differential diagnosis includes all tumorous and non-tumorous conditions of the spinal cord and meninges.
Atypical locations of the lesions
Atypical locations of the tuberculous lesion such as single vertebral affection, posterior complex disease are rare. These patients have pain of some standing with or without constitutional symptoms. These cases present more often with neurological complications. Clinically they have tenderness with or without midline or paramedian fluctuant swelling (posterior complex disease) or mild kyphus (single vertebral affection).
Treatment
Best treatment of neurological complications in tuberculous spine is the prevention of development of para/quadriplegia [1]. However, once tuberculous spine is complicated by neurological complications, early clinical diagnosis, confirmed by radiography, CT/CT myelogram and MRI and early effective treatment, can reverse paralysis and avert or minimize the potentially devastating effect of Pott’s paraplegia.
Paraplegia with active disease
The patients of TB spine with neurological complications have shown neural improvement on ATT alone and with radical surgery combined with chemotherapy. Dobson [18] reported neural improvement in 48 % of paraplegia improved neurologically by traditional conservative care in pre-antibiotic era. So, it is reasonable to expect a further high percentage of neurological recovery with the advent of effective modern chemotherapy. MRC trials have concluded that Pott’s paraplegia from active disease could be managed by conservative methods on ATT only [19–24]. MRC trials considered only limited disease with little kyphosis with mild to moderate paraparesis and presumably due to increase tension of the abscess and inflammatory edema. Drugs and rest arrest the pathological process and reduce tension and edema and relieve the compression, however, the response to conservative treatment is slow and its efficacy is doubtful, when compression is by large sequestrum and/or soft healing ridge. Tuli [15] has reported 38 % neurological recovery out of his 200 cases on 4–6 weeks of rest and ATT. While remaining 62 % were surgically decompressed. The difference in the chronicity of disease and neural complications, individual physical condition, bacterial drug sensitivity, and improper selection of patient for non-operative treatment may be the reasons for variable neural recovery.
The group of surgeons advocating universal surgical extirpation in all cases of tuberculous paraplegia reported the neural recovery between 75 and 84 % [25–28]. They believed the quality and speed of neural recovery will be better than non-operative treatment. Surgical decompression will remove fibrous barrier to drug penetration and diagnosis will be established beyond doubt. The opinion in developed countries goes for universal surgical extirpation on the pretext that the most common cause of paraplegia in tuberculous spine is pressure on the front of dura. The removal of pressure produces not only a high rate of cure, but the paralysis usually resolves rapidly after adequate removal of the compressing agent. Thus it would seem unfair to allow a patient to lie paralyzed, for perhaps some weeks awaiting cure from non-operative care when an operative decompression could produce complete recovery in a matter of days (Griffith [3]).
Tuli [15, 29] has advocated middle path regimen which was followed because of compulsion in Indian subcontinent. The patients reaching at paraplegic state having very poor general health, anemia and many of them having associated pulmonary tuberculosis were unfit for major surgery. While waiting for their turn and fitness for surgery, many of them (38 %) started showing neurological recovery on complete antitubercular therapy, bed rest and nutritious diet in 4–6 weeks [29]. The patient who did not recover was taken up for surgical decompression. The present author has also observed that 25–30 % patients start showing neurological recovery on similar line of treatment [1]. Tuli presented his cases in pre-CT and pre-MRI era with missed diagnosis. The improved imaging and percutaneous method of obtaining tissue diagnosis has reduced instances of missed diagnosis.
The antitubercular drugs reach the pus and granulation tissues in a concentration well above the MIC. The compression in tuberculous spine and neurological complications is a slowly developing process and spinal cord keep on adjusting to this slowly developing compression unless mechanical causes such as pathological subluxation/dislocation have resulted in an acute compression. A short delay in surgical decompression does not significantly alter the long-term recovery of neurological function in a gradually developing neural deficit provided by on MRI if one excludes a panvertebral disease and compression with little fluid component.
A judicious combination of conservative therapy and operative decompression when needed should form a comprehensive integrated course of treatment for TB spine with neurological complications. Tubercular liquid pus, granulation tissue, caseous tissue causing compression and inflammatory edema are amenable to non-operative treatment. These patients may show neural recovery on non-operative treatment, and surgical decompression may be avoided. The patient showing relatively preserved cord with evidence of edema/myelitis with predominant fluid collection in extradural space on MRI resolves on non-operative treatment. The patients with extradural compression of mixed or granulomatous natures showing entrapment spinal cord should be undertaken for early surgical decompression. The benefit of surgical decompression should be given to all patients even for long standing paraplegia. The neurological recovery has been observed even where decompression was performed up to 11–12 months after developing paraplegia [1, 12].
Indications of surgery in tuberculous spine with neurological deficit
The early surgical decompression is indicated when neural complication has developed during conservative treatment or remaining stationary with no neural improvement after fair trial of conservative treatment (3–4 weeks) or neural deficit getting worse on non-operative treatment. The patients who have pretreatment long segment (4 or more vertebral disease) disease with kyphosis are likely to progress with final kyphosis of 60° or more or already have 60° kyphosis should be undertaken for decompression and kyphosis correction.
The rapid onset paraplegia indicates mechanical accident (pathological subluxation/dislocation), hence the patient needs to be evaluated for instability. The patients with severe paraplegia (flaccid paraplegia, paraplegia in flexion, complete sensory loss, and complete loss of motor power for more than 6 months) should be undertaken for decompression. The patient of spinal tumor syndrome is to be decompressed to establish the diagnosis. The paraplegia with neural arch affection, recurrent paraplegia and paraplegia accompanied by uncontrolled spasticity of such severity that reasonable rest and immobilization are impossible and massive prevertebral abscess at upper cervical spine causing difficult deglutition/respiration in the patient are other clinical indications for surgical decompression.
The paraplegia with panvertebral involvement (disease of both anterior and posterior column) as observed on plain radiograph with associated scoliosis and/or severe kyphosis or showing destruction of all components of VB should be undertaken for instrumented stabilization besides anterior decompression, since instability besides mechanical compression is the contributing factor for paraplegias. Patient of TB paraplegia with MRI observation of extradural compression consisting of granulation/caseous tissue with little fluid component compressing spinal cord circumferentially and constricting the cord or compression with sequestrated disc with the features suggestive of cord edema/myelitis or myelomalacia should be undertaken for early surgical decompression.
Surgical decompression (anterior or posterior)
Surgical decompression should include full exposure of the front of the dura mater at the apex of kyphosis. Anterior decompression allows direct access to the focus of the disease, abscesses can be evacuated and all pathologic lesions can be excised and kyphosis can be corrected to some extent if stabilized with autologous bone grafting. Laminectomy for decompression in anterior disease is to be condemned [1]. It removes only the healthy component of vertebral column in anterior disease, thus, rendering the spine unstable as found in panvertebral involvement. The deterioration of neural deficit, increase in kyphosis, and pathological dislocation are well documented after laminectomy in anterior disease and in all such instance instrumented stabilizations are mandatory [30].
Laminectomy as surgical decompression is indicated in isolated neural arch affection and in the compressive myelopathy by spinal tumor syndrome. The shortening of posterior column with anterior decompression is procedure undertaken while kyphosis correction is indicated. This leaves the spine grossly unstable and instrumented stabilization is required. The posterior transpedicular anterior decompression is described where anterior decompression is performed through pedicle of apex VB after laminectomy, this leaves spine grossly unstable, hence posterior instrumentations added to give stability. It looks unwise to resect posterior, only healthy component of the vertebrae and create unstable situation necessitating instrumented stabilization, in short segment anterior disease needing only anterior decompression.
Debridement of spinal focus involves removal of all pus, caseous tissue, and sequestra but without the removal of unaffected or viable bone except to provide adequate access to the focus and to decompress the cord. The radical surgery developed by Hodgson and Stock [25] involves radical excision of tuberculous focus with repair of resultant gap with autologous bone grafting. The excision of bone is carried out until the dura mater is uncovered and until healthy, bleeding cancellous bone was exposed upward and downward with surface suitable for reception of bone graft. It may require exposure of even proximal and distal healthy vertebral body. This involves the removal of intervertebral disks at the limit (or limits) of the resection and of the end plate of the vertebrae immediately above and/or below the diseased area and healthy cancellous surfaces being cut in the vertebral bodies above and/or below. Upadhyay [30–33] observed equally good neurological recovery following radical and debridement surgeries with no incidence of reactivation or recurrence of tuberculosis on long-term follow-up of 112 patients. He observed improvement in deformity angle by 5° or more after radical surgery in 40 % patients, while 53 % patients showed deterioration after debridement surgery. The normal lordosis was maintained in lumbar spine after radical surgery in all, while only in 63 % after debridement. Correction achieved after surgery at 6 months evaluation was practically maintained up to final evaluation. The limitation of this study was that all cases were mainly of two vertebral diseases. Jenkin et al. [34] showed 46.4 % increase in kyphosis from admission to 10 years follow-up in children managed by anterior decompression and fusion. Rajasekaran and Soundarapandian [35] observed, in 81 cases who were operated by radical surgery after a minimum follow-up of 8 years, which 59 % had either some correction of kyphosis or it remained the same as in preoperative stage [36]. All these patients had limited surgical excision of bone, resulting in a small post-debridement defect that needed a short graft. Nineteen percent patients had worsening of kyphosis angle <20° and 22 % had increase in kyphosis angle of more than 20°. This increase in kyphosis was observed more in patients who had extensive involvement of the vertebral bodies that had resulted in a large post-debridement defect necessitating a graft particularly in a patient having thoracic spine affection and marked kyphosis before treatment.
Chen et al. [37] while managing 50 patients of TB spine has calculated preoperative VB loss. Anterior radical debridement with bone grafting was performed if loss was <2 VB heights. If VB loss was more than 2, besides following radical anterior debridement and bone grafting, second stage instrumented posterior spinal fusion was undertaken. He could achieve average correction of kyphosis of 10° (1–44°) with 20 % of his cases had deterioration of kyphosis. Tuli followed up his 118 cases with only debridement surgery for 2–6 years (mean 3.2 years). Angle of kyphosis increased by 10–30° in 19 %, more than 30° in 4 %, and in remaining 77 %, the kyphosis either remained static as preoperatively or decreases, or if increased it was <10°. Jain et al. [1, 38] reported 30 cases of TB spine with neurological complications, with anterolateral decompression. At final follow-up at 2 years, out of 40 cases, 34 (85 %) had no significant change in kyphotic deformity while 4 had increase in kyphosis between 11 and 20° and 2 more than 20°. Two patients maintained the pretreatment kyphosis. Thus, there is no great advantage of radical surgery over debridement surgery when we consider correction of kyphosis deformity.
In a tubercular lesion, the areas of bone, which are infiltrated inflamed, recover and reconstitute under drug treatment. Ischemic and infarcted bones recover and reconstitute as the disease activity subsides and the circulation of lesion improves. The areas of necrosis are past recovery and harbor tubercular bacilli, and for these areas operation in addition to drugs is essential. We need to remove that part of viable bone which allows us to remove all pus, caseous tissue and sequestra, to decompress spinal cord and whatever gap thus created should be bridged by bone grafts to achieve possible correction of kyphosis. The excessive excision of bone up to healthy bleeding bone leaves a large bone defect to be bridged by a long graft. The bone graft attains strength once it gets vascularised and incorporated. Before attaining incorporation, it may slip or break and may lead to neurological non-recovery or deterioration. However, debridement where entire body is not excised leaves a more stable spine. The concomitant posterior instrumentation is indicated in TB spine with neurological complications with panvertebral disease, disease of 4 or more vertebral bodies (long segment disease) and when correction of kyphosis is undertaken.
Surgical approaches to tubercular spine
The surgical approach to the spine in tuberculosis depends on the availability of appropriate facilities and trained personnel and also on the nature of the case. In cervical and lumbar spine, the lesion is explored and excised by anterior approach. In dorsal spine, the lesion can be accessed by thoracotomy, extra pleural (anterolateral) approach, and posterior transpedicular approach.
Thoractomy approach [25] is an operation of extensive disease, it requires a good experienced surgical team, chest surgeon (may not be), excellent operation theater set-up, trained personnel managing postoperatively, and intensive care facilities and should not be undertaken lightly even where a good surgical facility exists. In an excellent set-up, 6 % postoperative deaths have been reported in a moderate paraplegic patient. In severe paraplegia, the percentage of postoperative death rose to 11 % [39, 40]. Jain et al. reported 64 extrapleural anterolateral decompressions in 63 cases of TB spine with paraplegia with no major complications. 57/63 showed complete (n−54) and partial (n−3) neural recovery. In another study, he modified the incision to a T shaped incision while performing extrapleural anterolateral decompression and performed concomitant posterior Hartshill instrumentation and/or kyphus correction [38, 40, 41].
Spinal tuberculosis patients are generally anemic and have evidence of healing/active pulmonary tuberculosis, and due to compromised lung condition, thoracotomy increases the risk of post-operative complications with paralyzed intercostals. The extrapleural (anterolateral) approach can be undertaken even in a compromised general condition as it is a simpler and safer technique, and the exposures of spinal cord in kyphotic spine are easier as the surgeons are working lateral to an acute angle. However, the extrapleural approaches have steep learning curve. If concomitant posterior instrumented stabilization is to be performed, then separate posterior approach is required. However, with extrapleural anterolateral approach, posterior Hartshill fixation can be performed by the same approaches [40, 41].
The anterior decompression and fusion and posterior instrumentation can also be performed by posterior midline incision by transpedicular approach. The anterior decompression is performed through the pedicle of apex vertebra followed by posterior stabilization spanning two healthy vertebrae on each side of the lesion in dorsal and one in lumbar spine [42]. The spine can be stabilized by Hartshill instrumentation [43] or by pedicle screw system [44]. The advantage of this procedure is that anterior decompression and posterior instrumentation can be performed by one incision and approach, but the disadvantage is that we need to excise the healthy posterior complex so that the instrumented stabilization is a must. In a long segment (4 or more VB disease), the span of instrumentation is long. This is a good approach if anterior decompression for neurologic complication and correction of kyphotic deformity are indicated.
Prognosis in tuberculous para/quadriplegia
Surgery of tuberculous paraplegia/quadriplegia poses certain difficulties and anxiety for surgeons. Before planning the surgical procedures, the patient’s relatives must be warned that operation may be technically most difficult, and results may be most unpredictable as far as neurological outcome.
The younger patients with good nutritional state tolerate surgery well and are likely to show good neural recovery and healing. The paraplegia with active disease has better chance of neural recovery than those with healed disease. The cases with long standing paraplegia are likely to show poor outcome but still surgery should be done as this is the only chance for neural recovery. The rapid progression of paraplegia would have poor chance of neural recovery in comparison to slowly progressive neural deficit.
The preserved volume of spinal cord with edema/myelitis of cord on MRI would show a good neural recovery. Cord showing myelomalacia with reduction in cord volume would show a poor neural recovery. On surgical decompression, if pus is drained out (wet lesion) along with extradural compression of granulation tissue, the patient is likely to show better neural recovery in comparison to thick inspissated pus, caseous tissue, fibrous tissue, bony sequestra and disk (dry lesion).
Paraplegia with healed disease
The patients late onset paraplegia (paraplegia with healed disease) usually had extensive spinal tuberculosis in past but made an apparent recovery and remained symptomless apart from increasing kyphosis [2–4]. After a long period (4–40 years), paraplegia sets in, usually incomplete but sometimes severe. The severity of kyphotic deformity has not been correlated with development of neural deficit. Tuli [45] believed that chances of late onset paraplegia is more if the initial lesion heals with kyphosis of 60° or more.
This type of paraplegia is usually seen in patients with severe kyphosis in dorsal and dorsolumbar spine (Fig. 1a). It is attributed to the stretching of spinal cord over internal salient. There may indeed be a very sharp ridge at the angle of kyphosis, and the cord in its membrane may be found stretched across it like a violin string across a fiddle bridge. The prolonged anterior impingement on the cord by sharp osseous ridge or possibly from constriction caused by fibrosis around the neural element may produce interstitial gliosis (intrinsic damage) of the spinal cord (Fig. 1b, c). Persistent low grade infection with increasing kyphosis or reactivation of quiescent disease at the apex of kyphosis or away may add inflammation and/or mechanical compression.
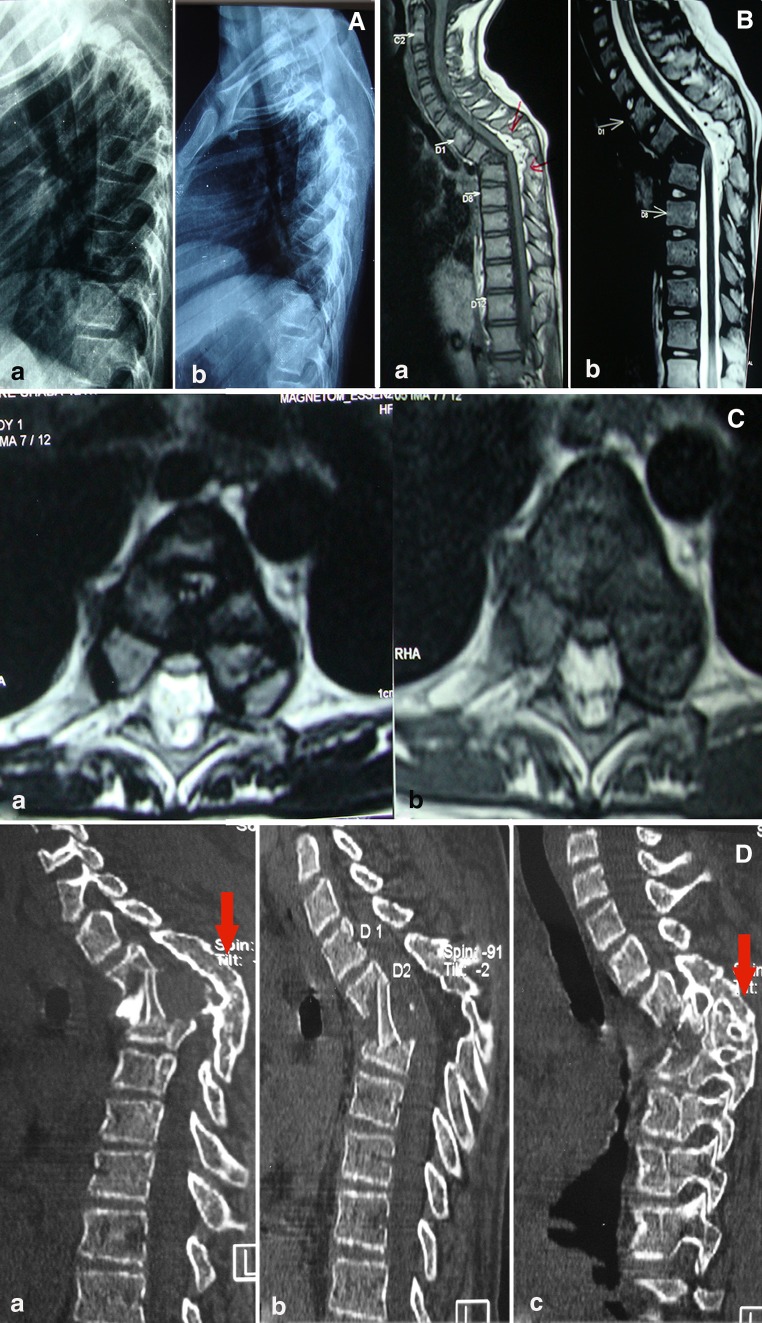
Fig. 1.
a Pre-operative lateral X-ray of dorsal spine (a) show healed TB of spine (D2–4) showing severe kyphotic deformity. Similar post operative X-ray (b) after surgery shows adequate decompression and minimal correction of kyphosis. b T1WI (a) and T2WI (b) midsagittal MRI shows healed vertebral body (D2–4) with severe kyphosis and showing internal salient indenting the spinal cord. The bright signal intensity (T2WI) on spinal cord at apex of kyphosis is suggestive of cord edema. c Axial T1WI (a) and T2WI (b) MRI images show shrunken cord with a cord edema at apex of deformity. d Post-operative reconstructed right parasagittal (a) and left parasagittal (c) image of CT scan show adequate anterior decompression with bone graft in situ. Obliteration of posterior facet joints (solid arrow) suggestive of spontaneous posterior fusion. Similar mid sagittal (b) CT scan image show adequate anterior decompression and interbody bone graft
Management
It is important to know whether the patient has paraplegia with healed lesion or reactivation/recrudescence. Clinically, paraplegia due to reactivation/recrudescence is severe and has a relatively rapidly developing course as compared to late onset paraplegia with healed lesion [46]. Similarly, such paraplegia with reactivation/recrudescence responds early and better to treatment than paraplegia with healed lesions.
Hsu [46] reported 22 cases of late onset paraplegia. Twelve cases had active infection at initial kyphosis out of which nine showed complete neural recovery, and three recovered partially with average recovery time 6.8 months after surgical decompression. The remaining 10 had paraplegia with hard bony ridge compressing the cord showed neural deterioration (n = 2), and neurapraxia of cord (n = 4) which slowly improved and two had CSF fistula after decompression surgery. The recovery time was variable, but in severe paraplegia recovery up to 24 months was observed. The selection of treatment in such cases was being helped by detailed imaging of the internal gibbus. The present author studied 17 of such cases with MRI and found active disease at kyphosis or proximal/distal to apex of kyphosis in 10 [46]. The cases responded to non-operative treatment. Rest of the cases had healed disease. The anterior transposition of cord was performed in one severe paraplegic and in two moderate paraplegics. All three cases made no neural recovery.
Anterior decompression with removal of internal gibbus is the treatment of choice (Fig. 1d) in such cases, though it seldom produces complete recovery as compared to paraplegia with reactivation and is fraught with risk of deterioration of neural deficit. The spinal cord has already exhausted the physiological reserve and inadvertent handling of spinal cord may produce deterioration of paraplegia. These cases with severe kyphosis, with costovertebral impingement, have poor pulmonary reserve, and are bad risk for major undertaking such as anterior decompression. Tuli [8] has suggested removal of internal gibbus only in those cases who have moderate to severe paraplegia, while mild paraplegics should not be decompressed.
Hsu [46] was of the opinion that patient with mild or moderate paraplegia should perhaps be treated by stabilization of spine only to prevent further progression of kyphosis and paraplegia, while decompression should be reserved for severe paralysis. The objective of surgical decompression here is not neural recovery but prevention of deterioration of neural deficit and progression of paraplegia, however, if any neural recovery that occurs is a bonus to the patient. Anterior decompression with removal of internal gibbus (internal kyphectomy) can be done by thoracotomy approach, but as they have severe kyphosis, extrapleural costotransversectomy approach is easier and gives a direct access to apex of kyphosis without jeopardizing the already compromised lung function [47]. We also believe that a long standing kyphosis in the posterior complex undergoes spontaneous fusion (Fig. 1d), hence the spine remains relatively stable in spite of anterior kyphectomy.
Correction of severe kyphosis for prevention of late onset paraplegia
The best form of treatment of late onset paraplegia is the prevention of development of severe kyphosis, hence either the initial lesion be diagnosed before a kyphosis develops or if patient reports with severe kyphosis or likely to develop 60° or more at the healing of lesion, then such cases should be undertaken for correction of kyphosis in active disease [48–50]. Once a kyphosis develops, correction is difficult and hazardous and there is an associated risk of neural deterioration if the correction of kyphosis is undertaken once patient had paraplegia with healed disease.
Conflict of interest
None.
References
- 1.Jain AK. Treatment of tuberculosis of the spine with neurologic complications in symposium on Osteo-articular tuberculosis. Clin Orthop Rel Res. 2002;398:75–84. doi: 10.1097/00003086-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Crenshaw AH (ed) (1987) Tuberculosis of spine, Campbell’s operative orthopaedics, vol 4, 7th edn. CV Mosby, St. Louis, pp 3326–3342
- 3.Griffiths DL (1979) The treatment of spinal tuberculosis. In: Mc Kibbin B (ed) Recent advances in orthopaedics, vol 3, pp 1–17
- 4.Griffiths DL, Sededon HL, Roaf R. Pott’s paraplegia. London: Oxford University Press; 1956. [Google Scholar]
- 5.Seddon HJ. Pott’s paraplegia, prognosis and treatment. Br J Surg. 1935;22:769–799. doi: 10.1002/bjs.1800228813. [DOI] [Google Scholar]
- 6.Seddon HJ (1956) Pott’s paraplegia. In: Platt H (ed) Modern trends in orthopaedics. Series II. Butterworth and Co, London, pp 230–234
- 7.Hodgson AR, Skinsnes OK, Leong CY. The pathogenesis of Pott’s paraplegia. JBJS. 1967;49A:1147–1156. [PubMed] [Google Scholar]
- 8.Tuli SM. Neurological complications in tuberculosis of skeletal system. New Delhi: Jaypee Brothers Medical Publishers Pvt. Ltd.; 2010. p. 251. [Google Scholar]
- 9.Hoffman EB, Crosier JH, Cremin BJ. Imaging in children with spinal tuberculosis—a comparison of radiography, computed tomography and magnetic resonance imaging. JBJS. 1993;75B:233–239. doi: 10.1302/0301-620X.75B2.8444943. [DOI] [PubMed] [Google Scholar]
- 10.Jain AK, Agarwal AN, Mehrotra G. Correlation of canal encroachment with neurological deficit in tuberculosis of spine. Int Orthop. 1999;23(2):85–86. doi: 10.1007/s002640050313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain AK. Tuberculosis of spine: a fresh look to an old disease. J Bone Joint Surg (Br) 2010;92(7):905–913. doi: 10.1302/0301-620X.92B7.24668. [DOI] [PubMed] [Google Scholar]
- 12.Jain AK, Jena A, Tuli SM (2000) Correlation of clinical course with magnetic resonance imaging in tuberculous myelopathy. Neurol India 48(2):132–139 [PubMed]
- 13.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia. 1969;7(3):179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 14.Jain AK, Sinha S. Evaluation of paraplegia grading systems in tuberculosis of the spine. Spinal Cord. 2005;43(6):375–380. doi: 10.1038/sj.sc.3101718. [DOI] [PubMed] [Google Scholar]
- 15.Tuli SM. Treatment of neurological complications in tuberculosis of the spine. JBJS. 1969;51a:680–692. [PubMed] [Google Scholar]
- 16.Jain AK, Dhammi IK, Kumar S, Singh S. Intraspinal tubercular granuloma. Indian J Orthop. 2003;37(3):182–185. [Google Scholar]
- 17.Babhulkar SS, Tayado WB, Babhulkar SK. Atypical spinal tuberculosis. JBJS. 1984;66B:239–242. doi: 10.1302/0301-620X.66B2.6707060. [DOI] [PubMed] [Google Scholar]
- 18.Dobson J. Tuberculosis of the spine; an analysis of the results of conservative treatment and of the factors influencing the prognosis. J Bone Joint Surg Br. 1951;33-B:517–531. doi: 10.1302/0301-620X.33B4.517. [DOI] [PubMed] [Google Scholar]
- 19.Medical Research Council (1974) A controlled trial of anterior spinal fusion and debridement in surgical management of tuberculosis of the spine in patients on standard chemotherapy—a study in Hong Kong. Br J Surg 61(11):853–866 [DOI] [PubMed]
- 20.Medical Research Council (1976) A 5-year assessment of controlled trials of inpatient and outpatient treatment and of plaster of Paris jackets for tuberculosis of the spine in children on standard chemotherapy. JBJS 58b:339–411 [DOI] [PubMed]
- 21.Medical Research Council (1973) A controlled trial of ambulant out-patient treatment and in patient rest in bed in the management of tuberculosis of the spine in young Korean patients on standard chemotherapy. JBJS 55B:678–697 [PubMed]
- 22.Medical Research Council (1978) A 5-year assessment of controlled trials of ambulatory treatment, debridement and anterior spinal fusion in the management of tuberculosis of the spine—studies in Bulawayo (Rhodesia) and in Hong Kong: VI Report. JBJS 60B:163–177 [DOI] [PubMed]
- 23.Medical Research Council (1985) A 10-year assessment of controlled trials of inpatients and outpatient treatment and of plaster of Paris jackets for tuberculosis of the spine in children on standard chemotherapy. JBJS 67B:103–110 [DOI] [PubMed]
- 24.Medical Research Council (1982) A 10-year assessment of controlled trial comparing debridement and anterior spinal fusion in the management of tuberculosis of the spine in patients on standard chemotherapy in Hongkong. J Bone Joint Surg 64B:393–398 [DOI] [PubMed]
- 25.Hodgson AR, Stock FE. Anterior spinal fusion for the treatment of tuberculosis of the spine. JBJS. 1960;42A:295–310. [Google Scholar]
- 26.Kohli SB. Radical surgical approach to spinal tuberculosis. J Bone Joint Surg. 1967;49B:668–673. [PubMed] [Google Scholar]
- 27.Goel MK. Treatment of Pott’s paraplegia by operation. J Bone Joint Surg. 1967;49B:674–681. [PubMed] [Google Scholar]
- 28.Lifeso RM, Weaver P, Harder EH. Tuberculous spondylitis in adults. JBJS. 1985;64A:1405–1413. [PubMed] [Google Scholar]
- 29.Tuli SM. Results of treatment of spinal tuberculosis by “middle-path” regime. J Boone Joint Surg (Br) 1975;57B:13–23. [PubMed] [Google Scholar]
- 30.Rand C, Smith MA. Anterior spinal tuberculosis—paraplegia following laminectomy. Ann R Coll Surg Eng. 1989;71:105–109. [PMC free article] [PubMed] [Google Scholar]
- 31.Upadhyay SS, Seff P, Saji MJ, et al. Surgical management of spinal tuberculosis in adults. Clin Orthop Relat Res. 1994;302:173–182. [PubMed] [Google Scholar]
- 32.Upadhyay SS, Saji MJ, Sell P, et al. Longitudinal changes in spinal deformity after anterior spinal surgery for tuberculosis of the spine in adult. Spine. 1994;19:542–549. doi: 10.1097/00007632-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Upadhyay SS, Saji MJ, Sell P, et al. The effect of age on the change in deformity after radical resection and anterior arthrodesis for tuberculosis of the spine. JBJS. 1994;76A:701–708. doi: 10.2106/00004623-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Jenkin DH, Hodson AR, Yan AC, Dwyer AP, O’Mahoney G. Stabilization of the spine in the treatment of severe spinal tuberculosis in children. Clin Orthop Relat Res. 1975;110:69–80. doi: 10.1097/00003086-197507000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Rajasekaran S, Soundarapandian S. Progression of kyphosis in tuberculosis of the spine treatment by anterior arthrodesis. JBJS. 1989;71A:1314–1323. [PubMed] [Google Scholar]
- 36.Rajasekaran S, Shanmugasundaram TK. Prediction of the angle of gibbus deformity in tuberculosis of the spine. JBJS. 1987;69A:503–508. [PubMed] [Google Scholar]
- 37.Chen WJ, Chen CH, Shich CH. Surgical treatment of tuberculous spondylitis: 50 patients followed for 2–8 years. Acta Orthop Scand. 1995;66:137–142. doi: 10.3109/17453679508995507. [DOI] [PubMed] [Google Scholar]
- 38.Jain AK, Agarwal P, Singh S, Arora A. Extrapleural anterolateral decompression in spinal tuberculosis. J. Bone Joint Surg. 2004;86-B(7):1027–1031. doi: 10.1302/0301-620X.86B7.14546. [DOI] [PubMed] [Google Scholar]
- 39.Adendroff JJ, Boke EJ, Lazarus C. Tuberculosis of the spine result of management of 300 patients. JR Coll Edinburg. 1987;32:152–155. [PubMed] [Google Scholar]
- 40.Adendroff JJ, Boke EJ, Lazarus C. Pott’s paraplegia. S Afr Med J. 1987;71:427–428. [PubMed] [Google Scholar]
- 41.Jain AK, Dhammi IK, Prasad B, Mishra P. Simultaneous anterior decompression and posterior instrumentation of the tuberculous spine using an anterolateral extrapleural approach. J Bone joint Surg (Br) 2008;90-B:1477–1481. doi: 10.1302/0301-620X.90B11.20972. [DOI] [PubMed] [Google Scholar]
- 42.Guven O, Kumano K, Yalcin S, Karahan M, Tsuji S. A single stage posterior approach and rigid fixation for preventing kyphosis in the treatment of spinal tuberculosis. Spine. 1994;19:1039–1043. doi: 10.1097/00007632-199405000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429–436. doi: 10.1038/sj.sc.3101185. [DOI] [PubMed] [Google Scholar]
- 44.Lee SH, Sung JK, Park YM. Single-stage transpedicular decompression and posterior instrumentation in treatment of thoracic and thoracolumbar spinal tuberculosis: a retrospective case series. J Spinal Disord Tech. 2006;19:595–602. doi: 10.1097/01.bsd.0000211241.06588.7b. [DOI] [PubMed] [Google Scholar]
- 45.Tuli SM. Current concepts: severe kyphotic deformity in tuberculosis of the spine. International Orthop (Sicot) 1995;19:327–331. doi: 10.1007/BF00181121. [DOI] [PubMed] [Google Scholar]
- 46.Hsu LCS, Cheng CC, Leong JCY. Pott’s paraplegia of late onset—the causes of compression and results after anterior decompression. JBJS. 1988;70B:534–538. doi: 10.1302/0301-620X.70B4.3403593. [DOI] [PubMed] [Google Scholar]
- 47.Wong YW, Leong JC, Luk KD. Direct internal kyphectomy for severe angular tuberculous kyphosis. Clin Orthop Relat Res. 2007;460:124–129. doi: 10.1097/BLO.0b013e31805470db. [DOI] [PubMed] [Google Scholar]
- 48.Jain AK, Maheshwari AV, Jena S. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117–123. doi: 10.1097/BLO.0b013e318073bd29. [DOI] [PubMed] [Google Scholar]
- 49.Jain AK, Dhammi IK, Jain S, Mishra P (2010) Kyphosis in spinal tuberculosis—prevention and correction. Indian J Orthop 44(2):127–136 [DOI] [PMC free article] [PubMed]
- 50.Yau ACMC, Hsu LCS, O’Brien JP, et al. Tuberculous kyphosis—correction with spinal osteotomy, halo-pelvic distraction, and anterior and posterior fusion. JBJS. 1974;56A:1419–1434. [PubMed] [Google Scholar]