Abstract
According to WHO estimates, in 2010 there were 8.8 million new cases of tuberculosis (TB) and 1.5 million deaths. TB has been classically associated with poverty, overcrowding and malnutrition. Low income countries and deprived areas, within big cities in developed countries, present the highest TB incidences and TB mortality rates. These are the settings where immigration, important social inequalities, HIV infection and drug or alcohol abuse may coexist, all factors strongly associated with TB. In spite of the political, economical, research and community efforts, TB remains a major global health problem worldwide. Moreover, in this new century, new challenges such as multidrug-resistance extension, migration to big cities and the new treatments with anti-tumour necrosis alpha factor for inflammatory diseases have emerged and threaten the decreasing trend in the global number of TB cases in the last years. We must also be aware about the impact that smoking and diabetes pandemics may be having on the incidence of TB. The existence of a good TB Prevention and Control Program is essential to fight against TB. The coordination among clinicians, microbiologists, epidemiologists and others, and the link between surveillance, control and research should always be a priority for a TB Program. Each city and country should define their needs according to the epidemiological situation. Local TB control programs will have to adapt to any new challenge that arises in order to respond to the needs of their population.
Keywords: Epidemiology, Pott Disease, Risk factors, Spine, Tuberculosis
Introduction
Tuberculosis (TB) has been classically associated with poverty, overcrowding and malnutrition. Low income countries and deprived areas, within big cities in developed countries, present the highest TB incidences and TB mortality rates. These are settings where immigration, important social inequalities, HIV infection and drug or alcohol abuse may coexist, all factors strongly associated with TB [1].
According to the recent World Health Organization (WHO) report [1], since 2006 there has been a decreasing trend in the global number of TB cases. However, in 2010 there were an estimated 8.8 million new TB cases. TB remains the second leading cause of death in the world after HIV, with over 1 million deaths among HIV negative subjects and 0.35 million deaths among HIV/TB coinfected patients. The percentage of multidrug-resistant TB (MDR-TB: resistant to at least isoniazid and rifampin) among new TB cases seems to be stable at an estimated 3.4 %, whilst 20 % of previously treated cases are MDR-TB [1].
As recently pointed out by Keshavjee et al. [2], avoiding most deaths from TB should be possible with the current tools. However, only one in ten people with MDR-TB have access to effective treatment. The lack of adequate TB treatment, poor adherence, low treatment completion rates and absence of effective TB prevention and control programs, lead to the development of drug resistances and, consequently, to complicated management and uncertain clinical prognosis.
Tuberculosis remains a major global health problem, declared by the WHO, a global public health emergency in 1993. The efforts to find new diagnostic methods and resistance detection tools, new drugs and treatment regimens, in addition to the fight against poverty and concomitant factors, open a new way to greatly reduce the burden of TB, a disease as old as humanity.
Tuberculosis epidemiological situation
Natural history and pathogenesis
Tuberculosis transmission occurs through droplet nuclei containing Mycobacterium tuberculosis, which are expelled by smear-positive pulmonary TB patients when coughing and sneezing, and remain suspended in the air. Inhalation of such aerosols may lead to infection. After close contact with an infectious case, 30–50 % of exposed susceptible contacts acquire latent TB infection (LTBI). This can be determined by the tuberculin skin test (TST) or/and Interferon Gamma Release Assays (IGRAs) [3–5].
After this first infection, active TB may occur immediately. However, for the majority of cases, the initial infection remains clinically silent and microbiologically latent. Approximately 10 % of the infected individuals will progress to active TB during their lives, 5 % in the first two years, and therefore could become the source of infection for others. With adequate treatments, <1 % of TB cases will die.
Besides M. tuberculosis bacilli, other factors that contribute to TB incidence are all the conditions that could alter host cellular immunity, and therefore, increase the risk of developing active TB. These conditions include HIV infection, extremes of age, diabetes, alcohol, severe malnutrition and anti-tumour necrosis alpha factor (TNF-α) treatment. Therefore, preventing patients with LTBI from developing active disease is an important step to break the cycle of transmission and decrease the overall burden of TB worldwide [6–8].
Osteoarticular TB and Pott’s disease
Most of TB cases are pulmonary cases. Extrapulmonary TB (EP) and mixed TB are less frequent [9]. In Europe, osteoarticular TB represents the 8–15 % of EP, and it has been observed more frequently in native groups in higher age ranges [10] and in young immigrants coming from Asia and Sub-Saharan Africa. In half of theses cases, the vertebral column has been affected [11, 12].
Pott disease is an infrequent type of EP that affects the vertebral column. It results from the haematogenous dissemination of a previous TB focus, normally pulmonary TB. It is a chronic disease with slow progression that attends with granulomas, usually in high lumbar or low dorsal vertebrae. The infection can disseminate to the disc space and when two vertebrae are affected, the nutrition to the intervertebrae disc is interrupted. This can lead to the vertebrae shortening and eventually to spine collapse and medullar injury [13].
As in other cities with a good TB control, bone TB is rare in Barcelona. However, there is no accurate information about the incidence and management of Spine TB in the city. The TB Program is focused on control, and pulmonary TB is a priority. From 2000 to 2010, 126 osteoarticular cases were reported (2–3 % of all TB cases, 7 % of those with an EP), 78 men (62 %) and 48 among women (38 %). Most of the cases were Spanish-born older patients and foreign-born patients, mainly coming from India–Pakistan and usually young. The diagnostic delay was 90 days and in relation to risk factors, 8 (6.3 %) were HIV-infected, 6 (4.8 %) injecting drug-users (IDU), 37 (29 %) smokers, and 12 (9.5 %) alcohol abusers. The majority of the patients received a TB treatment longer than 6 months.
TB epidemiology in the world, Europe and big cities
More than 2,000 million people, one out of three persons in the world, are infected with M. tuberculosis. According to the WHO estimates, in 2010 there were 8.8 million new cases of TB (64 % of which amongst men) and 1.5 million deaths due to TB. Incidence rates have been decreasing since 2002. However, in 2008 there were approximately 440,000 cases of resistant TB were MDR [1, 14, 15]. In 2010, the 3,500,000 new cases in the South-East Asian Region constituted 40 per cent of the global incidence of TB. The African Region had 2,300,000 cases or 26 per cent of the total. The African, South-East Asian, and Western Pacific Regions made up 85 per cent of the total cases in the world, whereas the Eastern Mediterranean, European Regions and The Americas claimed only 650,000 (7 per cent) and 420,000 (5 per cent), and 270,000 (3 per cent) cases, respectively.
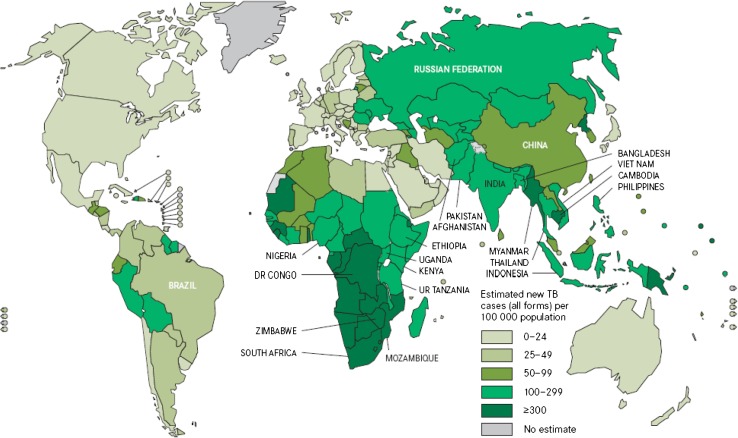
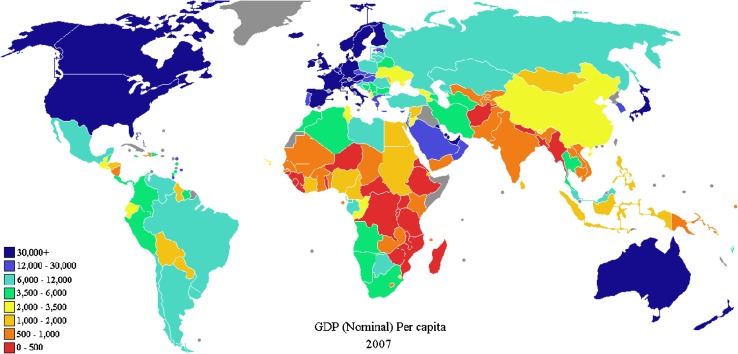
Figure 1 shows the estimated TB incidence in the world in 2009 and Fig. 2 the gross domestic product per capita in the world during 2007. There is a close relationship, as an overlap, among the poverty presentation in the world (Fig. 2) and the estimated TB incidence (Fig. 1), if both maps are compared. This is an easy and simple way to see that TB, as we will discuss later, is usually associated with poverty.
Fig. 1.
Estimated TB incidence rates in the world, 2010. Source: Global Tuberculosis Control 2011. WHO, 2011
Fig. 2.
Gross domestic product per capita in the world, 2007. Source: International Monetary Fund, 2008
The TB incidence for the European region countries ranges, according to WHO data 2010, from <5 per 100,000 to over 200 per 100,000, underlying the high differences in the continent among the Eastern and Western countries. The ECDC/WHO surveillance report 2009 described the situation in the European Region as a mixed epidemiological picture [16]. For example, the European Region has 5.6 % of new detected and relapsed TB cases in the world, the majority of them from the high-priority countries located in the Eastern Region [1, 16]. On one hand, TB notifications have been decreasing since 2005; while on the other, TB rates in these 18 high-priority countries is still around 8 times higher than in the rest of Europe [1]. It is important to note that it is estimated that Europe detection rate is 79 % of all TB cases.
Another concern in the East European region is the high percentage of M/XDR TB and the prevalence of HIV infection. The percentage of MDR TB is overwhelming with the highest percentages of MDR amongst new and re-treatment TB cases (12 and 37 %, respectively). MDR remained most frequent in the Baltic States (17.4–28 %) and Romania (11.2 %) in which XDR reporting had the highest numbers (22 cases total). Regarding to HIV, the prevalence among TB cases is 3.9 %. Although it is known that the HIV associated to TB notifications in the European Region are underreported, estimated detection rates of 46 % [1, 16].
Migrations have been increasing for the last 20 years. In 2010, over 200 million people (3.1 % of the world’s population) were estimated to live in a country different from that where they were born. As migration tends to take the direction from less economically developed countries, where TB infection and disease are more prevalent, to richer ones, it has become an important factor of TB control in the latest. In fact, about 25 % of TB cases in Europe are amongst immigrants and in some European cities this percentage has reached over 50 % [17].
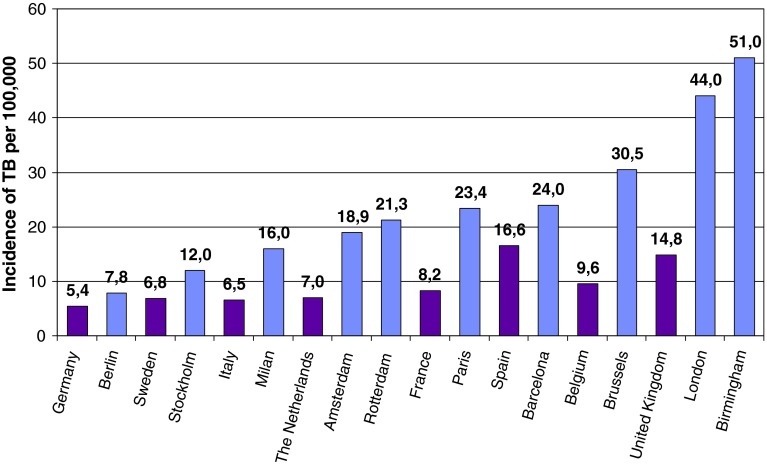
Tuberculosis affects vulnerable populations like HIV- infected people, drug abusers, homeless, immigrants in a disproportional way. These populations tend to live in urban areas affecting TB epidemiology of large cities, in which incidence rates might be higher than the national incidence rate [18]. Great variability in different cities within the same country has also been observed. For example, high incidences were detected in 2009 in Manchester (59.1/100,000) and in London (44.4/100,000) compared to other English cities [19, 20]. Figure 3 shows TB rates in some European cities and countries.
Fig. 3.
Incidence rates in some European cities and countries. Source: Urban TB control in the EU. Results questionnaire meeting urban TB control, 10th December 2010, Stockholm ECDC
TB and the HIV influence
The emergence of HIV has had an unprecedented impact on the epidemiology of infectious diseases in general and particularly on TB. In fact, HIV infection and TB are the pandemics which cause a higher number of deaths in the world annually, estimated at 1.8 and 1.2–1.5 million deaths, respectively. Of the 8.8 million incident TB cases in 2010, 1.1 million (13 %) were among people living with HIV [1]. Thus, the emergence of HIV has not only increased TB incidence and TB associated mortality but it also has made the diagnostics of TB more difficult [21]. TB/HIV coinfection affects mainly young adults in the most productive years of life, having a significant social and economical impact.
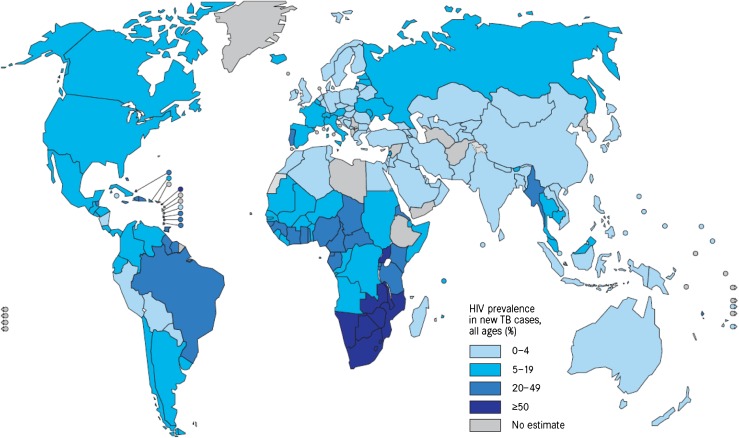
One-third of the HIV-infected world population also has LTBI. While the risk of developing TB among those infected only with M. tuberculosis is about 10 % during their lifetime, among HIV positive patients infected with MTB it is of the order of 10 % annually [22, 23]. Thus, the spread of the HIV infection has contributed to the extension of TB, which is the main cause of mortality in HIV patients. TB is, also, the most frequent AIDS defining illness globally. Due to its impact on individual health and its potential for transmission in the community, this comorbidity must be considered and treated as a global problem of maximum priority for public health. Figure 4 shows the HIV prevalence among new TB cases during 2010.
Fig. 4.
HIV prevalence among new tuberculosis cases, 2010. Source: Global Tuberculosis Control. WHO, 2011
Although everyone is susceptible to disease, neither of the epidemics affects all countries nor people equally. HIV, as mentioned before regarding TB, has also been associated with poverty. The higher prevalences of HIV infection among TB patients and LTBI, are found in low income settings, especially in Sub-Saharian Africa, and in run-down areas in big cities due to influence of IDUs [23, 24].
The impact of antiretrovirals on TB development and survival
After the identification of AIDS in 1981, it was observed that patients had a low survival rate, for which there was no other treatment than the one for the associated opportunistic diseases. The association between TB and HIV became more evident in 1987 when EP-TB in HIV-infected patients was included as an AIDS defining illness and even more so in 1994 when pulmonary TB in HIV-infected was also included.
During the period from 1987 to 1994, prevention and treatment of opportunistic infections improved survival [24]. The first antiretroviral, zidovudine, was approved by the Food and Drug Administration in 1987 and authorized for AIDS treatment in 1990 [25]. Monotherapy with this drug contributed to the initial increase of survival, which meant the beginning of antiretroviral therapy and the tearing of the idea that AIDS was a synonym of death. Nevertheless, AIDS was, together with MDR-TB and treatment abandonment, the most important predictive factor [26, 27].
The introduction of the nucleoside reverse transcriptase inhibitors in 1994 and the association of these to the protease inhibitors in 1996 increased survival in a drastic way [28–30]. The expansion in the use of combinations of 3 or more drugs since 1999 was the major influence factor [31]. This therapy was called highly active antiretroviral treatment (HAART), and the access and adherence to it by HIV-infected TB patients was the key to the improvement of their survival.
With HAART, the prevention measures and the improvement and access to the health services have made it, so that in a few years after the identification of HIV, the infection that used to cause death in a few months has become a chronical disease. Nevertheless, this change has not been observed in places where HAART coverage is low. Still today, countries that do not have access to HAART and in places where the treatment is not universal or free of cost, survival has not improved as much. The improvement in the immune system of these patients has decreased the risk of developing TB in a 70–90 %. However, despite HAART, HIV continues to be the main risk factor for TB.
TB, HIV and the influence of drug abuse
The influence of drug abuse on the epidemiology of TB is well known in developed and developing countries even before the HIV epidemics. Socio-economic factors, such as poor living conditions, homelessness, incarceration, poverty, tobacco use and alcohol abuse, place people who use drugs at higher risk for developing TB [1]. Drug use was described as an important TB risk factor for LTBI and incidence of TB disease whether drugs were injected or not. However, since the emergence of the HIV epidemic, the HIV-induced immunosuppression is considered the most important risk of progression to active TB disease [32]. Several studies have shown that IDU are at greater risk for TB infection and disease relative to other HIV-associated transmission groups. High prevalence of TB co-infection is usually reported among HIV-positive IDUs, particularly in prison.
As we will comment, the basis of an effective TB control programme is identification and treatment of the cases. Studies have reported that IDU have difficulty completing medical evaluations and adhering to treatment. Even when barriers to healthcare access are overcome, adherence to long treatment can be problematic for drug users. Some effective strategies are: outreach programs to engage substance abusers in nonmedical settings, such as prisons and the streets, active screening programs for HIV, C hepatitis, and TB, increased and broadened clinician expertise, knowledge and avoidance of drug interactions, access to microbiology services, treatment facilities, drugs and support for all diagnosed patients, TB screening/diagnosis includes medical history, chest X-ray, TST, and, if available, IGRA strategies to insure treatment adherence. All of these strategies require structural changes directed at comprehensive prevention and treatment programs and increased collaboration and integration of needed services for substance abusers [33, 34].
TB and other risk factors: the examples of tobacco and poverty
It is estimated that 30 % of the adult world population (1.250 million approximately) are smokers, 45 % of men (around 1.000 million) and 12 % of women (250 million). As commented, TB presents a strong association with multiple socioeconomical factors, such as malnutrition, alcohol abuse, drug abuse, tobacco consumption and social inequality. The literature that links, for example, poverty and tobacco is extensive. The majority of TB cases are in low or intermediate income countries where tobacco, HIV infection and AIDS amongst others are common factors. There is a concordance between the geographical TB distribution and poverty and the presence of tobacco consumption in the five continents; concordance that follows dynamic and similar patterns in both cases [35–37].
It is estimated that, by 2030, 80 % of deaths caused by tobacco consumption will be in low or intermediate income countries. It is important to highlight that the economically impoverished social class groups will account for the higher numbers of smokers in high income countries [38]. According to WHO (2009), close to 70 % of smokers live in low or intermediate income countries [39]. The Chinese population consume approximately 30 % of the world’s cigarettes and India is the country in the world with the highest tobacco production and consumption with a total of 182 million smokers.
In the last few years, there has been a number of publications which highlight the important relationship between TB (active and latent) and tobacco use [40–44]. In a meta-analysis, published in 2007, Slama et al. evaluated the quality of the published works. The association between being a smoker and the development of TB was measured though 25 items, found this association in two cohort studies, in six case-control studies and in two cross-sectional studies; six of them showed a dose-response effect. The meta-analysis for the high quality studies found that tobacco consumption was associated with a superior to two combined risk to present TB, independently from localization and compared with those who had never smoked [45]. Other revisions published in the same year found similar levels of evidence [46, 47]. In relation to tobacco consumption and LTBI, data does not seem as concluding [45–47]. Something similar happens in respect to passive smoking individuals and TB [45–51] and encourages the need for more studies.
Consciousness-raising campaigns against tobacco and the implementation of restrictive policies, especially in high income settings, have made the tobacco industry focus its attention on more permissive societies with a lower income. This fact will lead to an increase in the burden of disease and premature deaths in low income countries with high population growth rates and low quality and difficult to access health services. The impact of the increase in tobacco consumption will have to be followed-up. Moreover, this factor will have to be considered in TB control programs. Only with a multidisciplinary approach where measures that observe coadjutant factors it will be able to impact on disease that kills every year close to 2 million people [1].
Drug resistance and TB
Recent data confirm the high rates of TB caused by resistant strains in Eastern Europe and Central Asia [52]. This would mean 650,000 cases of MDR-TB amongst the 12 million cases in the world for 2010 [1]. The use of antimicrobials, which do not fit the sensibility of the causal agents and low adherence and completion of treatments, together with poor TB control programs and lack of access to drugs have been appointed as associated factors in the increase of resistances [53]. In order to favour and standardize the management of resistant forms, the WHO has developed guidelines. In these, it highlights the importance of early detection and diagnose of resistant cases, the establishment of an efficacious treatment and the development of an adequate prevention that allows, together with the epidemiological surveillance, monitoring and evaluation of national programs, to avoid the transmission of new cases [54]. Only 60 % of countries have available notification data [1].
European- and global-resistance numbers vary according to the analysed countries. Only one in every ten MDR-TB patients has access to adequate treatment. Treatment success in these patients varies between 25 and 82 % which states the great existing variability. As mentioned, Eastern Europe presents the highest TB incidences in the European continent and it is also the region with higher resistance rates (up to 65 % in Moldova or 60 % in Belarus) [1]. Up to 19.4 % of the strains in Moldova present a primary MDR profile, 15.4 % in Estonia and 14.2 % in Kazakhstan. Seven of the 10 countries with higher rates of MDR strains are European (Moldova, Estonia, Kazakhstan, Latvia, Armenia, Lithuania, and Georgia) [54]. A trend towards stability can be observed in the American region, while a decrease is the trend in Mediterranean Europe, Southeast Asia and Western Pacific regions [1].
It is estimated that there are 25,000 new cases of XDR-TB every year. In 2010, 69 countries reported at least one XDR-TB case [1]. These strains are resistant to isoniazid, rifampin, and also to quinolones and to at least one second-line-injected drug for TB treatment. XDR-TB represents an important and increasing health problem with complicated treatment, management and prognosis [55].
In the city of Barcelona, during 2009, 35 of the 285 culture positive patients presented some sort of resistance. Primary resistance to isoniazid was 6.9 %, 5.2 % in Spanish born and 8.7 % in immigrants. Rifampin resistance was found in only 1.9 % samples (0.7 and 3.1 % in Spanish-born and immigrants, respectively). Only 1.1 % of cases presented MDR, 0.7 % in Spanish-born and 1.6 % subjects born abroad. During 2009, no XDR cases were identified [56].
In the last few years, public consortiums, together with the pharmaceutical industry and private partners, joined together in the research of better and more simple early diagnosis methods, new drugs and drug combinations for the efficacious treatment of these patients [57, 58]. The use of already known drugs for new indications (such as linezolid, thioridazine and fluoroquinolones) associated with new research antimicrobials (new rifamicin derivatives, azoles, nitroimidazoles, or diarylquinolones) could offer new therapeutical possibilities in the next few years [59–61].
The influence of TB programs
Biggs already highlighted in 1914 that public health can be purchased and that each community can determine its own mortality rate [62]. Reichman [63] added that each country can decide how much TB it wants according to the resources it invests in the control of this disease.
Tuberculosis control is based on complicated programs that require the completion of long treatments and contact tracing. In the US, the dismantling of the control programs during the 80s’ was accompanied by an increase of the incidence, and subsequently, as they were reimplemented, a progressive control of TB was observed [64]. New York City also experienced a difficult epidemiological situation. From 1978 through 1992, the number of TB patients nearly tripled due to HIV infection, drug resistances and abandonment of TB programs, but fortunately they were able to apply strong control measures (DOT, control of nosocomial transmission, etc.) and the situation reversed [65]. The peak on incidence was observed in 1992 (52.0/100,000) declining to 10.8/100,000 in 2008 [66].
In Barcelona, the TB program began in 1987 with Public Health Nurses (PHN) who performed the follow-up of patients and the contact tracing. This program also developed an active epidemiologic surveillance system (the notification of cases was encouraged, microbiological results and hospital discharges were controlled, and the AIDS and TB registers were linked) [67]. At the early stages, the influence of IDU and HIV infection on TB was observed, and the incidence increased to its peak in 1992 (68/100,000) [67]. Early on, DOT for patients with predictors of poor adherence (homeless, IDU, prisoners) was implemented. A strong coordination between TB programs in prisons and the city methadone program was very useful to achieve a high level of completion among IDU. Due to DOT and the indirect effect of HAART, TB incidence started to decrease, but with the massive immigration flow that took place since 2000, the incidence decline was lowered. Since 2003, community health workers (CHW) were incorporated to the program to help PHN in the follow-up and contact tracing. CHW visit patients at home, in the hospital, in DOT facilities and act as translators and cultural mediators [68].
In TB programs, the coordination among clinicians, microbiologists, epidemiologists, and others, and the link between surveillance, control and research should always be a priority since it enhances the coordination of health workers involved in TB control. Furthermore, with the current economic crises, TB programs should be based on improvements in health organization. It is important to evaluate TB programs every year to identify gaps in services and in data monitoring. TB programs should assess a few indicators (TB incidence, diagnostic delay, treatment completion, coverage of contact tracing, and TB meningitis in children under 4 years) in order to facilitate annual evaluations [69, 70].
Tuberculosis control needs efficient programs based on improving organisational aspects. To take advantage of other resources for communicable diseases (HIV, etc.) and the cooperation with other programs will be essential. In this way, each country and city needs to define its needs according to the epidemiological situation, but the ratio of 1 nurse per 40 TB cases is a very good starting point in developed countries. It is crucial to evaluate TB programs every year to early detect gaps and adapt them to the needs of the changing populations.
Conclusions
Tuberculosis represents a health problem with a very high incidence and associated deaths worldwide. Only one in ten MDR-TB patients receive an appropriate treatment and the poor implementation of efficacious and rapid diagnostic methods for resistance detection hinders the correct treatment of MDR-TB. However, a global decrease in TB has been observed during the last decade as a consequence of hard work in implementing prevention and control campaigns.
Tuberculosis remains an important Public Health problem in big cities in developed countries, as well. For this reason, National Health Services in each country should prioritize the TB Prevention and Control Programs in big cities. TB programs should also be aware of the impact of new inflammatory disease treatments (anti-TNF) and other high prevalent disease, such as diabetes. The number of people with diabetes is increasing worldwide and it has been associated with higher risks of TB and adverse TB treatment outcomes that could complicate TB care and control [71].
One of the most important components of the Stop TB Strategy launched by the WHO in 2006 to reduce the disease burden by 2015 in the context of the Millennium Development Goals was to enhance the Direct Observed Treatment Strategy (DOTS). Other components of the Stop TB Strategy are: the need to address the global problems of the co-epidemics TB/HIV and drug-resistant TB, the importance of engaging all care providers in TB control and care and of contributing to strengthening health systems, the role of communities and people with TB, and the fundamental role of research and development for new diagnostics, drugs and vaccines.
In the next few years, TB should benefit of other global health programs such as fight against poverty, tobacco consumption, immigrant assistance or HIV. Maintaining the TB program in a Public Health structure that also performs the surveillance and control of other reportable diseases can be crucial to achieve long time resources and management skills. Local TB control programs will have to adapt to any new challenge that arises in order to respond to the needs of their population.
Conflict of interest
None.
Contributor Information
Juan-Pablo Millet, Email: jmillet@aspb.es.
Joan A. Caylà, Email: jcayla@aspb.cat
References
- 1.World Health Organization (2011) Global Tuberculosis Control. http://www.who.int/tb/publications/global_report/en/
- 2.Keshavjee S, Harrington M, Gonsalves G, Chesire L, Farmer PE. Time for zero deaths from tuberculosis. Lancet. 2011;378(9801):1449–1450. doi: 10.1016/S0140-6736(11)61521-3. [DOI] [PubMed] [Google Scholar]
- 3.Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc. 1954;69(5):724–732. doi: 10.1164/art.1954.69.5.724. [DOI] [PubMed] [Google Scholar]
- 4.Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50(1):90–106. [PubMed] [Google Scholar]
- 5.van Geuns HA, Meijer J, Styblo K. Results of contact examination in Rotterdam, 1967–1969. Bull Int Union Tuberc. 1975;50(1):107–121. [PubMed] [Google Scholar]
- 6.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 7.Advisory Council for the Elimination of Tuberculosis (ACET) (1999) Tuberculosis elimination revisited: obstacles, opportunities, and a renewed commitment. MMWR Recomm Rep 48(RR-9):1–13 [PubMed]
- 8.Institute of Medicine (US) Committee on the Elimination of Tuberculosis in the United States (2000) Endling neglect: the elimination of tuberculosis in the United States, National Academy Press, Washington, DC [PubMed]
- 9.Orcau À, Caylà JA, Martínez JA. Present epidemiology of tuberculosis. Prevention and control programs. Enferm Infecc Microbiol Clin Suppl. 2011;1:2–7. doi: 10.1016/S0213-005X(11)70011-8. [DOI] [PubMed] [Google Scholar]
- 10.Houshian S, Poulsen S, Riegels-Nielsen P. Bone and joint tuberculosis in Denmark: increase due to immigration. Acta Orthop Scand. 2000;71(3):312–315. doi: 10.1080/000164700317411942. [DOI] [PubMed] [Google Scholar]
- 11.Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J. 2008;31(1):99–105. doi: 10.1183/09031936.00020607. [DOI] [PubMed] [Google Scholar]
- 12.Talbot JC, Bismil Q, Saralaya D, Newton DA, Frizzel RM, Shaw DL. Musculoskeletal tuberculosis in Bradford—a 6-year review. Ann R Coll Surg Engl. 2007;89(4):405–409. doi: 10.1308/003588407X183328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.García-Lechuz JM, Julve R, Alcala L, Ruíz-Serrano MJ, Muñoz P. Espondilodiscitis tuberculosa o enfermedad de Pott: experiencia en un hospital general. Enferm Infecc Microbiol Clin. 2002;20(1):5–9. doi: 10.1016/S0213-005X(02)72723-7. [DOI] [PubMed] [Google Scholar]
- 14.United Nations, Department of Economic and Social Affairs, Population Division (2009) Trends in International Migrant Stock: the 2008 revision (United Nations database, POP/DB/MIG/Stock/Rev.2008)
- 15.Rieder HL, Zellweger JP, Raviglione MC, Keizer ST, Migliori GB. Tuberculosis control in Europe and international migration. Eur Respir J. 1994;7:1545–1553. doi: 10.1183/09031936.94.07081545. [DOI] [PubMed] [Google Scholar]
- 16.Tuberculosis Surveillance Report in Europe 2009. European Centre for Disease Prevention and Control (ECDC) and WHO regional office for Europe
- 17.Marin M, Moreno R, Gil M, Bueso MJ, Romeu MA, Gonzalez F. Impacto de la inmigración desde Rumanía en la tuberculosis de un area mediterránea. Enf Emerg. 2010;12:115–120. [Google Scholar]
- 18.Cayla JA, Orcau A Control of tuberculosis in large cities in developed countries: an organisational problem. BMC Medicine (in press) [DOI] [PMC free article] [PubMed]
- 19.Bothamley GH, Kruijshaar ME, Kunst H, Woltmann G, Cotton M, Saralaya D, Woodhead MA, Watson JP, Chapman ALN. Tuberculosis in UK cities: workload and effectiveness of TB control programmes. BMC Public Health (in press) [DOI] [PMC free article] [PubMed]
- 20.Tuberculosis in the UK. Report on tuberculosis surveillance in the UK 2010 Health Protection Agency
- 21.Gatell JM, Zulaica D, Del Romero J. Cómo promover y facilitar el diagnóstico precoz de la infección por el VIH-1. Enf Emerg. 2010;12:121–124. [Google Scholar]
- 22.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320:545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 23.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 24.Decker CF, Masur H. Current status of prophylaxis for opportunistic infections in HIV-infected patients. AIDS. 1994;8:11–20. doi: 10.1097/00002030-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Fischl MA, Richman DD, Grieco MH, Gottlieb MS, Volberding PA, Laskin OL, Leedom JM, Groopman JE, Mildvan D, Schooley RT, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 26.Leroy V, Salmi LR, Dupon M, Sentilhes A, Texier-Maugein J, Dequae L, Dabis F, Salamon R. A cohort study in Bordeaux, France, 1988–1994. The Groupe d’Epidémiologie Clinique du Sida en Aquitaine (GECSA) Am J Epidemiol. 1997;15:293–300. doi: 10.1093/oxfordjournals.aje.a009105. [DOI] [PubMed] [Google Scholar]
- 27.Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223–1228. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 28.García de Olalla P, Martínez-González MA, Caylà JA, Jansà JM, Iglesias B, Guerrero R, Marco A, Gatell JM, Ocaña I, Barcelona AIDS–TB Study Group Influence of highly active anti-retroviral therapy (HAART) on the natural history of extra-pulmonary tuberculosis in HIV patients. Int J Tuberc Lung Dis. 2002;6:1051–1057. [PubMed] [Google Scholar]
- 29.Leonard MK, Larsen N, Drechsler H, et al. Increased survival of persons with tuberculosis and human immunodeficiency virus infection, 1991–2000. Clin Infect Dis. 2002;34:1002–1007. doi: 10.1086/339448. [DOI] [PubMed] [Google Scholar]
- 30.Thseng SH, Shyong-Jiang DD, Hoi HS, Lo HY, Hwang KP. Effect of free treatment and surveillance on HIV-infected persons who have tuberculosis, Taiwan, 1993–2006. Emerg Infect Dis. 2009;15:332–334. doi: 10.3201/eid1502.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hung CC, Chen MY, Hsiao CF, Hsieh SM, Sheng WH, Chang SC. Improved outcomes of HIV-1-infected adults with tuberculosis in the era of highly active antiretroviral therapy. AIDS. 2003;17:2615–2622. doi: 10.1097/00002030-200312050-00008. [DOI] [PubMed] [Google Scholar]
- 32.Selwyn PA, Hartel D, Lewis VA, Schoenbaum EE, Vermund SH, Klein RS, et al. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989;320(9):545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- 33.Deiss RG, Rodwell TC, Garfein RS. Tuberculosis and illicit drug use: review and update. Clin Infect Dis. 2009;48(1):72–82. doi: 10.1086/594126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Centre for Disease Prevention and Control and European Monitoring Centre for Drugs and Drug Addiction (2011) Prevention and control of infectious diseases among people who inject drugs. Stockholm: ECDC. http://www.ecdc.europa.eu/en/publications/Publications/111012_Guidance_ECDC-EMCDDA.pdf)
- 35.WHO Report on the global tobacco epidemic (2011). http://whqlibdoc.who.int/publications/2011/9789240687813_eng.pdf
- 36.Shafey O, Eriksen M, Ross H, Mackay J (2009) El atlas del tabaco. Sociedad Americana del Cáncer 3:18–21
- 37.Ministerio de Sanidad. Encuesta Nacional de Salud de España (1987–2006).http://msps.es/estadEstudios/estadisticas/encuestaNacional/encuesta2006.htm
- 38.Leung CM, Leung KC, Hon KE, Kong AY. Fighting tobacco smoking—a difficult but not impossible battle. Int J Environ Res Public Health. 2009;6:69–83. doi: 10.3390/ijerph6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization (2007) The Union against tuberculosis and lung diseases. A WHO/The Union monograph on TB and tobacco control: joining efforts to control two related global epidemics; WHO/HTM/TB/2007.390:21–23
- 40.Alcaide J, Altet MN, Plans P, Parrón I, Folguera L, Saltó E, Domínguez A, Pardell H, Salleras L. Cigarette smoking as a risk factor for tuberculosis in young adults: a case–control study. Tuber Lung Dis. 1996;77:112–116. doi: 10.1016/S0962-8479(96)90024-6. [DOI] [PubMed] [Google Scholar]
- 41.Leng CC, Li T, Lam TH, Yew WW, Law WS, Tam CM, Chan WM, Chan CK, Ho KS, Chang KC. Smoking and tuberculosis among the elderly in Hong Kong. Am J Respir Crit Care Med. 2004;170:1027–1033. doi: 10.1164/rccm.200404-512OC. [DOI] [PubMed] [Google Scholar]
- 42.Lienhardt C, Fielding K, Sillah JS, Bah B, Gustafson P, Warndorff D, Palayew M, Lisse I, Donkor S, Diallo S, Manneh K, Adegbola A, Aaby P, Bah-Sow O, Bennett S, McAdam K. Investigation of the risk factors for tuberculosis: a case–control study in three countries in West Africa. Int J Epidemiol. 2005;34:914–923. doi: 10.1093/ije/dyi100. [DOI] [PubMed] [Google Scholar]
- 43.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan. Prospective cohort study. Am J Respir Crit Care Med. 2009;180:475–480. doi: 10.1164/rccm.200904-0549OC. [DOI] [PubMed] [Google Scholar]
- 44.Jee SH, Golub JE, Jo J, LlS Park, Ohrr H, Samet JM. Smoking and risk of tuberculosis incidence, mortality, and recurrence in South Korean men and women. Am J Epidemiol. 2009;170:1478–1485. doi: 10.1093/aje/kwp308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slama K, Chiang CY, Enarson DA, Hassmiller Fanning A, Gupta P, Ray C. Tobacco and tuberculosis: a qualitative systematic review and meta-analysis. Int J Tuberc Lung Dis. 2007;11:1049–1061. [PubMed] [Google Scholar]
- 46.Lin HH, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. doi: 10.1371/journal.pmed.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke. A systematic review and meta-analysis. Arch Intern Med. 2007;167:335–342. doi: 10.1001/archinte.167.4.335. [DOI] [PubMed] [Google Scholar]
- 48.den Boon S, Verver S, Marais BJ, Enarson DA, Lombard CJ, Bateman ED, Irusen E, Jithoo A, Gie RP, Borgdorff MW, Beyers N. Association between passive smoking and infection with Mycobacterium tuberculosis in children. Pediatrics. 2007;119(4):734–739. doi: 10.1542/peds.2006-1796. [DOI] [PubMed] [Google Scholar]
- 49.Leung CC, Lam TH, Ho KS, Yew WW, Tam CM, Chan WM, Law WS, Chan CK, Chang KC, Au KF. Passive smoking and tuberculosis. Arch Intern Med. 2010;170(3):287–292. doi: 10.1001/archinternmed.2009.506. [DOI] [PubMed] [Google Scholar]
- 50.Altet MN, Alcaide J, Plans P, Taberner JL, Saltó E, Ll Folguera, et al. Passive smoking and risk of pulmonary tuberculosis in children immediately following infection. A case–control study. Tub Lung Dis. 1996;77:537–544. doi: 10.1016/S0962-8479(96)90052-0. [DOI] [PubMed] [Google Scholar]
- 51.Singh M, Mynak ML, Kumar L, Mathew JL, Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005;90(6):624–628. doi: 10.1136/adc.2003.044255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization/International Union Against Tuberculosis and Lung Disease (2007) Global project on anti-tuberculosis drug resistance surveillance. Anti-tuberculosis drug resistance in the world: report no. 4. Geneva, Switzerland: WHO. http://www.who.int/tb/publications/2008/drs_report4_26feb08.pdf
- 53.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest. 2006;130:162–272. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization (2011) Guidelines for the programmatic management of drug-resistant tuberculosis. http://www.who.int/tb/challenges/mdr/programmatic_guidelines_for_mdrtb/en/index.html [PubMed]
- 55.Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015. Report 2011. http://www.who.int/tb/features_archive/world_tb_day_mdr_report_2011/en/
- 56.La tuberculosi a Barcelona. Informe 2009. http://www.aspb.es/quefem/docs/Tuberculosi_2009.pdf
- 57.Chan ED, Iseman MD. Multidrug-resistant and extensively drug-resistant tuberculosis: a review. Curr Opin Infect Dis. 2008;21:587–595. doi: 10.1097/QCO.0b013e328319bce6. [DOI] [PubMed] [Google Scholar]
- 58.Ruiz-Manzano J, Blanquer R, Calpe JL, Caminero JA, Caylà J, Domínguez JA, García JM, Vidal R. Diagnóstico y tratamiento de la tuberculosis. Normativa SEPAR. Arch Bronconeumol. 2008;44:551–566. doi: 10.1157/13126836. [DOI] [PubMed] [Google Scholar]
- 59.Sarkar S, Suresh MR. An overview of tuberculosis chemotherapy—a literature review. J Pharm Pharmaceut Sci. 2011;14:148–161. doi: 10.18433/j33591. [DOI] [PubMed] [Google Scholar]
- 60.Singla R, Caminero JA, Jaswal A, Singla N, Gupta S, Bali RK, Behera D (2011) Linezolid, an effective, safe and cheap drug in MDR-TB treatment failure in India. Eur Respir J (Epub ahead of print) [DOI] [PubMed]
- 61.Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soonlingen D. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! Int J Antimicrobiol Agents. 2010;35:524–526. doi: 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 62.Our motto. Health News. February 1914;30:table of contents. Monthly Bulletin: New York State Department of Health
- 63.Reichman LB. Defending the Public’s health against tuberculosis. JAMA. 1997;278:865–867. doi: 10.1001/jama.1997.03550100091046. [DOI] [PubMed] [Google Scholar]
- 64.Reichman LB. The U shaped curve of concern. Am Rev Resp Dis. 1991;148:741–742. doi: 10.1164/ajrccm/144.4.741. [DOI] [PubMed] [Google Scholar]
- 65.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York city—turning the tide. N Engl J Med. 1995;333(4):229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 66.New York City, Department of Health and Mental Hygiene (2009) Annual Summary 2008: New York city is stopping TB. New York, NY. http://www.nyc.gov/html/doh/downloads/pdf/tb/tb_annualsummary08.pdf
- 67.Marco A, Caylà JA, Serra M, Pedro R, Sanrama C, Guerrero R, Ribot N. Predictors of adherence to tuberculosis treatment in a supervised therapy programme for prisoners before and after release. Study Group of Adherence to Tuberculosis Treatment of Prisoners. Eur Respir J. 1998;4:967–971. doi: 10.1183/09031936.98.12040967. [DOI] [PubMed] [Google Scholar]
- 68.Ospina JE, Orcau A, Millet JP, Caylà JA, Casals M, Rius C, Sanchez F (2009) Effectiveness of community health workers for the control of tuberculosis. Int J Tubec Lung Dis 13(12) suppl 1 (Abstract book. 40th World Conference on Lung Health of the IUATL)
- 69.Rodrigo T, Caylà JA, Galdós-Tangüis H, García de Olalla P, Brugal MT, Jansà JM. Proposing indicators for evaluation of tuberculosis control programmes in large cities based on the experience of Barcelona. Int J Tuberc Lung Dis. 2001;5:432–440. [PubMed] [Google Scholar]
- 70.Gillman A, Berggren I, Bergström SE, Wahlgren H, Bennet R. Primary tuberculosis infection in 35 children at a Swedish day care center. Pediatr Infect Dis J. 2008;27:1078–1082. doi: 10.1097/INF.0b013e31817e83f4. [DOI] [PubMed] [Google Scholar]
- 71.World Health Organization (2011) Collaborative framework for care and control of tuberculosis and diabetes. http://whqlibdoc.who.int/publications/2011/9789241502252_eng.pdf [PubMed]