Abstract
Sublingual administration of certain buffered propranolol may improve the rate and extent of absorption compared to oral administration. The main objectives of this study were to (1) compare the plasma propranolol concentrations (Cp-prop) following sublingual administration of a specially buffered formulation (Promptol™) to that following oral administration of Inderal® and (2) evaluate the utility of a special pharmacokinetic model in describing the Cp-prop following sublingual administration. Eighteen healthy volunteers received 10 mg sublingual Promptol™ or oral Inderal®. Multiple Cp-prop were determined and their pharmacokinetics compared. Additional data following sublingual 40 mg Promptol™ or Inderal® were utilized for evaluation of a special advanced compartmental absorption and transit (ACAT) model. For model simulation, the physicochemical parameters were imported from AMET predictor, whereas the pharmacokinetic parameters were calculated and optimized by Gastroplus®. Based on this model, the quantity of drug absorbed via buccal/sublingual mucosa was estimated. Cp-prop was higher at earlier times with 3-fold greater relative bioavailability following sublingual Promptol™ compared to that from oral Inderal®. The special ACAT model provided excellent goodness of fit of Cp-prop-time curve and estimated a 56.6% increase in absorption rate from Promptol™ and higher initial Cp-prop compared to the regular formulation. The modified ACAT model provided a useful approach to describe sublingual absorption of propranolol and clearly demonstrated an improvement of absorption of Promptol™. The sublingual 10 mg Promptol™ achieved not only a similar systemic exposure as 30 mg oral Inderal® but an earlier effective Cp-prop which may be advantageous for certain clinical conditions.
Key words: ACAT, buffered, pharmacokinetics, propranolol, sublingual
INTRODUCTION
Propranolol, a nonselective β1 and β2-adrenergic antagonist, has been used widely in the treatment of various cardiovascular diseases, including essential hypertension, ischemic heart disease, atrial fibrillation, and paroxysmal tachycardia for more than 50 years (1). Propranolol is also often used for as-needed or short-term treatment of performance/situational anxiety with accompanying tachycardia, e.g., day surgery, public speaking/performance, air travel, and taking examinations (2–6). More recently, propranolol has also been shown to be effective in the prevention of post-traumatic stress disorder symptoms (7,8).
For most of the clinical uses, oral administration of an immediate release tablet, e.g., Inderal®, is the primary mode of administration. However, the oral route is not well suited for acute conditions requiring rapid onset of action (e.g., for rapid control of tachycardia). Sublingual drug delivery may offer an attractive and convenient route of administration for such use. Because of thin mucosa (190 μm) and excellent blood flow in the sublingual region, rapid absorption leading to the rapid onset of action is possible following sublingual administration of propranolol, in view of its molecular size and lipophilicity (9). In addition, the high liver first-pass effect of propranolol can also be avoided with the sublingual route, leading to a higher bioavailability, since the drug can be absorbed directly into the systemic circulation (10). Not surprisingly, sublingual propranolol administration has been demonstrated to result in a higher bioavailability than that following oral administration of the conventional tablet (Inderal®) (11).
In an attempt to further improve sublingual absorption of propranolol, a patented buffered propranolol tablet (Promptol™) based on “pHmax” technology has been reported (12). By incorporating suitable buffering agents in the formulation, the saliva pH can be targeted (at pHmax) to yield maximal drug solubility and permeability when the tablet is administered sublingually. Thus, such a tablet may potentially achieve rapid and improved plasma concentration leading to rapid action (e.g., reduce heart rate and controlling anxiety). A proof of this concept has been demonstrated in a previous pilot study in eight human subjects (13).
Despite the proof of concept, the advantage of sublingual Promptol™ in bioavailability and absorption compared to the current standard practice of oral administration of the conventional tablet is unknown. Thus, the present study was carried out to compare 10 mg sublingually administered propranolol tablet (buffered specially formulation, Promptol™) to the orally administered immediate release tablet (Inderal®), using a randomized cross-over design in 20 healthy subjects.
Because there is no suitable pharmacokinetic model to describe drug kinetics following sublingual administration, we utilized a new approach, a modified advanced compartmental absorption and transit (ACAT) model which was developed previously for gastrointestinal (GI) absorption (14), to describe and simulate the concentration–time curve as well as estimate the percentage of drug absorbed following sublingual administration (see “METHODS” below).
METHODS
Clinical Study Design and Subjects
The study was conducted using a single-dose, two-treatment, two-period, two-sequence, randomized, crossover design. Twenty healthy subjects were enrolled in this study and were assigned to two groups corresponding to two dosing sequences. All volunteers were fully advised of the nature, purpose, procedures, and possible risks of this study. An acknowledgement of the receipt of this information and the participant’s freely tendered offer to volunteer was obtained by signing the informed consent form, which was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee.
The subjects were all male, nonsmokers, 18 to 60 years old, and within 15% of ideal weight. They were all in good health based on medical history, physical examination, electrocardiogram evaluation, and routine laboratory tests (including blood chemistry, hematology, and urine analysis). No evidence of any major organic/systemic disease including lung, hepatic, and cardiac disease was identified within 3 months, and no blood donation was involved within 4 months prior to the study. All participants were required not to take ethanol or caffeinated beverages during each study period (6 h prior to the study and during the study). All the subjects did not receive β-blockers or similar drugs in this class within 4 weeks before the study. Standard meals were provided no less than 4 h after drug administration. Alcohol was not allowed for the 24 h period before the study and until the last sample was collected. Subjects were monitored carefully at the study site at the time of study, and all adverse reactions were recorded.
Treatments
Promptol™ tablet 10 mg (batch no. DDC100105) administered to the study subjects was manufactured in compliance with GMP by Marching Pharmaceutical Ltd., Hong Kong. The reference product, Inderal® brand of propranolol tablet 10 mg administered, was manufactured by AstraZeneca Co., UK.
Each subject underwent two sessions. During each session, they received either a single sublingual dose of Promptol™ tablet 10 mg or a single oral dose of Inderal® tablet 10 mg. For sublingual administration of Promptol™ tablet, the medication was placed under the tongue, and at 15 min, the saliva (with dissolved drug) was swallowed with a glass of water (approximately 240 ml). For oral administration, the Inderal® tablet 10 mg was swallowed with a glass of water (approximately 240 ml). Each treatment was separated by a 7-day washout period. Additionally, blood pressure and heart rate were assessed at baseline, 120, 240, and 600 min after dose administration.
Venous blood samples were collected from each subject at pre-dose (0 min) and then at 5, 10, 17, 30, 45, 60, 90, 120, 150, 180, 240, 300, 360, 480, and 600 min post-dose. Each sample (4 ml) was collected via a catheter placed in the forearm vein and then stored in a lithium heparin tube. Blood samples were centrifuged immediately at 4,000 rpm for 10 min at 5°C, and then the separated plasma was transferred into two polypropylene tubes and stored at −80°C until assay.
Assay
Plasma concentrations of propranolol were determined by a high-performance liquid chromatographic (HPLC) method as described previously (13). The HPLC system consisted of a Waters 600E HPLC pump and a Waters 464 fluorescence detector (Waters, USA) and a reversed-phase Thermo BDS Hypersil C18 column (5 μm, 250 × 4.6 mm I.D.). The fluorescence detector was operating at λex = 285 nm, λem = 345 nm; gain was set at 100; and the attenuation was set at 1. The mobile phase consisted of 0.05 M KH2PO4 (pH 3.0, adjusted by 85% H3PO4), methanol, and acetonitrile (42:43:15) and was delivered at a flow rate of 1 ml/min. The system was operated at the ambient temperature.
The validity of the assay method was assessed according to FDA guidance, with regard to the linearity, sensitivity, repeatability, stability, precision, and accuracy. The calibration curve of propranolol was linear over the concentration range of 0.50–50 ng/ml. The correlation coefficient (r2) was greater than 0.99 with different runs. For quality control samples at concentrations of 5, 10, and 50 ng/ml, the intra-day relative standard derivation (RSD) values were 1.19–9.62%, whereas the inter-day RSDs were 3.93 to 9.58%. The accuracy (% bias) ranged from −6.54 to 12.83%. The lower limit of quantification was found to be 0.5 ng/ml for propranolol.
Pharmacokinetic Analysis
The plasma concentration–time data were analyzed by non-compartmental method (with the aid of computer program WinNonlin, version 2.1, SCI software). The major pharmacokinetic parameters were:
Cmax: peak drug concentration, obtained directly from the original concentration–time data
Tmax: time to peak drug concentration, obtained directly from the original concentration–time data
AUC0–last: area under the concentration–time curve from time zero to the last sampling time t obtained by trapezoidal rule
AUC0–∞: area under the concentration–time curve from time zero to infinity, where AUC0–∞ = AUC0–t + Ct/λz
T1/2: terminal elimination half-life, calculated as 0.693/λz, where λz was the terminal phase elimination rate constant
F: relative bioavailability for the sublingual Promptol™ tablet to oral Inderal® tablet was determined as F = AUCSL, 0–∞/AUCPO, 0–∞
Estimation of Drug Absorbed via Oral Mucosa (Buccal/Sublingual)
The computational program Gastroplus (version 6.0, Simulations Plus, Inc., Lancaster, CA, USA) was utilized for fitting of the data (see “Methods”) with slight modification. The program consisted of a system of coupled linear and nonlinear rate equations to simulate the drug’s in vivo profile in the body based on the original ACAT model which described the human gastrointestinal tract drug transit in nine compartments (stomach, seven segments of small intestine, and colon) with the following equation to estimate drug mass absorbed (Mabs).
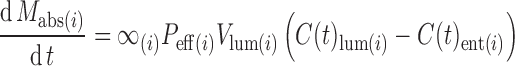 |
where α(i) is the absorption scale factor (ASF) in compartment i (nominal value is surface/volume, which is 2/Ri (Ri = radius of compartment i)), Peff(i) is the permeability in compartment I, Vlum(i) is volume of lumen, and C(t)lum(i) and C(t)ent(i) are lumen and enterocyte concentration, respectively. The values for these parameters were reported previously (15,16). From the original ACAT model, additional compartment (oral cavity) was incorporated with the parameters being set as pH of 7.4 and transit time of 15 min, and the user defined ASF of 0.7 which was previously tested to generate the best fit (the oral cavity pH 7.4 was an estimated mean value of pHmax 7.6 and oral mucosal pH). This modified model was capable of simulating drug concentration following sublingual as well as gastrointestinal (oral) absorption (with drug at oral cavity being 0). The values for the physicochemical parameter (pKa, solubility, particle size, particle density, etc.) were based on the experimental data as well as the previous publications (see Table I) (13).
Table I.
Summary of the Input Parameters Employed for the Gastroplus Simulation
| Parameter | Valuea | |
|---|---|---|
| Physiochemical | 1. Molecular weight (Da) | 259.4 |
| 2. Solubility (mg/ml) | 1.66 (at pH 10.5) | |
| 3. Log P | 2.93 | |
| 4. pK a | 9.23 | |
| Formulation | 5. Mean precipitation time (s) | 900 |
| 6. Drug particle density (g/L) | 1.2 | |
| 7. Effective particle radium (mm) | 25 | |
| Pharmacokinetics | 8. Dose (mg) | 8.8, 35.2 |
| 9. Dose volume (mL) | 1 | |
| 10. Body weight (kg) | 65.5 | |
| 11. Simulation time (h) | 7b | |
| 12. Diffusion coefficient (×10−5 cm2/s) | 0.83 | |
| 13. P eff (×10−4 cm/s) | 3.74c | |
| 14. Unbound percent in plasma (%) | 8.08 | |
| 15. Blood/plasma concentration ratio | 1 | |
| 16. First pass extraction (%) | 62.89d | |
| 17. Clearance (L/h/kg) | 1.08 | |
| 18. Volume distribution (L/kg) | 1.12 | |
| 19. Elimination half-life (h) | 2.91 | |
| 20. K 12 (1/h) | 13.24 | |
| 21. K 21 (1/h) | 4.60 | |
| 22. V 2 (L/kg) | 3.22 |
aExcept for item 8 (dose), the values of parameters 1–15 are input values based on ADMET predictor (version 2.3.0) used by Gastroplus (version 6.0), whereas items 16–22 are optimized values generated to achieve best fit after using initial values estimated from IV propranolol (see (17))
bFor sublingual administration, total simulation duration is 7 h with 0.25 h sublingual absorption, followed by swallowing of saliva with dissolved drug, i.e., gastrointestinal absorption
cPeff stands for effective permeation
dReduced to 47% with 40 mg dose administration
The human physiologic parameters were generated using the program’s internal population estimates for Age-Related Physiology module. The initial pharmacokinetic parameters, e.g., ke, Vd, K12, K21, V2, were obtained from published intravenous dose (17) and the initial value of first pass extraction was estimated by incorporating the published oral dose with the intravenous data (13). The initial value of sublingual permeation was obtained from the published sublingual dose (11). The main plasma concentration data were recovered from the Cp-prop concentration curve, and the pharmacokinetic parameters were fitted using two-compartment mode which was able to provide the best fit.
The modified ACAT model with the initial values mentioned above was utilized to describe the mean plasma propranolol concentration–time curve of sublingual Promptol™ (10 mg and 40 mg), Inderal® (40 mg), and oral Inderal® (10 mg). For model fitting, the Cp-prop data of sublingual 10 mg Promptol™ and oral 10 mg Inderal® were obtained from the current study. The additional sublingual 40 mg Inderal and Promptol™ Cp-prop data were obtained from our previous study (13) which was also a cross-over study in healthy Chinese subjects. To carry out the simulation, an assumption was made that there was saturation of liver metabolism at higher doses. Thus, the first pass extraction for the 40 mg doses was reduced from 62% to 47% to account for saturation of propranolol metabolism (see Table I). In addition, because of the special buffering effect of the Promptol™ formulation, its absorption rate from the sublingual area for Promptol™ was adjusted (using a higher value of absorption scale factor as that generated by in the GastroPlus® Oral Cavity ACAT model). The rest of parameters remained same for all data simulations. The quantity of drug absorbed via buccal/sublingual mucosa was then estimated and compared as described by the model equation. The % absorption versus time obtained by the ACAT model was further compared to that estimated from a different method, the Wagner–Nelson method (18).
Statistical Analysis
Analysis of variance (ANOVA) was performed on logarithmically transformed Cmax, AUC0–last, and AUC0–∞. The sequence effect was tested using the between-subject effect as an error term. All other effects (subject, period, and treatment) were tested against the residual error from the ANOVA. Differences in plasma concentration and pharmacokinetic parameters between Promptol™ tablet and Inderal® tablet were compared using paired-samples T test. A p < 0.05 was considered statistically significant for all tests. The similarity factor (f2, see following equation) was employed to compare the % absorption profiles of Wagner–Nelson method versus that from the ACAT model with the f2 value between 50 and 100 being considered similar.
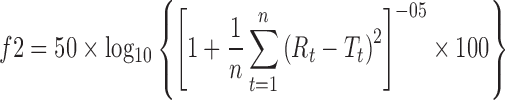 |
The residual analysis and quantile–quantile plot (QQ plot) were utilized to exam the quality of model. The weighted absolute residuals with weighting factor of 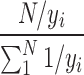 (yi denotes the ith observed value) versus predicted values were plotted, and no systematic trend in residuals was considered to be unbiased. The residuals ordered from the smallest to the largest against Φ−1((i − 0.5)/n) (Φ−1 denotes the ith percentile of the normal distribution) and a linear QQ plot was considered to be normality.
(yi denotes the ith observed value) versus predicted values were plotted, and no systematic trend in residuals was considered to be unbiased. The residuals ordered from the smallest to the largest against Φ−1((i − 0.5)/n) (Φ−1 denotes the ith percentile of the normal distribution) and a linear QQ plot was considered to be normality.
RESULTS
Twenty male Chinese subjects enrolled in the study and 18 subjects completed both sessions of the study. Two subjects were excluded from the study due to the abnormal results in medical test and participation in extensive clinical trial prior to current study.
No serious adverse drug reactions were observed. All subjects experienced and tolerated transient mucosal irritation/tingling and anesthetic sensation under the tongue following sublingual administration of Promptol™ tablet 10 mg. No one withdraws from observed study due to this transient discomfort.
The mean propranolol plasma concentration (Cp-prop) curve following each formulation is presented in Fig. 1. Promptol™ tablet (administered sublingually) achieved a significantly higher and earlier CP-Prop than the Inderal® Tablet (administered orally). At 5 and 10 min, Promptol™ tablet reached a mean CP-prop of 1.69 and 8.04 ng/ml, respectively, in contrast to undetectable concentration at these times following Inderal® (Fig. 2). The values of individual AUC0–15 were much higher following sublingual 10 mg Promptol™ than that following oral Inderal® (Table II).
Fig. 1.
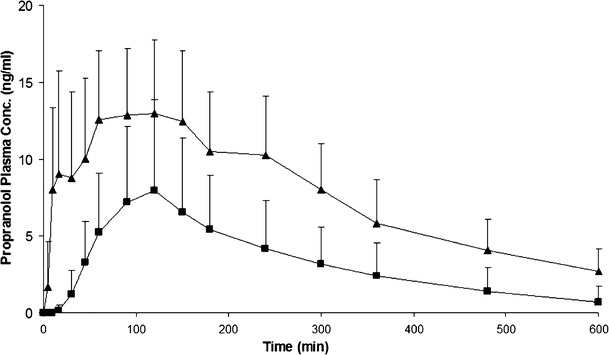
Mean propranolol plasma concentration–time profiles following a sublingual dose of Promptol™ tablet 10 mg (triangle) versus an oral dose of Inderal® tablet 10 mg (square) in 18 subjects. Data were represented as mean + SD, n = 18
Fig. 2.
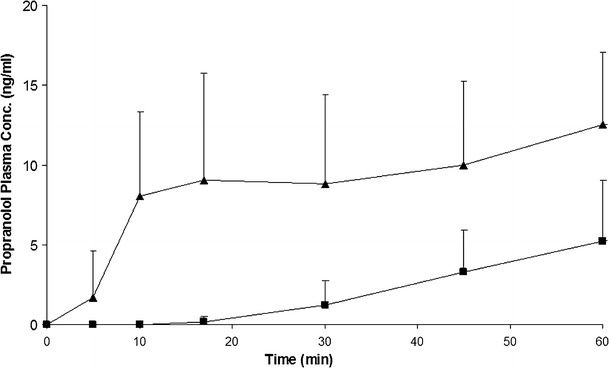
Mean propranolol plasma concentration–time profiles from 0 to 60 min following a sublingual dose of Promptol™ tablet 10 mg (triangle) versus an oral dose of Inderal® tablet 10 mg (square) in 18 subjects. Data were represented as mean + SD, n = 18
Table II.
Individual Comparison of AUC0–15 Following Sublingual 10 mg PromptolTM with Oral 10 mg Inderal®
| Sub | AUC0–15 (ng min/ml) | |
|---|---|---|
| Sublingual 10 mg Promptol™ | Oral 10 mg Inderal® | |
| #1 | 42.8 | 0 |
| #2 | 37.1 | 0 |
| #3 | 69.4 | 3.3 |
| #4 | 65.2 | 0 |
| #5 | 93.6 | 1.8 |
| #6 | 120.9 | 0 |
| #7 | 217.8 | 0 |
| #8 | 69.7 | 0 |
| #9 | 47.3 | 0 |
| #10 | 145.7 | 0 |
| #11 | 9.0 | 0 |
| #12 | 23.9 | 0 |
| #13 | 51.2 | 1.6 |
| #14 | 13.0 | 0 |
| #15 | 13.0 | 0 |
| #16 | 77.0 | 0 |
| #17 | 80.0 | 0 |
| #18 | 72.2 | 0 |
AUC area under the concentration–time curve
The mean values of the pharmacokinetic parameters are summarized in Table III. Significantly higher Cmax, AUC0–last, and AUC0–∞ (p < 0.05) were observed following sublingual administration, suggesting that the bioavailability and the absorption from Promptol™ tablet 10 mg were significantly higher and faster than that from the orally administered Inderal® tablet 10 mg (see also absorption plot, Fig. 3).
Table III.
Comparison of Pharmacokinetic Parameters Between Promptol™ Tablet 10 mg and Inderal® Tablet 10 mg
| PK parameters | Promptol™ tablet (10 mg, test) | Inderal® tablet (10 mg, reference) | p value |
|---|---|---|---|
| T max (min) | 102 ± 67 | 107 ± 33 | 0.772 |
| C max (ng/ml) | 15.5 ± 5.6 | 8.6 ± 5.8 | 0.000 |
| AUC0–last (μg min/ml) | 4.60 ± 1.66 | 1.95 ± 1.40 | 0.000 |
| AUC0–∞ (μg min/ml) | 5.36 ± 2.04 | 2.26 ± 1.65 | 0.000 |
| T 1/2 (min) | 184 ± 38 | 171 ± 55 | 0.336 |
| Relative F (%) | 300 ± 127 | N/A | N/A |
T max time to peak drug concentration, C max peak drug concentration, AUC 0–last area under the concentration–time curve from time zero to the last sampling time t obtained by trapezoidal rule, AUC 0–∞ area under the concentration–time curve from time zero to infinity, T 1/2 terminal elimination half-life, F relative bioavailability for the sublingual Promptol™ tablet to oral Inderal® tablet, N/A not applicable
Fig. 3.
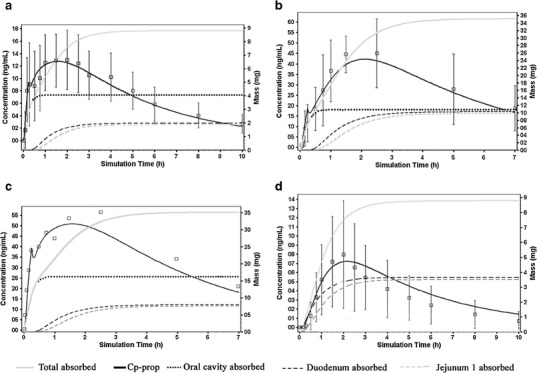
The % absorption of propranolol in human tissue simulated using GastroPlus following sublingual administration of (a) 10 mg Promptol™, (b) 40 mg Inderal®, (c) 40 mg Promptol™, and (d) oral administration of 10 mg Inderal®. The open square boxes are the observed Cp-prop data (mean ± SD)
There were no significant sequence and period effects for Cmax, AUC0–last, and AUC0–∞ using ANOVA. However, a significant subject effect was observed for all three pharmacokinetic parameters, suggesting that the absorption and disposition of propranolol exhibit relatively large inter-individual variability. Meanwhile, a significant treatment effect was also observed on all these parameters due to the significant differences on these pharmacokinetic parameters between the two formulations.
The modified ACAT model described the plasma concentration profiles of oral and sublingual administration of propranolol (10 mg) sufficiently well as demonstrated by goodness of fit for all four doses using visual criteria (Fig. 3) (19). No systematic trend was observed in the plot of absolute residuals versus the predicted Cp-prop values which suggested that the simulated values by the model were unbiased (Fig. 4). In addition, the resulting quantile–quantile plots showed approximate linearity between the expected normal residuals and the observed calculated residuals with no curvature which indicated that the residuals were consistent with the normal distribution. Based on this model, faster absorptive rate and greater mass of absorption were identified with sublingual Promptol™ when compared to sublingual or oral Inderal® (Figs. 3 and 5).
Fig. 4.
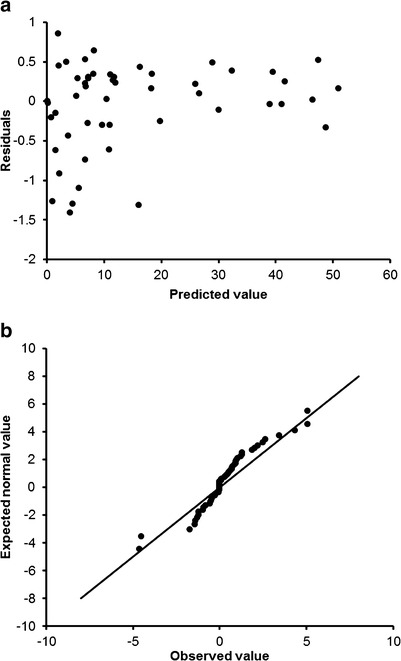
Weighted sample residuals plot (top) and the normal quantile–quantile plot (bottom)
Fig. 5.
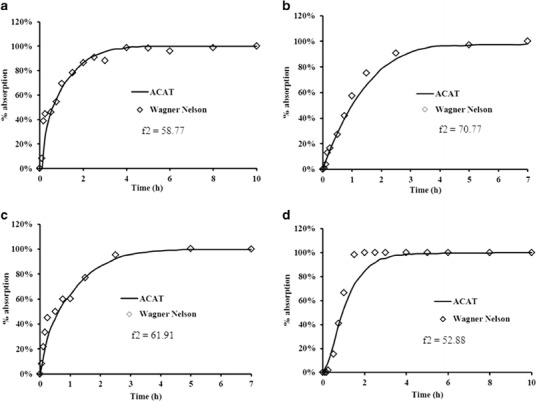
Comparison of cumulative % absorbed using Wagner-Nelson technique versus ACAT simulation. (a) 10 mg sublingual Promptol™; (b) 40 mg sublingual Inderal®; (c) 40 mg sublingual Promptol™; (d) 10 mg oral Inderal®
Following sublingual Promptol™ 10 mg, the total amount attributable to sublingual absorption was 4.1 mg (46.6% of total, Table IV), which was substantial considering only 15 min sublingual time (under the tongue) before swallowing the drug dissolved in the saliva. In comparing sublingual absorption of 40 mg Promptol™ to Inderal, the total amount of sublingual absorption was 16.4 mg (46.7% of total) for Promptol™ versus 10.6 mg (30% of total) for Inderal® (Table III). This represented 55% higher absorption from Promptol™ compared to Inderal®.
Table IV.
The Mass Absorption in Different Tissues
| Tissue | Inderal® (10 mg oral) | Promptol™ (10 mg SL) | Inderal® (40 mg SL) | Promptol™ (40 mg SL) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mass (mg) | % | Mass (mg) | % | Mass (mg) | % | Mass (mg) | % | ||
| Oral cavity | Sublingual mucosa | N/A | N/A | 4.1 | 46.6 | 10.6 | 30.1 | 16.4 | 46.7 |
| GI tract | Duodenum | 3.7 | 42.0 | 2.0 | 22.7 | 10.3 | 29.3 | 7.8 | 22.2 |
| Jejunum 1 | 3.5 | 39.8 | 1.9 | 21.6 | 9.8 | 27.9 | 7.5 | 21.4 | |
| Jejunum 2 | 1.0 | 11.4 | 0.5 | 5.7 | 2.5 | 7.1 | 2.4 | 6.8 | |
| Others | 0.6 | 6.8 | 0.3 | 3.4 | 2.0 | 5.7 | 1.1 | 6.0 | |
| Total | 8.8 | 8.8 | 35.2 | 35.2 | |||||
The amount of propranolol free base is 8.8 and 35.1 mg in 10 and 40 mg of propranolol · HCl, respectively
SL sublingual, N/A not applicable, GI gastrointestinal
The consistency of the ACAT model in estimating absorption of propranolol at different gastrointestinal segments, following swallowing of drug dissolved in saliva from sublingual administration of different doses and formulations, is shown in Table V. The observed consistency of data from GI absorption of these two formulations from separate occasions further confirmed the reliability of the ACAT model. Comparison of these absorption plots and f2 values by ACAT versus Wagner–Nelson technique is shown in Fig. 5. All of the f2 values were greater than 50, which indicated that there was no significant difference in the absorption curves estimated by these two techniques.
Table V.
Contribution of Absorption at Different Segments of the Gastrointestinal Tract Relative to Total Gastrointestinal Absorption
| Segments | Inderal® (10 mg oral) (%) | Promptol™ (10 mg SL) (%) | Inderal® (40 mg SL) (%) | Promptol™ (40 mg SL) (%) | |
|---|---|---|---|---|---|
| GI tract | Duodenum | 42.0 | 42.6 | 41.9 | 41.5 |
| Jejunum 1 | 39.8 | 40.4 | 39.8 | 39.9 | |
| Jejunum 2 | 11.4 | 10.6 | 10.2 | 12.8 | |
| Others | 6.8 | 6.4 | 8.1 | 5.9 |
The portion for total gastrointestinal absorption form different products was set as 100% regardless of the dose absorbed sublingually
SL sublingual, GI gastrointestinal
DISCUSSION
The results of the present study showed that sublingual Promptol™ achieved significantly earlier and higher Cp-prop compared to that from Inderal® tablet without any observed serious adverse effects. These results confirmed the ability of sublingual Promptol™ (based on pHmax technology) to achieve more desirable Cp-prop values, which could be advantageous for certain clinical conditions, e.g., rapid control of paroxysmal tachycardia/situational anxiety.
Following sublingual Promptol™, double Cp-prop peaks were observed. The first peak occurred at a mean time of 17 min and second peak at about 120 min (see Fig. 3). The first peak is most likely due to rapid sublingual absorption and the second peak due to primarily GI absorption following swallowing of the drug dissolved in saliva. In contrast, only one peak (at about 120 min) was observed following oral administration of Inderal® reflecting usual pattern of GI absorption of immediate release tablets.
To verify the contribution of the sublingual Promptol™ to rapid absorption and higher Cp-prop, the rate and total mass of propranolol absorbed via the oral cavity should ideally be determined. However, no simple experimental technique exists that can easily measure the amount of drug absorbed via the sublingual mucosa in human subjects. Kates estimated the absorption of a given dose of sublingually administered propranolol by determining the salivary drug content at different time points (i.e., estimate from sublingual dose administered minus the amount unabsorbed). However, such data do not indicate amount absorbed sublingually due to retention of drug in the mucosa (20,21). Our physiologically based pharmacokinetic model (the ACAT model) allows the estimation of the amount of systemic plasma drug absorbed via the oral mucosa at any given time. The data generated from this model showed substantial sublingual (oral cavity) absorption which contributed to the early rise as well as higher Cp-prop when compared to that following oral administration, with absorption only from the GI tract (Figs. 3 and 4).
The present work to our knowledge is the first report of a kinetic approach to estimate the absorption of drug from oral mucosa following the usual sublingual route of administration in human subjects (i.e., placing the sublingual tablet under the tongue until dissolved and then swallowing the saliva containing the remaining dissolved drug). The ability of the ACAT model to generate consistent absorption data (among different conditions as well as between different dosage forms) together with excellent concordance of the absorption plot with that from the Wagner–Nelson technique (Fig. 4) are indications of the model’s capability and reliability in estimating absorption of drug via sublingual administration. The current modified ACAT model not only can estimate propranolol absorption from the sublingual site but also from the GI tract.
The observed improvement in the rate and extent of propranolol absorption following sublingual Promptol™, compared to that following oral Inderal®, is most likely attributed to superior drug absorption from the special formulation technology (pHmax technology) (13) in combination with sublingual route of administration. The rationale for pHmax technology (buffering technique not aimed at maximizing unionized species but optimum ratio of unionized to ionized specie for maximum solubilization and mucosal permeation) has been validated previously (13). The results of the present study further confirm its utility. When using the ACAT model, the estimated sublingual absorption rates were 0.83 and 0.53 mg/min for Promptol™ versus Inderal® sublingual administration (see Fig. 3b, c), reflecting an increase of 56.6% from the Promptol™ tablet. This difference corresponded to a mean Cp-prop of 37.6 versus 17.5 ng/ml at 15 min following sublingual Promptol™ and Inderal® tablet, respectively (see Fig. 3b, c). These data clearly demonstrated the enhanced absorption from Promptol™, consistent with the expectation from the pHmax technology.
Sublingual administration can lead to improved absorption (bioavailability) compared to oral administration for a highly permeable drug like propranolol. This was clearly demonstrated by Duchateau et al. in a previous study in healthy subjects (11). In that study, although the peak time following these two routes of administration was similar (at about 2 h), careful inspection of the representative drug concentration–time curve showed substantially higher concentration at early time points following sublingual compared to oral Inderal® 10 mg (11). However, in comparison with our data, sublingual Promptol™ 10 mg appeared to achieve even a higher Cp-prop at the early time points, consistent with improved mucosal permeation/absorption using the pHmax technology.
Based on comparative bioavailability of AUC0–t or AUC0–∞ ratios, the mean bioavailability of the 10-mg sublingual Promptol™ tablet is about 3.00 ± 1.27-fold of 10 mg oral Inderal®. Further analysis of AUC ratios from individuals receiving 10 mg sublingual Promptol™ to corresponding AUC × 3 of 10 mg oral Inderal® (assuming dose proportionality, as per previous publications (22)) using two-sided one tail T tests showed the geometric mean ratio for AUC0–last to be 92.9% (90% CI, 83.6–119.8%) and AUC0–∞, 91.70% (90% CI, 82.6–117.4%). Such data analysis consistently showed the overall bioavailability from sublingual Promptol™ to be 3-fold greater compared to oral Inderal®. Furthermore, the AUC0–15 min data (Table II) showed convincingly a significantly higher early concentration with Promptol™. Taken together, our study results may provide useful application in certain clinical conditions. For example, in situational/performance anxiety with tachycardia, a 30-mg oral dose propranolol (Inderal®) is recommended for its short-term treatment (23); an earlier response, potentially within 10 min (compared to 30 min with an oral dose), may be possible with Promptol™ and concurrently achieve a concentration needed for heart rate reduction (about 3 ng/ml (24)).
Currently, intravenous propranolol (1 mg/ml) is recommended for acute treatment of tachycardia for urgent conditions (25). Previous study showed that a 10% reduction in heart rate can be expected at 5 min after a 0.05 mg/kg intravenous dose (26) with a corresponding Cp-prop about 16.6 ng/ml (27). Similar concentration can be reached at about 7 min after 40 mg sublingual Promptol™ (Fig. 3), while it would take more than 1 h to achieve such similar concentration if administered orally. Thus, sublingual Promptol™ may be considered as an alternative to intravenous propranolol in certain patient settings where a 50-min lag time is to be avoided (if administered orally). This potential benefit of early effect is consistent with observation of a 15% reduction in heart rate as early as 10 min with the use of 40 mg sublingual propranolol reported by others (28).
Sublingual Promptol™ may cause minor local irritation (mild but tolerable, numbness, and tingling sensations) which were experienced by majority of our study subjects. Similar side effects and tolerance were also observed by others receiving sublingual propranolol (10–40 mg) (11,28). In view of this easily tolerated effect and no other major observed problems in our study subjects, we believe that a short-term use of sublingual Promptol™ 10 mg tablet is likely to be relatively safe (in patients without cardiac decompensation) and could provide advantage therapeutically in certain situations. Previous experience of oral propranolol at doses of 500 mg or greater in many patients further attests to this safety projection (29).
CONCLUSION
Sublingual administration of Promptol™ tablet 10 mg (for 15 min followed by swallowing of dissolved drug in saliva) resulted in a significantly quicker absorption, higher plasma concentration, and greater AUC (about 3-folds) as compared to orally administered Inderal® tablet 10 mg. The observed improvement in Cp-prop following sublingual Promptol™ was attributed to the rapid and substantial absorption of propranolol from oral cavity, based on estimation/simulation of a physiology-based model, ACAT model, which was able to reliably predicted the drug absorption from sublingual mucosal oral cavity and gastrointestinal segments over time. The above results support the potential utility of sublingual Promptol™ to improve therapy in certain condition, e.g., acute use for situational/performance anxiety and tachycardia associated with various conditions for which oral propranolol is indicated.
Acknowledgments
This study was supported by an external grant from Comprehensive Drug Enterprises Ltd., Hong Kong Science Park, Hong Kong.
Conflict of Interest
Yanfeng Wang and Benjamin T.K. Lee are employees of the company, and Moses S.S. Chow is a shareholder of the company.
Footnotes
Yanfeng Wang and Zhijun Wang contributed equally to this work.
References
- 1.Frishman WH. β-Adrenoceptor antagonists: new drugs and new indications. New Engl J Med. 1981;305(9):500–506. doi: 10.1056/NEJM198108273050907. [DOI] [PubMed] [Google Scholar]
- 2.Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav. 1998;59(2):387–397. doi: 10.1016/S0091-3057(97)00457-7. [DOI] [PubMed] [Google Scholar]
- 3.Fourneret P, Desombre H, de Villard R, Revol O. Interest of propranolol in the treatment of school refusal anxiety: about three clinical observations. Encephale. 2001;27(6):578–584. [PubMed] [Google Scholar]
- 4.Gruber RP, Roberts C, Schooler W, Pitman RK. Preventing postsurgical dissatisfaction syndrome after rhinoplasty with propranolol: a pilot study. Plast Reconstr Surg. 2009;123(3):1072–1078. doi: 10.1097/PRS.0b013e318199f63f. [DOI] [PubMed] [Google Scholar]
- 5.Mazzuero G, Galdangelo F, Zotti AM, Bertolotti G, Tavazzi L. Effects of propranolol, atenolol, and chlordesmethyldiazepam on response to mental stress in patients with recent myocardial infarction. Clin Cardiol. 1987;10(6):293–302. doi: 10.1002/clc.4960100601. [DOI] [PubMed] [Google Scholar]
- 6.Mealy K, Ngeh N, Gillen P, Fitzpatrick G, Keane FB, Tanner A. Propranolol reduces the anxiety associated with day case surgery. Eur J Surg. 1996;162(1):11–14. [PubMed] [Google Scholar]
- 7.Taylor F, Cahill L. Propranolol for reemergent posttraumatic stress disorder following an event of retraumatization: a case study. J Trauma Stress. 2002;15(5):433–437. doi: 10.1023/A:1020145610914. [DOI] [PubMed] [Google Scholar]
- 8.Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54(9):947–949. doi: 10.1016/S0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 9.Mathias NR, Hussain MA. Non-invasive systemic drug delivery: developability considerations for alternate routes of administration. J Pharm Sci. 2010;99(1):1–20. doi: 10.1002/jps.21793. [DOI] [PubMed] [Google Scholar]
- 10.Squier CA, Hall BK. In-vitro permeability of porcine oral mucosa after epithelial separation, stripping and hydration. Arch Oral Biol. 1985;30(6):485–491. doi: 10.1016/0003-9969(85)90095-0. [DOI] [PubMed] [Google Scholar]
- 11.Duchateau GSMJE, Zuidema J, Merkus FWHM. Bioavailability of propranolol after oral, sublingual, and intranasal administration. Pharm Res. 1986;3(2):108–111. doi: 10.1023/A:1016345504153. [DOI] [PubMed] [Google Scholar]
- 12.Chow M, Zuo JZ, Wang Y. Method of enhancing absorptions of transmucosal administration formulations. USA patent, 2008: US7329416.
- 13.Wang Y, Zuo Z, Chen X, Tomlinson B, Chow MSS. Improving sublingual delivery of weak base compounds using pHmax concept: application to propranolol. Eur J Pharm Sci. 2010;39(4):272–278. doi: 10.1016/j.ejps.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11(2):217–224. doi: 10.1208/s12248-009-9098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukacova V, Woltosz W, Bolger M. Prediction of modified release pharmacokinetics and pharmacodynamics from < b > <i > in vitro </b > immediate release, and intravenous data. AAPS J. 2009;11(2):323–334. doi: 10.1208/s12248-009-9107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolger M, Lukacova V, Woltosz W. Simulations of the nonlinear dose dependence for substrates of influx and efflux transporters in the human intestine. AAPS J. 2009;11(2):353–363. doi: 10.1208/s12248-009-9111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olanoff LS, Walle T, Cowart TD, Walle UK, Oexmann MJ, Conradi EC. Food effects on propranolol systemic and oral clearance: support for a blood flow hypothesis. Clin Pharmacol Ther. 1986;40(4):408–414. doi: 10.1038/clpt.1986.198. [DOI] [PubMed] [Google Scholar]
- 18.Shargel L, Wu-Pong S, Yu ABC. Applied biopharmaceutics & pharmacokinetics, 5th edn. New York: McGraw-Hill Medical Publishing Division; 2004. [Google Scholar]
- 19.Boxenbaum HG, Riegelman S, Elashoff RM. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- 20.Kates RE. Absorption kinetics of sublingually administered propranolol. J Med. 1977;8(6):393–402. [PubMed] [Google Scholar]
- 21.Henry JA, Ohashi K, Wadsworth J, Turner P. Drug recovery following buccal absorption of propranolol. Br J Clin Pharmacol. 1980;10(1):61–65. doi: 10.1111/j.1365-2125.1980.tb00502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomeni R, Bianchetti G, Sega R, Morselli PL. Pharmacokinetics of propranolol in normal healthy volunteers. J Pharmacokinet Biopharm. 1977;5(3):183–192. doi: 10.1007/BF01065394. [DOI] [PubMed] [Google Scholar]
- 23.Bruce TJ, Saeed SA. Social anxiety disorder: a common, underrecognized mental disorder. Am Fam Physician. 1999;60(8):2311–2320. [PubMed] [Google Scholar]
- 24.Pine M, Favrot L, Smith S, McDonald K, Chidsey CA. Correlation of plasma propranolol concentration with therapeutic response in patients with angina pectoris. Circulation. 1975;52(5):886–893. doi: 10.1161/01.CIR.52.5.886. [DOI] [PubMed] [Google Scholar]
- 25.AHFS Drug Information. Bethesda, MD Authority of the Board of the American Society of Health-system Pharmacists; American Hospital Formulary Service; 2012.
- 26.Julius S, Pascual AV, London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation. 1971;44(3):413–418. doi: 10.1161/01.CIR.44.3.413. [DOI] [PubMed] [Google Scholar]
- 27.Weiss YA, Safar ME, Chevillard C, Frydman A, Simon A, Lemaire P, et al. Comparison of the pharmacokinetics of intravenous dl-propranolol in borderline and permanent hypertension. Eur J Clin Pharmacol. 1976;10(6):387–393. doi: 10.1007/BF00563074. [DOI] [PubMed] [Google Scholar]
- 28.Mansur Ade P, Ramires JA, Avakian SD, de Paula RS, Pileggi F. [Comparison of the effects of diazepam, nifedipine, propranolol and a combination of nifedipine and propranolol, by sublingual administration, in patients with hypertensive crisis] Arq Bras Cardiol. 1991;57(4):313–317. [PubMed] [Google Scholar]
- 29.Johnston GD. Dose–response relationships with antihypertensive drugs. Pharmacol Therapeut. 1992;55(1):53–93. doi: 10.1016/0163-7258(92)90029-Y. [DOI] [PubMed] [Google Scholar]


