Abstract
The immunogenicity profile of a biotherapeutic is determined by a multitude of product and patient-related risk factors that can influence the observed incidence and clinical consequences of immunogenicity. Pre-existing antibodies, i.e., biotherapeutic-reactive antibodies present in samples from treatment-naïve subjects, have been commonly observed during immunogenicity assessments; however their relevance in terms of the safety and efficacy of a biotherapeutic is poorly understood. An American Association of Pharmaceutical Scientists-sponsored survey was conducted to gather information about the prevalence, nature, and consequences of pre-existing antibodies in clinical and nonclinical studies. The survey results indicate that pre-existing antibodies against a variety of biotherapeutics (e.g., mAbs, fusion proteins) are frequently encountered, especially in the context of autoimmune diseases, but that the methods and approaches used to detect, characterize, and report these antibodies vary. In most cases, pre-existing antibodies did not appear to have clinical consequences; however, a few of the respondents reported having observed an effect on pharmacokinetic, pharmacodynamic, safety, and/or efficacy parameters. The findings from this survey are an important first step in evaluating the potential risks associated with the presence of pre-existing antibodies and highlight the importance of standardizing the approaches for detection and characterization of these antibodies. Cross-industry sharing of case studies and relevant data collection will help better inform biotherapeutic risk/benefit profiles and provide deeper understanding of the biological consequences of pre-existing antibodies.
KEY WORDS: immunogenicity risk, pre-existing antibodies, standardization
INTRODUCTION
Immunogenicity assessments (anti-drug antibody (ADA) testing) for a biotherapeutic molecule usually follow a tiered approach wherein a sample is tested in a screen assay followed by a confirmation and titer assay. The ADA results are interpreted based on the assay cutpoint and established criteria to define treatment induced ADA. However, baseline serum samples from treatment-naïve animals and human subjects sometimes exhibit pre-existing reactivity that is related to the presence of drug-reactive antibodies. The impact of pre-existing antibodies on the safety and/or efficacy of biotherapeutic products is poorly understood. In the case of Cetuximab, pre-existing IgE antibodies to a carbohydrate (gal alpha (1–3) gal) moiety caused serious hypersensitivity reactions (1), whereas in the case of Panitumumab, pre-existing antibodies did not exert impact on post treatment ADA induction (2). With a growing number of biotherapeutics in clinical development, it is becoming increasingly important to understand the impact of pre-existing antibodies on the risk–benefit profile of a biotherapeutic.
A cross-industry team was formed in association with the American Association of Pharmaceutical Scientists (AAPS) Therapeutic Protein Immunogenicity Focus Group (TPIFG) to assess whether pre-existing antibodies represent a risk factor for immunogenicity. In March 2012, the team launched a survey on this topic for the AAPS TPIFG and Ligand Binding Assay Bioanalytical Focus Group members, with a goal to gather and share information on the prevalence and characteristics of clinical and nonclinical pre-existing antibodies, and the impact of pre-existing antibodies on treatment-induced ADA, safety, pharmacokinetics (PK), and pharmacodynamics (PD) of a biotherapeutic. Following are the summarized results of the survey.
PREVALENCE AND CHARACTERIZATION OF PRE-EXISTING ANTIBODIES
The survey was completed by 70 scientists, involved in biotherapeutic product development at various pharmaceutical and biotech companies. Pre-existing reactivity in the ADA assay appeared to be common and was observed at least once by 74% of the respondents. Most of the respondents (70%) reported conducting further investigations into the nature of the reactivity to determine whether it was antibody mediated and/or was caused by other reactive components/molecules (to distinguish from pre-existing antibodies). Of those who have conducted investigations into the nature of the reactivity, 46% observed drug reactive pre-existing antibodies only occasionally, whereas 32% observed this antibody-mediated reactivity at least 50% of the time. The extent of characterization of pre-existing reactivity in treatment-naïve subjects varied depending on the prevalence and associated risk level attributed to the biotherapeutic.
The most common method used to characterize pre-existing antibodies was the use of competitive inhibition assays performed by 53% respondents, followed by protein A/G immunodepletion (30%) and neutralizing activity assays (26%). However, respondents also reported performing isotyping (19%), epitope mapping (19%), relative affinity assessments (1%), and dilutional linearity characterizations (9%; Fig. 1).
Fig. 1.
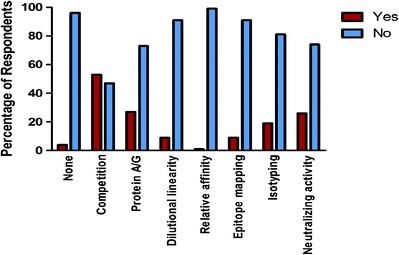
Characterization of pre-existing antibodies. Competition: Performance of confirmatory assay in presence of excess of drug; Protein A/G: Depletion of immunoglobulins; Dilutional linearity: Serial dilution of study samples; Relative affinity: Determination of anti-drug antibody (ADA) affinity to the drug; Epitope mapping: Identification of ADA binding site(s); Isotyping: Determination of ADA Ig class(es); Neutralizing activity: Assessment of ADA inhibition of the drug’s biological activity
SPECIFICITY OF PRE-EXISTING ANTIBODIES
Pre-existing antibodies, as detected and characterized in immunogenicity assessments, were found to be reactive to the protein framework as well as the glycan structures of a biotherapeutic. Clinically, the most commonly reported sources of pre-existing antibodies were nonspecific immunoglobulins (37%) and rheumatoid factor (21%). The presence of heterophilic antibodies, anti-carbohydrate, and anti-Fab antibodies was also observed (Fig. 2). Among biotherapeutic modalities, human monoclonal antibody-based products (37%) were most often associated with pre-existing antibodies, while fusion and homologues of endogenous proteins, chimeric, and alternative mAb scaffolds were each cited by less than 10% of respondents.
Fig. 2.
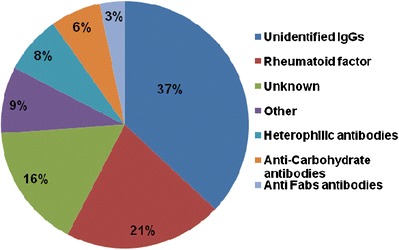
Specificity of pre-existing antibodies identified in clinical samples
Pre-existing antibodies in clinical studies were most often detected in autoimmune disease patient samples (85%) followed by oncology (8%) and metabolic (3%) indications. In nonclinical studies, pre-existing antibodies were mostly due to unknown reactivity (44%) or unidentified specificity IgGs (9%) as well as heterophilic antihuman antibody, anti-Fab, and anti-carbohydrate antibodies. A small percentage of respondents indicated not having observed pre-existing antibodies during nonclinical investigations.
IMPACT OF PRE-EXISTING ANTIBODIES
Although the majority of the respondents did not observe a noticeable impact of pre-existing antibodies on safety or efficacy parameters, 15.2% and 10.7% of respondents reported a potential impact of pre-existing antibodies on safety in clinical and non-clinical studies, respectively. Similarly, only 10% reported an effect on clinical efficacy and 20.7% reported an effect on nonclinical PD markers. Pre-existing antibodies were observed to impact the PK of a biotherapeutics in nonclinical studies by 32.3%of respondents and in clinical studies by 24.2% of the responders. Respondents observed that pre-existing antibodies were sometimes associated with an increase in titer of treatment induced ADA (25% for nonclinical and 32.3% for clinical studies; Fig. 3).
Fig. 3.
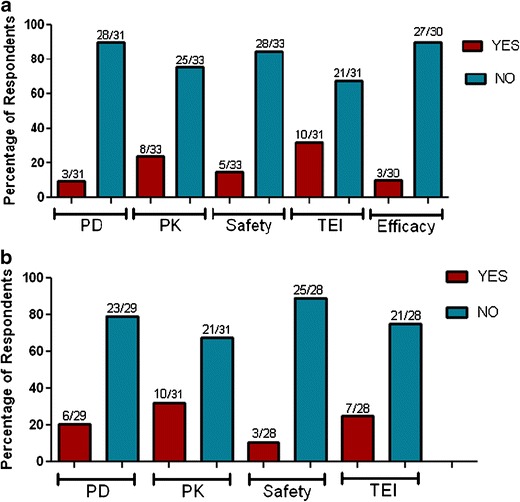
Impact of pre-existing antibodies on PK, PD, safety, efficacy (clinical), and treatment emergent ADA. a Clinical, b non-clinical. TEI treatment emergent immunogenicity
PRE-EXISTING ANTIBODY DATA REPORTING
Detection of pre-existing antibodies is largely dependent on the selection of the bioanalytical ADA assay cut point. In the survey, the use of the 95th percentile (5% false positive rate), and removal of outliers in statistical calculations were reported as the most commonly used criteria for establishing the screening cut point for both clinical and nonclinical ADA assays (Fig. 4). In order to establish screen cut points for clinical ADA assays, 53% of respondents indicated they used samples from the disease-specific population, whereas 11% of respondents indicated they used samples from healthy volunteers.
Fig. 4.
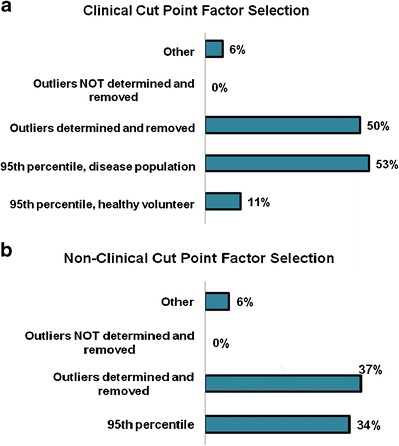
Pre-existing antibody data reporting in clinical and nonclinical evaluations. a Cut-point factor selection criteria in clinical evaluations. b Cut-point factor selection criteria in non-clinical evaluations
The approaches for reporting nonclinical and clinical pre-existing antibodies appeared to be similar. Eighty percent of respondents indicated reporting the incidence of pre-existing antibodies along with treatment-induced ADA incidence, and including the identified impact of pre-existing antibodies on PK, PD, safety, efficacy, and immunogenicity in the study reports. However, there was discrepancy in how to report the ADA incidence for the subjects with pre-existing antibodies. Half of the respondents (52% clinical and 58% nonclinical) indicated that they included the pre-dose positive subjects that did not have an associated post-dose increase in ADA levels in the final reported immunogenicity incidence.
STRATEGIES TO ADDRESS PRE-EXISTING ANTIBODIES
During clinical immunogenicity assessments, 46% of respondents did not perform any additional characterization of pre-existing antibodies. Use of confirmatory/characterization assays to provide additional information about the nature and/or specificity of the pre-existing antibodies and post-treatment ADA was reported by 14% and 23% of respondents, respectively. Other approaches, such as eliminating subjects with pre-existing antibodies, raising the cut point factor to exclude detection of the pre-existing antibodies, and balancing the frequency of patients with pre-existing antibodies among study cohorts were also reported albeit by a low number of respondents (Fig. 5a).
Fig. 5.
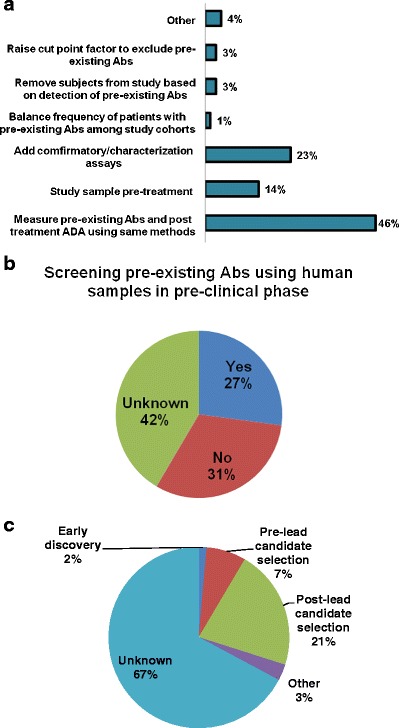
Immunogenicity risk management and mitigation. a Strategies currently in use to address pre-existing antibodies (Abs). b Screening pre-existing antibodies using human samples in pre-clinical phase to proactively manage/mitigate risk and impact associated with pre-existing antibodies. c Stages at which human sample pre-existing antibody screening was performed
Notably, 27% of respondents reported implementing proactive strategies to manage/mitigate the potential impact of pre-existing antibodies. Gaining an early sense of the prevalence of pre-existing antibodies in a given disease by screening commercially available human samples was one example. This strategy was reportedly implemented after biotherapeutic lead candidate selection by 21% of respondents and prior to the lead candidate selection by 7% of the respondents (Fig. 5b, c).
DISCUSSION
Overall, the survey results indicated that pre-existing antibodies were observed during clinical and nonclinical immunogenicity assessments. Although the methods used to characterize and report pre-existing antibodies varied, the reported clinical prevalence of pre-existing antibodies appeared higher in autoimmune indications relative to metabolic diseases and oncology. The majority of respondents reported no impact of pre-existing antibodies on treatment-induced immunogenicity, PK, PD, safety, and/or efficacy.
The survey results and the observed trends are based on the individual experience of the respondents. Given that some respondents reported observing an impact of pre-existing antibodies on either PK or safety, there is value in further understanding the nature of these antibodies. Standardization of the cutpoint selection and methods used to measure and characterize pre-existing antibodies is needed to enable better assessment of the prevalence, characteristics, and impact of these antibodies. Furthermore, questions related to the potential impact of pre-existing antibodies on treatment-induced immunogenicity, the lack of regulatory or industry guidance, and the need for standardization were identified as the top three gaps/barriers towards improving our understanding of the clinical impact of pre-existing antibodies. Prospective and retrospective evaluation of approaches taken for determining and characterizing pre-existing antibodies may also help refine the assessment of the potential inherent risk associated with these antibodies.
CONCLUSION
Guided follow-up efforts are needed to further enhance our understanding of the occurrence and specificity of pre-existing biotherapeutic-reactive antibodies, and standardize the reporting criteria and approaches for these antibodies. More importantly, there is value in assessing the overall impact of these antibodies on the immunogenicity, PK, PD, efficacy, and safety of a biotherapeutic treatment. Collectively, these efforts can help direct the formulation of proactive and fit-for-purpose immunogenicity risk management/mitigation strategies.
ACKNOWLEDGMENTS
We would like to thank all survey participants for filling out the survey, and TPIFG and LBABFG steering committee members for reviewing the survey questionnaire and providing constructive suggestions. We thank Maria Nadeau, Scott Didawick for their assistance in launching the survey and compiling the survey results.
REFERENCES
- 1.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med. 2008;358:1109–17. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weeraratne D, Chen A, Pennucci JJ, et al. Immunogenicity of panitumumab in combination chemotherapy clinical trials. BMC Clin Pharmacol. 2011;11:17. doi: 10.1186/1472-6904-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


