Abstract
Isoniazid (INH) and rifampicin (RIF) are the first-line drugs for antituberculosis (anti-TB) chemotherapy. The levels of serum transaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] are abnormal in 27% of patients undergoing INH and RIF treatments and in 19% of patients undergoing treatment with INH alone. Cytochrome P450 2E1 (CYP2E1) metabolizes many toxic substrates, including ethanol, carbon tetrachloride, and INH, which ultimately results in liver injury. The objective of this study was to screen for CYP2E1 inhibitors in vitro and investigate whether the selected compound could prevent INH/RIF-induced hepatotoxicity in vivo. We screened 83 known compounds from food and herbal medicines as inhibitors of CYP2E1. The hepatotoxic dose of INH/RIF was 50/100 mg kg−1 day−1. Hepatotoxicity was assessed using galactose single-point (GSP) method (a quantitative measurement of liver function), histopathological examination of the liver, malondialdehyde (MDA) assay, and measurement of AST and ALT activities. Kaempferol inhibited CYP2E1 activity in mice by 0.31- to 0.48-fold (p < 0.005). Mice with INH/RIF-induced hepatotoxicity showed significantly abnormal serum levels of AST and ALT, and GSP value, and these values could be decreased by the administration of kaempferol (p < 0.005). Kaempferol significantly reduced the depletion of hepatic glutathione and prevented the increase in MDA formation in mice. Furthermore, kaempferol did not affect the anti-TB effects of INH/RIF. To our knowledge, this is the first report of kaempferol’s utility as an adjuvant for preventing CYP2E1-mediated hepatotoxicity induced by drugs such as INH and RIF.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-013-9490-6) contains supplementary material, which is available to authorized users.
Key words: galactose single-point method, hepatotoxicity, isoniazid, kaempferol, rifampicin
INTRODUCTION
Hepatic cytochrome P450 (CYP450) enzymes are an important superfamily of hemoproteins that are responsible for the monooxygenation of various xenobiotics, including therapeutic drugs, environmental pollutants, carcinogens, as well as many endogenous substrates such as steroids, prostaglandins, arachidonic acid, and leukotrienes. Many popular herbal medicines have been routinely used or marketed as dietary supplements, such as St. John’s wort and Ginkgo biloba (1). Flavonoids and their derivatives can be metabolized by CYP450 isozymes and/or directly inhibit CYP450 activities (2,3).
A variety of flavonoids are substrates of CYP450 monooxygenases, and CYP-mediated oxidation seems to play only a minor role in the in vivo metabolism of flavonoids (5). The excretion of flavonoids or their conjugated metabolites may involve transport by transporters such as multidrug resistance-associated proteins 1 and 2 or breast cancer resistance protein (5,6). Unconjugated flavonoid aglycones may be substrates of the drug efflux transporter P-glycoprotein (multidrug-resistant 1) (7).
Among the various CYP isoforms, isoform CYP3A4 plays a prominent role in the metabolism of ∼50% of all prescribed drugs (7,8). The substrate specificity of CYP3A4 widely overlaps that of P-glycoprotein (9). A number of studies have shown flavonoid-induced inhibition of drug-metabolizing enzymes such as CYP3A4 or drug transporters such as P-glycoprotein. Compared to other isoforms, CYP2E1 is usually relevant to only minor metabolic reactions of certain drugs (10). Interestingly, oral administration of high doses of the flavones wogonin, bacalein, or the baicalein glucuronide baicalin from Scutellaria radix decreased hepatic protein expression and enzyme activity of CYP2E1 in mice (10,11).
CYP2E1 can be induced by ethanol, drugs such as isoniazid (INH), and hydrocarbons (12,13). CYP2E1 is responsible for the biotransformation of therapeutic agents [e.g., acetaminophen, INH, and rifampicin (RIF)], procarcinogens (e.g., N-nitrosamine), and endogenous compounds (e.g., fatty acids and ketone bodies) (14–16). Antituberculosis (anti-TB) drug-induced hepatitis is one of the most prevalent drug-induced liver injuries in many countries (17,18). CYP2E1-mediated INH metabolism may result from oxidative stress through the production of toxic metabolites or free radicals and lead to drug-induced hepatotoxicity (15,16).
INH has been widely used for the treatment of both active and latent forms of Mycobacterium tuberculosis infections. Mild and transient increases in serum transaminase levels [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] occur in 10–20% of the patients taking INH, and severe hepatotoxicity occurs in approximately 1–3% of patients (17,18). RIF is another primary component in the treatment and prophylaxis of TB. Serum transaminase levels increased in 27% of patients taking INH/RIF and in 19% of patients taking INH alone (17). The primary pathway of INH metabolism involves acetylation by N-acetyltransferase (NAT2) in the liver to generate acetyl-INH. Acetyl-INH undergoes hydrolysis to form acetyl-hydrazine and the nontoxic substance isonicotinic acid (INA), which is oxidized by CYP2E1 to form reactive acylating hepatotoxins, possibly acetyldiazene, or its breakdown products consisting of an acetyl radical, an acetylonium ion, and a ketene (16,17) [Electronic Supplementary Materials (ESM) Fig. 1]. Polymorphisms of NAT2, which have been identified in the population, lead to humans being either rapid or slow acetylators. In rapid acetylators, more than 90% of INH is excreted as acetyl-INH, whereas in slow acetylators, 67% of INH is excreted as acetyl-INH; a greater percentage of INH is excreted as unchanged drug in the urine (19,20). Slow acetylators shunt some INH to a secondary metabolic pathway of hydrolysis through amidase and produce hydrazine. Both acetylhydrazine and hydrazine, which are generated by rapid and slow acetylators, respectively, are capable of participating in reactions that generate oxidative stress (e.g., free radicals). Hydrazine may induce CYP450 (specifically CYP2E1) and thus increase the production of additional hepatotoxins. Thus, hepatotoxicity may occur in both rapid and slow acetylators, although for slightly different reasons (21,22).
The pathogenesis of INH- and RIF-induced hepatitis in humans is unclear, and several mechanisms have been postulated. Timbrell et al. (23) have reported that acetylhydrazine is the toxic metabolite responsible for liver injury caused by INH. RIF is a potent inducer of CYP2E1, and it could aggravate INH-induced hepatotoxicity by increasing the production of toxic metabolites such as hydrazine in man and rats (16,24,27). A few studies have shown that hepatic CYP2E1 activity highly correlates with INH-induced hepatotoxicity (15,17). Previous studies have described that the synergistic effects of RIF contribute to INH-induced hepatotoxicity, probably through the induction of CYP2E1, amidase, or other enzymes (17,24–27).
Recent studies have proposed that oxidative stress is one of the mechanisms responsible for INH- and RIF-induced liver injury (19). INH- and RIF-induced hepatic injury occurs through the induction of oxidative stress (20). In rats, hepatic glutathione (GSH) levels significantly decreased in response to INH or hydrazine. Hydrazine reacts with a sulfhydryl group, which results in GSH depletion within the hepatocytes and leads to cell death (12,28,29). The molecular mechanism of hydrazine-induced hepatotoxicity has been attributed to oxidative stress due to the formation of reactive oxygen species (ROS) and carbonyl (16). These findings suggest that CYP2E1 could play an important role in the adaptive response against the CYP2E1-dependent oxidative stress caused by INH and RIF. Thus, a CYP2E1 inhibitor might be used as a potential adjuvant to reduce INH- and RIF-induced hepatotoxicity. However, whether the inhibition of CYP2E1 can protect against INH- and INH/RIF-induced hepatotoxicity remains unclear.
AST and ALT activities are the most commonly used biochemical markers of liver damage. However, they often lack sensitivity in the early stages of liver diseases and change only in advanced stages. The galactose single-point (GSP) method has been recommended by the US Food and Drug Administration in the guidance document for the industry, “Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design, Data Analysis, and Impact on Dosing and Labeling,” and has been successfully used to predict a variety of liver diseases (31–34).
Flavonoid compounds from commonly used food and herbal medicines are generally regarded as nontoxic and nonirritant components. To date, no study has reported whether flavonoid compounds can prevent INH/RIF-induced hepatotoxicity through CYP2E1 inhibition. Therefore, in this study, we aimed to examine whether a CYP2E1 inhibitor could prevent INH/RIF-induced hepatotoxicity in vitro and in vivo.
MATERIALS AND METHODS
Materials
All the organic solvents were high-performance liquid chromatography grade and were obtained from Tedia Company, Inc. (Fairfield, OH, USA). Chlorzoxazone (CZX), 6-hydroxyl-CZX (6-OH-CZX), diethyldithiocarbamate, thiobarbituric acid, INH, and RIF were purchased from Sigma-Aldrich Co. LLC (St. Louis, MO, USA). Galactose injection solution for GSP injection was prepared by the Nang-Kuang Pharmaceutical Co., Ltd. (Tainan, Taiwan, Republic of China).
Methods
Screening for CYP2E1 Inhibitors
Human Liver Microsomes
Histologically normal human livers were obtained from patients undergoing liver surgery. A pool of human liver microsomes (HLMs) was created from three individual liver donors. The study was approved by the Tri-Service General Hospital Institutional Review Board. The liver samples were frozen at −80°C until use. HLMs were prepared using the differential centrifugation technique (35), and the microsomal protein concentration was determined using the Lowry method (36).
Rat Liver Microsomes
Male Sprague–Dawley® (SD) rats were purchased from the National Laboratory Animal Center (Taipei, Taiwan). Rat liver microsomes (RLMs) were prepared from 3-g liver samples from SD rats (175–250 g) using the differential centrifugation technique. The final pellet was resuspended in 0.1 M phosphate buffer (pH 7.4) and the liver microsomes prepared. The liver microsomal protein concentration was determined using the Lowry method (36).
CYP2E1 Enzyme Activity Assays
CYP2E1 activity was determined by measuring the hydroxylation of CZX to 6-OH-CZX, as described previously by Kharasch et al. (37). All samples were tested in triplicate. For the determination of the percent inhibition values, each test compound was dissolved in 16.5, 33, and 66 μM concentrations. Enzymatic reaction was terminated by ice–acetonitrile, and the addition of 4-hydroxyl tolbutamide was used as an internal standard. The organic phase was evaporated to dryness and reconstituted into a mobile phase (methanol/water = 1:1) before liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis.
LC-MS/MS Analysis with CZX and 6-OH-CZX
An API 3000 triple-quadrupole mass spectrometer equipped with an ion spray (ESI) source was used to determine the CZX and 6-OH-CZX levels in both HLMs and RLMs. The chromatographic column was a phenyl column (100 × 2.1 mm, 3.5 μm; XBridge). The mobile phase that consisted of A/B (50:50, v/v) (solvent A, 0.1% formic acid in water; solvent B, 0.1% formic acid in methanol) was delivered by a Shimadzu LC-10AD pump at a flow rate of 0.5 mL/min. The ESI interface was used in the negative ion mode. The selected transitions of m/z were 167.9→131.9 for CZX and 184.0→119.9 for 6-OH-CZX.
Acute Toxicity Study of Kaempferol in Animals
An oral toxicity study was performed to evaluate the safety of the oral administration of kaempferol in CD-1 (ICR) mice.
CD-1 (ICR) mice were purchased from the National Laboratory Animal Center. Mice were randomized into four groups; each group consisted of six male rats and six female rats. The treated animals were administered kaempferol at doses of 100, 1,000, and 10,000 mg/kg, while the control mice were administered equal volumes of 0.5% carboxymethyl cellulose (CMC). The test solutions were freshly prepared by suspending kaempferol in 0.5% CMC on the day of dosing. The dose volume was 40 mL/kg (20 mL/kg twice a day). Mice were observed for four consecutive hours after dosing, followed by once daily observation for clinical signs and twice daily for mortality, which was continued for 14 days. Body weight was recorded on the dosing day (D1), at 1-week intervals (D8), and at the end of the study period (D15). Mice were killed by exposure to carbon dioxide before exsanguination and necropsy.
Hepatotoxicity Study in Mice
Animals
129/sv mice weighing 18–25 g were purchased from the National Laboratory Animal Center. All experiments were performed according to the National Institutes of Health Guidelines for the treatment of animals. All mice were maintained in an air- and humidity-controlled room with a 12-h light/12-h dark cycle and allowed access to food and water ad libitum throughout the experiment. All of the mice were anesthetized with ether and administered an intravenous injection of galactose. After 60 min, blood was taken from the tail vein to measure the blood concentration of galactose and GSP values.
Hepatotoxicity Studies
For evaluation of the hepatoprotective effects of the CYP2E1 inhibitor on INH/RIF-induced hepatotoxicity, the mice were randomized into the following four treatment groups for 3 weeks: (1) normal control group (NC, n = 12), which consisted of normal mice intraperitoneally (i.p.) injected with saline once daily at a volume of 10 mL/kg; (2) control group (INH/RIF, n = 12), which consisted of normal mice i.p. injected with INH/RIF in saline at doses of 50/100 mg/kg once daily at a volume of 10 mL/kg; (3) LK study group (Kaempferol-INH, n = 12), which consisted of normal mice i.p. injected with kaempferol in saline at 1.89 mg/kg and INH/RIF in saline at 50/100 mg/kg once daily at a volume of 10 mL/kg; and (4) HK-Study group (Kaempferol-INH, n = 12), which consisted of normal mice i.p. injected with kaempferol in saline at 3.78 mg/kg and INH/RIF in saline at 50/100 mg/kg once daily at a volume of 10 mL/kg. GSP analysis was performed 16 h after treatment at 0, 2, and 3 weeks to quantify residual liver function.
Blood Sampling
At the end of treatment, the animals were killed while under diethyl ether anesthesia. Blood was collected in heparin tubes from the heart of the mice and the plasma was separated by centrifugation at 13,000×g for 5 min at 4°C. Aliquots of the plasma were transferred to Eppendorf tubes and stored at −80°C until analysis.
Hepatic AST and ALT Level Determinations
Plasma enzyme activities (AST and ALT) were determined at 37°C using Synchron LXi 725 (Beckman Coulter, Inc., Brea, CA, USA) with kits provided by the manufacturer.
Measurement of GSH levels
GSH levels were determined using the method of Griffith (28). The contents of hepatic GSH were assessed by colorization of 5,5′-dithio-2-nitrobenzoic acid-reactive GSH. GSH values were expressed as micromoles per gram of liver.
Lipid Peroxidation Assay
Lipid peroxidation was quantified by measuring malondialdehyde (MDA) levels, as described by Ohkawa et al. (30). Hepatic MDA contents were assessed by detecting thiobarbituric acid-reactive MDA, which is an end product of the peroxidation of polyunsaturated fatty acids and related esters. MDA values were expressed as nanomoles per gram of liver.
Quantitative Testing of the Liver Function
The methods are briefly described as follows. Study animals received a rapid intravenous administration of 0.75 g/kg body weight galactose solution (G.S.P.® 0.4 g/mL). The solution was injected in 30 s. Dried blood specimens were taken from the tail vein 5, 10, 15, 30, 45, and 60 min after the injection. The GSP values were defined as the galactose blood concentration 60 min after the injection (32).
Liver Microsome Preparation and CYP2E1 Activity Assay
After administration of the doses, liver microsomes were prepared by homogenizing liver samples (0.1 g) obtained from the 129/sv mice (18–25 g) in a mechanical homogenizer as described previously. Liver microsomal protein concentration was determined using the Lowry method (36). CYP2E1 activity was determined by measuring the hydroxylation of CZX to 6-OH-CZX.
Histological Examinations
Immediately after the mice were killed, the livers were removed for histological analysis. For light microscopy, the liver specimens (n = 12 for each group) were fixed in 10% phosphate-buffered formalin, dehydrated, embedded in paraffin, and stained for histological observation. Liver histology was assessed by a pathologist that was blinded to the treatment groups or the corresponding liver biochemistries. Histological assessments were graded using the histological activity index (HAI) according to the criteria of Knodell et al. (38).
Pharmacokinetic Studies
Pharmacokinetics of INH, Acetyl-INH, INA, and RIF in Mice
After 3 weeks of treatment, mice in the NC, Control, LK, and HK groups were administered INH/RIF i.p. at a dose of 50/100 mg/kg body weight. Blood samples were collected in heparinized microcentrifuge tubes at intervals of 0, 0.08, 0.25, 0.5, 1, 1.5, 2, 4, 6, 12, and 24 h. Plasma was immediately obtained by centrifuging blood samples at 8,000 rpm for 10 min. Plasma samples were then stored at −80°C until use. The plasma samples of INH, acetyl-INH, INA, and RIF were analyzed using a validated LC-MS/MS method.
LC-MS/MS Analysis with INH, Acetyl-INH, INA, and RIF
An ACQUITYTM UPLCTM system with an ESI source was used to determine INH, acetyl-INH, INA, and RIF levels in the animal samples. C18 chromatographic column (100 × 2.1 mm, 1.7 μm; BEH) was used. Chromatographic separations were achieved by gradient elution with a mobile phase comprising water and methanol (both containing 0.1% formic acid) and by using an ACQUITYTM Binary Solvent Manager at a flow rate of 0.25 mL/min. The ESI interface was used in the positive ion mode. The selected transitions of m/z were 138.3→121.3 for INH, 180.2→138.1 for acetyl-INH, 124.1→79.9 for INA, and 823.6→791.6 for RIF. The calibration variability data that were obtained over concentrations of 0.5–100 μg/mL for INH and RIF and 0.25–25 μg/mL for acetyl-INH and INA showed a linear and reproducible curve. Within-run and between-run imprecision (coefficients of variation) values were 0.4–3.7% for INH, 5.1–9.2% for acetyl-INH, 4.1–6.2% for INA, and 3.8–9.3% for RIF; the lower limits of detection were 0.5 μg/mL for INH and RIF and 0.25 μg/mL for acetyl-INH and INA.
Pharmacokinetics of CZX and 6-OH-CZX in Mice
After administration of the treatments, the mice were given CZX orally at a dose of 500 mg. Blood samples were collected in heparinized microcentrifuge tubes at intervals of 0, 0.08, 0.25, 0.5, 1, 1.5, 2, 4, 6, and 8 h. Plasma samples were immediately obtained by centrifuging blood samples at 8,000 rpm for 10 min. The plasma samples were then stored at −80°C until use. At the final time point, the animals were killed while under diethyl ether anesthesia and the liver was removed immediately, frozen on dry ice, and stored at −80°C until use. Liver microsomes were prepared and CYP2E1-mediated CZX hydroxylation activity was determined. Plasma samples of CZX and 6-OH-CZX were analyzed using a validated LC-MS/MS method.
Anti-TB Efficacy Assays
M. tuberculosis strain H37Ra was grown in 7H9 liquid medium (Difco) supplemented with 0.05% Tween 80 and 10% bovine serum albumin dextrose–catalase enrichment (Difco) at 37°C for 3 weeks with occasional shaking. Mycobacterium smegmatis strain mc26 (MC2) was cultivated in a similar manner in the 7H9 medium at 37°C for 4 days. Next, to analyze the effects of INH and INH/RIF with and without kaempferol on the survival of Mycobacterium, M. tuberculosis H37Ra, or M. smegmatis, cells were resuspended in 7H9 liquid medium (pH 6.5) with 0.1 μg/mL INH and 0.1/0.5 μg/mL INH/RIF without or with 0.1 (LK), 1 (MK), and 10 μg/mL kaempferol (HK). When Mycobacteria were incubated to a cell density of 0.3 at OD600 at 37°C, aliquots of the cell suspension were removed, washed, and diluted before plating on 7H11 plates. Then, the plates were incubated at 37°C for 4 weeks for M. tuberculosis and for 5 days for M. smegmatis to determine the number of surviving Mycobacterium.
Statistical Analyses
All of the data were expressed as the mean ± standard deviation. The results were analyzed for statistical significance using one-way analysis of variance tests with the Statistical Package of the Social Science Program (ver. 13.0, IBM Corporation, Armonk, NY, USA). The least significant difference post hoc test of multiple comparisons was subsequently used to identify significant differences among the groups.
RESULTS
Screening for Inhibitors of CP450 2E1
Diethyldithiocarbamic acid (DDTC) is a well-known inhibitor of CYP2E1 (4). At a concentration of 100 μM, DDTC treatment resulted in 89.6% and 84.2% inhibition of CYP2E1 in HLM and RLM, respectively (measured with CZX as a CYPE21 substrate). On the basis of the observed inhibitory activities of pure DDTC, we screened 83 components from commonly used herbal medicines for CYP2E1 inhibition at concentrations of 66, 33, and 16.5 μM. The inhibitory activity of the compounds selected and DDTC are summarized in Tables I and II. Compared to the control groups, the groups treated with these compounds effectively inhibited CZX metabolism by at least 60%.
Table I.
Inhibition up to 60% of CYP2E1 Activity Observed in Human Liver Microsomes Using Selected Compounds from Food and Herbal Medicines at Varying Concentrations
| Selected component | Percent inhibition for each compound (%) | 6-Hydroxychlorzoxazone production of control group (ng/mL) | ||
|---|---|---|---|---|
| 66 μM | 33 μM | 16.5 μM | ||
| DDTC (positive control) | (100 μM) 89.6 ± 0.8 | (50 μM) 48.7 ± 2.3 | (10 μM) 7.8 ± 0.4 | 1,136.7 ± 128.6 |
| Nordihydroquaiaretic acid | 97.0 ± 0.2 | 67.7 ± 2.2 | 49.8 ± 2.4 | 983.0 ± 12.8 |
| trans-Cinnaldehyde | 92.8 ± 0.5 | 89.6 ± 1.5 | 49.8 ± 2.4 | 1,036.7 ± 75.1 |
| Daidzein | 86.8 ± 1.0 | 76.3 ± 2.3 | 73.5 ± 1.7 | 1,036.7 ± 75.1 |
| Isovitexin | 81.8 ± 1.3 | 67.6 ± 3.2 | 59.8 ± 1.4 | 1,136.7 ± 128.6 |
| Kaempferol | 79.2 ± 0.3 | 74.7 ± 0.6 | 66.5 ± 1.7 | 983.0 ± 12.8 |
| Disulfiram | 78.2 ± 0.4 | 75.8 ± 1.4 | 74.1 ± 1.1 | 983.0 ± 12.8 |
| β-Myrcene | 76.5 ± 2.2 | 75.5 ± 2.1 | 53.4 ± 4.9 | 1,176.7 ± 75.1 |
| Quercetin | 73.3 ± 1.6 | 53.0 ± 2.2 | 46.4 ± 4.7 | 1,036.7 ± 75.1 |
| (- (−)-Epigallocatechin-3-gallate | 72.2 ± 1.0 | 60.5 ± 2.1 | 50.2 ± 1.9 | 983.0 ± 12.8 |
| Limonene | 63.6 ± 2.7 | 38.1 ± 1.9 | 13.8 ± 2.0 | 1,283.3 ± 153.7 |
| Myricetin | 61.6 ± 0.9 | 59.2 ± 1.3 | 42.2 ± 2.5 | 1,176.7 ± 90.7 |
| Quercitrin | 61.0 ± 5.9 | 53.8 ± 3.5 | 33.5 ± 4.3 | 1,283.3 ± 153.7 |
| Luteolin-7-glucoside | 60.3 ± 1.1 | 55.9 ± 0.7 | 43.0 ± 5.1 | 1,136.7 ± 128.6 |
| Morin | 60.3 ± 1.6 | 52.1 ± 1.7 | 36.9 ± 1.6 | 1,283.3 ± 153.7 |
CYP2E1 cytochrome P450 2E1, DDTC diethyldithiocarbamic acid
Table II.
Inhibition up to 60% of CYP2E1 Activity Observed in Rat Liver Microsomes Using Selected Compounds from Food and Herbal Medicines at Varying Concentrations
| Selected component | Percent inhibition of each compound (%) | 6-Hydroxychlorzoxazone production of control group (ng/mL) | ||
|---|---|---|---|---|
| 66 μM | 33 μM | 16.5 μM | ||
| DDTC (positive control) | (100 μM) 84.2 ± 2.2 | (50 μM) 39.3 ± 1.1 | (10 μM) 6.4 ± 0.2 | 2,750.0 ± 163.7 |
| Kaempferol | 87.8 ± 1.3 | 80.0 ± 1.6 | 59.8 ± 3.3 | 2,780.0 ± 75.5 |
| trans-Cinnaldehyde | 86.8 ± 2.8 | 81.9 ± 0.9 | 66.4 ± 2.4 | 2,750.0 ± 163.7 |
| Nordihydroguaiaretic acid | 84.5 ± 1.3 | 72.7 ± 2.0 | 45.0 ± 3.9 | 2,780.0 ± 75.5 |
| Daidzein | 82.4 ± 0.6 | 78.7 ± 1.5 | 71.0 ± 1.6 | 2,740.0 ± 79.4 |
| β-Myrcene | 79.3 ± 1.7 | 72.7 ± 1.6 | 63.3 ± 0.7 | 2,750.0 ± 163.7 |
| Isovitexin | 78.6 ± 1.8 | 73.9 ± 2.7 | 57.7 ± 3.6 | 2,513.0 ± 85.0 |
| Morin | 77.5 ± 3.9 | 68.1 ± 1.0 | 55.1 ± 3.7 | 2,463.3 ± 102.1 |
| (−)-Epigallocatechin-3-gallate | 75.1 ± 2.0 | 57.6 ± 3.8 | 38.7 ± 3.2 | 2,780.0 ± 75.5 |
| Quercetin | 72.3 ± 2.6 | 56.8 ± 5.8 | 34.1 ± 2.0 | 2,740.0 ± 79.4 |
| Limonene | 69.0 ± 3.9 | 56.8 ± 2.7 | 29.1 ± 3.1 | 2,463.3 ± 102.1 |
| Disulfiram | 66.1 ± 2.5 | 58.1 ± 1.1 | 45.1 ± 2.7 | 2,813.3 ± 90.7 |
| Hesperedin | 64.0 ± 4.1 | 52.8 ± 2.2 | 46.5 ± 3.9 | 2,463.3 ± 102.1 |
| Quercitrin | 61.6 ± 2.5 | 49.5 ± 2.5 | 36.3 ± 3.9 | 2,463.3 ± 102.1 |
CYP2E1 cytochrome P450 2E1, DDTC diethyldithiocarbamic acid
Acute Toxicity of Kaempferol in Mice
All test animals survived the study period. Hypoactivity and piloerection were observed in male mice within 3 h after administration of a dose of 10,000 mg/kg kaempferol. Discolored (test article-like) stool was found in both genders 3–4 h after administration of 1,000 or 10,000 mg/kg of kaempferol. All clinical signs recovered on the second day. No statistically significant differences in body weight were observed between the treatment and control groups during the study period. No gross lesions were detected in any animal upon necropsy. These results may serve as a safety reference for human use (ESM Tables 1 and 2).
Hepatotoxicity in an Animal Model
Effects of Kaempferol on INH- and RIF-Induced Hepatotoxicity
The body weights and relative liver weights of the experimental animals were measured at the end of study. No statistically significant differences were observed between the treated and the control animals.
Intraperitoneal administration of INH/RIF at a dose of 50/100 mg kg−1 day−1 over a period of 3 weeks produced hepatotoxicity. A relatively normal liver architecture was found in the NC group (Fig. 1a). Hepatocytes within the liver parenchyma were arranged into one-cell-thick anastomosing plates that radiated from the central vein of the lobules. Hepatic sinusoids were found between two anastomosing plates of hepatocytes. Hepatocellular disintegration and vacuolation of the pericentral vein hepatocytes were observed in the INH/RIF group (Fig. 1b). Treatment of mice with kaempferol (LK, low dose) before injection with INH/RIF inhibited the formation of toxic intermediates and prevented hepatocellular damage. The liver tissue of kaempferol-INH/RIF-treated mice (Fig. 1c) showed histopathological features similar to those in the NC group (Fig. 1a). Doubling the dose of kaempferol to 3.78 mg kg−1 day−1 (HK, high dose) resulted in increased hepatoprotection with INH/RIF treatment (Fig. 1d).
Fig. 1.
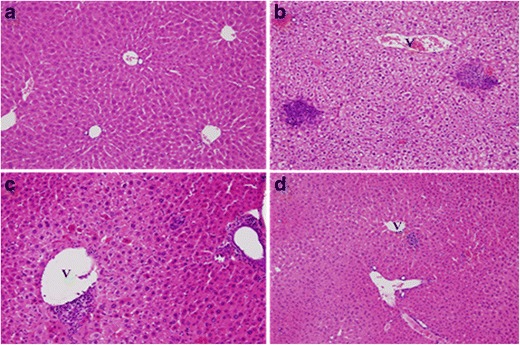
Photomicrography of liver sections from mice in the normal control (NC) group (a), control group (b), LK-Study group (c), and HK-Study group (d). a Mice in the NC group showed relatively normal morphology of the liver tissue. b Hepatocellular disintegration and vacuolation of the pericentral vein area in the isoniazid (INH)/rifampicin (RIF) group. c Histological sections of the liver tissue of mice treated with 1.89 mg/kg kaempferol–INH/RIF showed patterns similar to those of the NC group. d Histological sections of the liver tissue of mice treated with 3.78 mg/kg kaempferol–INH/RIF showed patterns similar to those of the NC group (hematoxylin and eosin stain, original magnification ×200). V central vein
Plasma AST and ALT activities significantly increased with INH/RIF treatment (AST activity increased from 80 ± 11 to 571 ± 295 IU/L, p < 0.005; ALT activity increased from 41 ± 13 to 364 ± 192 IU/L, p < 0.005), which indicated liver injury (Table III). In line with our observations of liver architecture, co-administration of kaempferol reduced the biochemical markers of INH/RIF-induced hepatotoxicity in a dose-dependent manner (Table III). These results showed that kaempferol was able to partially prevent INH/RIF-induced hepatotoxicity.
Table III.
GSP Levels, Hepatic AST and ALT Activities, and Total HAI Score in NC, INH/RIF-Control, LK-Study, and HK-Study Groups after 3 Weeks of Treatment
| Groups | GSP (mg/L) | AST (IU/L) | ALT (IU/L) | Total HAI score |
|---|---|---|---|---|
| Normal control (n = 9) | 177 ± 22 | 80 ± 11 | 41 ± 13 | 0.0 ± 0.0 |
| INH/RIF-control (n = 8) | 866 ± 339*** | 571 ± 295*** | 364 ± 192*** | 5.3 ± 2.1*** |
| Study-LK (n = 9) | 401 ± 178*** | 164 ± 78*** | 117 ± 62*** | 1.9 ± 1.1* |
| Study-HK (n = 9) | 245 ± 98*** | 89 ± 19*** | 48 ± 21*** | 1.6 ± 1.0** |
Data are shown as the mean ± SD. Each group contained at least eight mice. Statistical analysis: ANOVA and LSD test
GSP: ***p < 0.005 vs. NC group and vs. control group
AST and ALT: ***p < 0.005 vs. NC group and vs. control group
Total HAI score: ***p < 0.005 vs. NC group; *p < 0.05, **p < 0.01 vs. control group
GSP galactose single point, AST aspartate aminotransferase, ALT alanine aminotransferase, HAI histological activity index, NC normal control, INH isoniazid, RIF rifampicin
Histological assessments were performed by a pathologist with the HAI according to the criteria of Knodell et al. (38) (at least eight mice per group). The HAI score was significantly higher in the INH/RIF treatment group than in the control group (p < 0.005). Compared to the control group, groups with low (LK) and high (HK) doses of kaempferol showed a significant decrease in the HAI scores (p < 0.05 and p < 0.01, respectively; Table III).
Assessment of Residual Liver Function
GSP values were significantly higher in the INH/RIF group than in the NC group (p < 0.005; Table III). GSP values increased gradually with an increase in the length of treatment (INH/RIF group at 0 weeks, GSP = 184 ± 24 mg/L; at 3 weeks, GSP = 866 ± 339 mg/L, p < 0.005; data not shown). In addition, the groups that were co-treated with kaempferol (LK and HK) showed significantly lower GSP values than those shown by the groups treated with INH/RIF (p < 0.005; Table III).
CYP2E1 Activity in Liver Microsomes
INH/RIF significantly increased CYP2E1 activity; CYP2E1 activity was 2.27 times higher in the INH/RIF group than in the NC group (p < 0.005) after 3 weeks of treatment. Co-treatment with kaempferol (LK-Study group) resulted in a 50% decrease in CYP2E1 activity compared to that in the INH/RIF-induced hepatotoxicity group (p < 0.005). Doubling the dose of kaempferol to 3.78 mg kg−1 day−1 (HK-Study group) decreased CYP2E1 activity by 61% compared to that in the INH/RIF-induced hepatotoxicity group (p < 0.001; Fig. 2). No significant differences were observed between the NC and the LK- and HK-Study groups.
Fig. 2.
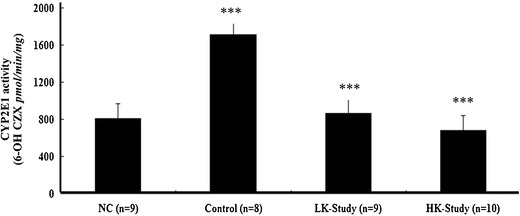
Hepatic cytochrome P450 2E1 (CYP2E1) activity in the normal control (NC), INH/RIF-Control, LK-Study, and HK-Study groups. Data are shown as the mean ± SD. Each group contained at least eight mice. ***p < 0.001, control vs. NC group; ***p < 0.001, study vs. control group
Pharmacokinetic Studies
Kaempferol Protected Against INH/RIF-Induced Oxidative Stress in the Liver
Hepatic GSH contents were significantly decreased the mice treated with INH/RIF (Table IV). Conversely, the levels of MDA, which are an index of lipid peroxidation, significantly increased in the livers of mice treated with INH/RIF (Table IV). The study groups treated with kaempferol showed a significant decrease in INH/RIF-induced hepatic GSH depletion. Furthermore, kaempferol prevented INH/RIF-induced lipid peroxidation.
Table IV.
Effect of Kaempferol on GSH and MDA Content in NC, INH/RIF-Control, LK-Study, and HK-Study Groups After 3 Weeks of Treatment
| Groups | GSH (umol/g liver) | MDA (nmol/g liver) |
|---|---|---|
| Normal control (n = 9) | 7.2 ± 2.2b | 107.2 ± 10.8b,g |
| INH/RIF-control (n = 8) | 4.6 ± 0.6a,d | 201.0 ± 24.4a,c,d |
| Study-LK (n = 9) | 5.8 ± 1.0 | 132.4 ± 15.1b,e,l |
| Study-HK (n = 9) | 7.1 ± 1.2b | 114.3 ± 16.9b,k |
Data are shown as the mean ± SD. Each group contained at least eight mice. Statistical analysis: ANOVA and LSD test
GSH glutathione, MDA malondialdehyde, NC normal control, INH isoniazid, RIF rifampicin
a p < 0.005 relative to NC
b p < 0.005 relative to C
c p < 0.005 relative to LK
d p < 0.005 relative to HK group
e p < 0.01 relative to NC
f p < 0.01 relative to C
g p < 0.01 relative to LK
h p < 0.01 relative to HK group
i p < 0.05 relative to NC
j p < 0.05 relative to C
k p < 0.05 relative to LK
l p < 0.05 relative to HK group
Kaempferol Did Not Affect the Plasma Levels of INH, Acetyl-INH, INA, or RIF
The plasma concentration–time curves of INH and RIF in mice treated with INH/RIF alone or with a combination of INH/RIF and kaempferol are shown in Fig. 3. No significant differences were observed in the pharmacokinetic parameters of INH, acetyl-INH, INA, or RIF in any of the tested conditions (Fig. 3). These results indicated that CYP2E1 inhibition did not influence the metabolism of INH. This may have been because of CYP2E1 acting further downstream in the INH metabolic pathway or from redundancy with other enzymes involved in the metabolism of INH, such as amidase or N-acetyltransferase 2.
Fig. 3.
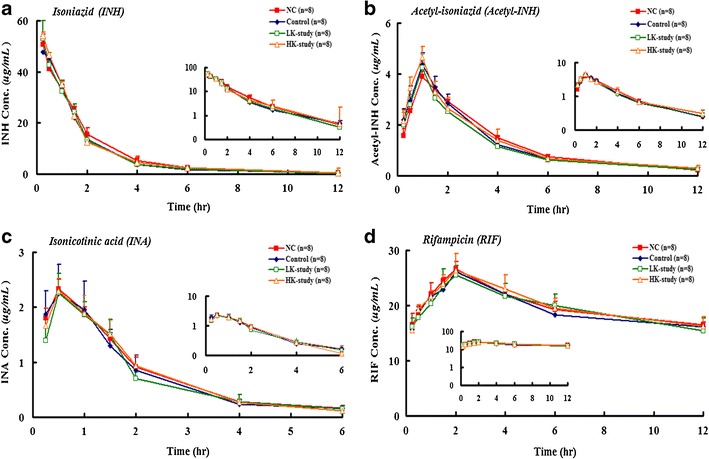
Plasma concentration time plots of isoniazid (INH) (a), acetyl-isoniazid (Acetyl-INH) (b), isonicotinic acid (INA) (c), and rifampicin (RIF) (d) for normal control (NC), INH/RIF-control, LK-Study, and HK-Study groups. Data are shown as the mean ± SD. Each group contained eight mice
Effects of Kaempferol on the Plasma Levels of CZX and 6-OH-CZX
6-OH-CZX and CZX hydroxylation activity correlated significantly with the levels of CYP2E1. ESM Figure 2 shows the plasma concentration–time plots of CZX and 6-OH-CZX in the NC, INH/RIF, LK-, and HK-Study groups. The INH/RIF group showed a higher area under the plasma concentration–time curve for 6-OH-CZX than did the NC group (42.58 ± 5.42 vs. 29.57 ± 2.27 h μg/mL, p < 0.005). The combination of kaempferol with INH/RIF significantly decreased the area under the curve (LK, 24.24 ± 0.81; HK, 18.94 ± 1.13 h μg/mL, p < 0.005), which confirmed the inhibition of CYP2E1 by kaempferol (Table V). However, kaempferol did not affect the plasma concentrations of INH, acetyl-INH, INA, or RIF. Consequently, we established that CYP2E1 acted further downstream in the metabolism of INH. The inhibition of CYP2E1 may decrease the production of toxic products such as free radicals during INH metabolism.
Table V.
Pharmacokinetic Parameters of CZX and 6-OH CZX in NC, INH/RIF-Control, LK-, and HK-Study Groups
| Parameters | NC | Control | LK-Study | HK-Study | |
|---|---|---|---|---|---|
| CZX | t 1/2 | 1.38 ± 0.41 | 1.52 ± 0.42 | 1.67 ± 0.19 | 1.39 ± 0.27 |
| T max (h) | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 | 0.25 ± 0.00 | |
| C max (μg/mL) | 20.81 ± 2.14 | 17.15 ± 1.14 | 24.96 ± 1.59c | 29.63 ± 2.50b,d,i | |
| AUCt (h μg/mL) | 26.35 ± 2.97 | 20.29 ± 1.24d | 35.18 ± 2.07b,g | 43.42 ± 3.11a,d,f | |
| AUCinf (h μg/mL) | 30.03 ± 3.97 | 23.34 ± 1.25a | 42.70 ± 2.98a,b | 49.95 ± 4.24a,d | |
| 6-OH CZX | t 1/2 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 | 0.50 ± 0.00 |
| T max (h) | 19.24 ± 1.61 | 24.83 ± 1.34 | 15.64 ± 0.88 | 12.78 ± 1.06 | |
| C max (μg/mL) | 29.57 ± 2.27 | 42.58 ± 5.42a | 24.24 ± 0.81b,d | 18.94 ± 1.13a,b,c | |
| AUCt (h μg/mL) | 32.47 ± 3.21 | 49.87 ± 5.45a | 28.18 ± 2.86b | 21.31 ± 2.01a,b,c | |
| AUCinf (h μg/mL) | 1.13 ± 0.13 | 2.10 ± 0.26a | 0.69 ± 0.05a,b | 0.44 ± 0.05a,b,c |
Data are shown as the mean ± SD. Each group contained eight mice. Statistical analysis: ANOVA and LSD test
CZX chlorzoxazone, 6-OH CZX 6-hydroxyl-CZX, NC normal control, INH isoniazid, RIF rifampicin
a p < 0.005 relative to NC
b p < 0.005 relative to C
c p < 0.005 relative to LK group
d p < 0.01 relative to NC
e p < 0.01 relative to C
f p < 0.01 relative to LK group
g p < 0.05 relative to NC
h p < 0.05 relative to C
i p < 0.05 relative to LK group
Anti-TB Efficacy Assays
Finally, we investigated whether co-treatment with kaempferol affected the efficacy of INH and RIF. We selected M. tuberculosis and M. smegmatis as the anti-TB test strains. The anti-TB efficacy of INH/RIF alone was about 90% against M. tuberculosis and M. smegmatis. No significant differences were observed in the survival of Mycobacteria between the groups treated with INH/RIF alone and those co-treated with kaempferol (Table VI). Therefore, the combination of 0.1–10 μg/mL kaempferol with INH/RIF did not affect the anti-TB activity of the latter in our assays. Thus, these findings suggested that kaempferol may effectively protect mice against INH/RIF-induced hepatotoxicity without interfering with the anti-TB efficacy of INH/RIF.
Table VI.
Effect of INH/RIF Treatment With or Without Kaempferol on the Survival of M. tuberculosis and M. smegmatis
| Bacterial species | Treatment | |||
|---|---|---|---|---|
| INH/RIF | INH/RIF-LK | INH/RIF-LK | INH/RIF-LK | |
| M. tuberculosis | 6.5 ± 1.4 | 6.0 ± 1.3 | 5.9 ± 1.2 | 6.1 ± 1.9 |
| M. smegmatis | 8.9 ± 2.2 | 9.2 ± 1.5 | 9.1 ± 1.8 | 8.5 ± 2.3 |
Survival (in percent): colony numbers of different treatment groups/control group × 100
Statistical analysis: ANOVA and LSD test
INH isoniazid, RIF rifampicin
***p < 0.005, **p < 0.01, *p < 0.05 compared to INH/RIF control group
DISCUSSION
Many popular herbal medicines have been routinely used or marketed as dietary supplements, such as St. John’s wort, G. biloba, garlic, and milk thistle. Some of these herbal medicines have significant pharmacological and therapeutic effects. However, several case reports have indicated that certain herbal medicines enhance or attenuate the efficiency of prescription drugs; in addition, herbal medicines markedly increase the levels or the activity of hepatic CYP450 isozymes (1,2). Commonly used herbal medicines have attracted a great deal of interest as physiologically functional foods and as sources for the development of drugs. In addition, flavonoid-induced effects on drug bioavailability have been shown in drug–drug interactions. This could raise concerns about the safety of flavonoid supplements and flavonoid-containing remedies that are not subject to legal regulations.
Zhang et al. have shown that extracts of G. biloba that contain quercetin, kaempferol, and isorhamnetin may influence the activity of hepatic CYP450 isozymes (2,3). Kaempferol (3,4′-5,7-tetrahydroxyflavone), which is the main flavonol found in the Chinese herb Kaempferia galangal, has also been found in many vegetables, fruits, and herbal medicines, including apple, citrus, broccoli, grapefruit, onion, tea, and G. biloba extract (39). Kaempferol possesses antioxidant, anti-inflammatory, antitumor, antiviral, and immunomodulatory properties in vitro and in vivo (1,40). In addition, Jeong et al. have shown the protective effects of oleanolic acid and puerarin against CCl4-induced hepatotoxicity that may arise from the prevention of CCl4 bioactivation through the inhibition of CYP2E1 (39,40).
We selected kaempferol as the model compound because the effect of CYP2E1 inhibition in humans was the same as that in rats in vitro. In addition, nordihydroquaiaretic acid, which is the most potent CYP2E1 inhibitor, has a mutagenic effect. Thus, it cannot be used in an in vivo study. In a preliminary study, we have described that the well-known CYP2E1 inhibitor, disulfiram, could not effectively prevent INH/RIF-induced hepatotoxicity in mice, as was shown by the significantly increased levels of AST and ALT and the total HAI score. Thus, on the basis of safety concerns and the inhibitory effects of CYP2E1, we selected kaempferol to show that a CYP2E1 inhibitor could reduce or even eradicate INH/RIF-induced hepatotoxicity in mice.
A logical approach to preventing INH/RIF-induced liver injury could be the potentiation of the antioxidant defense machinery of the host so as to guard the liver against these offending drugs. CYP2E1, which is involved in the disposal of ROS, exhibits significant alterations after INH/RIF treatment. An excessive buildup of ROS within the liver tissues leads to increased lipid peroxidation, which is evidenced by GSH depletion and an increase in MDA levels, in INH/RIF-induced hepatotoxic mice. Kaempferol prevented lipid peroxidation as well as that of normal control-treated mice. Kaempferol acts by scavenging the electrophilic metabolites and hydroxyl radicals and preventing their interactions with lipids (39). Among the CYP2E1 inhibitors selected, kaempferol was evaluated as a suitable hepatoprotective compound on the basis of the normal histopathological features of the mice liver as well as the normal levels of transaminases after co-administration of kaempferol with INH and RIF.
To our knowledge, this is the first study in which the protective effects of kaempferol against INH/RIF-induced hepatotoxicity through CYP2E1 inhibition have been reported. Such protection was detected both by diagnostic indicators of liver damage (AST and ALT levels and GSP value) and by histopathological analyses. The oxidative injury that was induced by INH and RIF was substantiated by histopathological findings. The protection by kaempferol in this study was reflected by the absence of histological lesions. This study further supported oxidative stress as the mechanism for INH/RIF-induced hepatotoxicity. In vivo, kaempferol significantly inhibited INH/RIF-induced CYP2E1 activity, as assessed by CZX hydroxylation (p < 0.001). Doubling the dose of kaempferol to 3.78 mg kg−1 day−1 significantly improved the hepatoprotective effects in mice treated with INH/RIF. All mice treated with kaempferol (doses of 100 mg/kg, 1 g/kg, and 10 g/kg) survived the study period, and no clinical symptoms were observed during an acute oral toxicity study (14 days). These results showed that kaempferol had no safety concerns, which indicated that it could be applied in a clinical trial in a future study. Thus, this study presents a novel approach to prevent INH/RIF-induced liver injury by the co-administration of kaempferol.
CONCLUSION
Kaempferol inhibited CYP2E1 activity by 60–88% in HLMs and RLMs in a dose-dependent manner. Kaempferol diminished or even eradicated INH/RIF-induced hepatotoxicity in mice. Moreover, kaempferol treatment prevented INH/RIF-mediated lipid peroxidation, as indicated by the reduction in hepatic GSH depletion and MDA formation. Pharmacokinetic studies with INH/RIF showed that co-treatment with kaempferol inhibited CYP2E1 activity, but did not affect the absorption of INH and RIF in mice. In addition, kaempferol treatment did not affect the anti-TB efficacy of INH/RIF. These results implied that kaempferol could be suitable for use during anti-TB therapy, pending further testing. Thus, kaempferol has a high potential for developing a novel INH/RIF formulation that does not cause hepatotoxicity and consequently for improving the compliance of TB patients who are taking anti-TB drugs.
Electronic Supplementary Materials
Below is the link to the electronic supplementary material.
(DOC 339 kb)
Acknowledgments
We are grateful to Drs. HS Lee and CB Hsieh from the Tri-Service General Hospital. The National Defense Medical Center supported this study.
Financial support
None.
Statement of Interest
No conflicts of interest are declared by all of the authors.
References
- 1.Zhang L, Wang Y, Zou P, Zhang H. Advances in clinical pharmacokinetics of herbal medicines. J US-China Med Sci. 2005;2:59–72. [Google Scholar]
- 2.Breinholt VM, Offord EA, Brouwer C, Nielsen SE, Brøsen K, Friedberg T. In vitro investigation of cytochrome P450-mediated metabolism of dietary flavonoids. Food Chemical Toxicol. 2002;40:609–16. doi: 10.1016/S0278-6915(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 3.Kane GC, Lipsky JJ. Drug–grapefruit juice interactions. Mayo Clin Proc. 2000;75:933–42. doi: 10.4065/75.9.933. [DOI] [PubMed] [Google Scholar]
- 4.Ono S, Hatanaka T, Hotta H, Satoh T, Gonzalez FJ, Tsutsui M. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of In vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 1996;26:681–93. doi: 10.3109/00498259609046742. [DOI] [PubMed] [Google Scholar]
- 5.Otake Y, Hsieh F, Walle T. Glucuronidation versus oxidation of the flavonoid galangin by human liver microsomes and hepatocytes. Drug Metab Dispos. 2002;30:576–81. doi: 10.1124/dmd.30.5.576. [DOI] [PubMed] [Google Scholar]
- 6.Gradolatto A, Canivenc-Lavier MC, Basly JP, Siess MH, Teyssier C. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab Dispos. 2004;32:58–65. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 7.Cermak R. Effect of dietary flavonoids on pathways involved in drug metabolism. Expert Opin Drug Metab Toxicol. 2008;4:17–35. doi: 10.1517/17425255.4.1.17. [DOI] [PubMed] [Google Scholar]
- 8.Wojnowski L, Kamdem LK. Clinical implications of CYP3A polymorphisms. Expert Opin Drug Metab Toxicol. 2006;2:171–82. doi: 10.1517/17425255.2.2.171. [DOI] [PubMed] [Google Scholar]
- 9.Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specific cities and tissue distribution of cytochrome P450 3A and P-glycoprotein. Implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–34. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- 10.Ueng YF, Shyu CC, Lin YL, Park SS, Liao JF, Chen CF. Effects of baicalein and wogonin on drug-metabolizing enzymes in C57BL/6J mice. Life Sci. 2000;67:2189–200. doi: 10.1016/S0024-3205(00)00809-2. [DOI] [PubMed] [Google Scholar]
- 11.Jang SI, Kim HJ, Hwang KM. Hepatoprotective effect of baicalin, a major flavone from Scutellaria radix, on acetaminophen-induced liver injury in mice. Immunopharmacol Immunotoxicol. 2003;25:585–94. doi: 10.1081/IPH-120026443. [DOI] [PubMed] [Google Scholar]
- 12.Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology. 2003;37:924–30. doi: 10.1053/jhep.2003.50144. [DOI] [PubMed] [Google Scholar]
- 13.Walubo A, Smith P, Folb PI. The role of oxygen free radicals in isoniazid-induced hepatotoxicity. Meth Find Exp Clin Pharmacol. 1998;20:649–55. doi: 10.1358/mf.1998.20.8.487491. [DOI] [PubMed] [Google Scholar]
- 14.Yue J, Peng RX, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. Acta Pharmacol Sin. 2004;25:699–704. [PubMed] [Google Scholar]
- 15.Nicod L, Viollon C, Regnier A, Jacqueson A, Richert L. Rifampicin and isoniazid increase acetaminophen and isoniazid cytotoxicity in human HepG2 hepatoma cells. Hum Exp Toxicol. 1997;16:28–34. doi: 10.1177/0960327197016001061. [DOI] [PubMed] [Google Scholar]
- 16.Tafazoli S, Mashregi M, O’Brien PJ. Role of hydrazine in isoniazid-induced hepatotoxicity in a hepatocyte inflammation model. Toxicol Appl Pharmacol. 2008;229:94–101. doi: 10.1016/j.taap.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 18.Vuilleumier N, Rossier MF, Chiappe A, Degoumois F, Dayer P, Mermillod B, Nicod L, Desmeules J, Hochstrasser D. CYP2E1 genotype and isoniazid-induced hepatotoxicity in patients treated for latent tuberculosis. Eur J Clin Pharmacol. 2006;62:423–9. doi: 10.1007/s00228-006-0111-5. [DOI] [PubMed] [Google Scholar]
- 19.Cederbaum AI. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J Gastroenterol Hepatol. 2006;21:S22–S25. doi: 10.1111/j.1440-1746.2006.04595.x. [DOI] [PubMed] [Google Scholar]
- 20.Attri S, Rana SV, Vaiphei K, Sodhi CP, Katyal R, Goel RC. Isoniazid and rifampicin induced oxidative hepatic injury protection by N-acetylcysteine. Hum Exp Toxicol. 2000;19:517–24. doi: 10.1191/096032700674230830. [DOI] [PubMed] [Google Scholar]
- 21.Gurumurthy P, Krishnamurthy M, Nazareth O. Lack of relationship between hepatic toxicity and acetylator phenotype and in South Indian patients during treatment with isoniazid for tuberculosis. Am Rev Respir Dis. 1984;129:58–61. doi: 10.1164/arrd.1984.129.1.58. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell JR, Thorgeisson UP, Black M, Timbrell JA, Snodgrass WR, Potter WZ, Jollow DJ, Keiser HR. Increased incidence of isoniazid hepatitis in rapid acetylators: possible relation to hydrazine metabolites. Clin Pharmacol Ther. 1975;18:70–79. doi: 10.1002/cpt197518170. [DOI] [PubMed] [Google Scholar]
- 23.Timbrell JA, Mitchell JR, Snodgrass WR, Nelson SD. Isoniazid hepatotoxicity: the relationship between covalent binding and metabolism in vivo. J Pharmacol Exp Ther. 1980;213:364–9. [PubMed] [Google Scholar]
- 24.Tasduq SA, Kaiser P, Sharma SC, Johri RK. Potentiation of isonoazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: a toxicity profile study. Hepatol Res. 2007;37:845–53. doi: 10.1111/j.1872-034X.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 25.Sarich TC, Adams SP, Petricca G, Wright JM. Inhibition of isoniazid-induced hepatotoxicity in rabbits by pretreatment with an amidase inhibitor. J Pharmacol Exp Ther. 1999;289:695–702. [PubMed] [Google Scholar]
- 26.Shen C, Meng Q, Zhang G, Hu W. Rifampicin exacerbates isoniazid-induced toxicity in human but not in rat hepatocytes in tissue-like cultures. Br J Pharmacol. 2008;153:784–91. doi: 10.1038/sj.bjp.0707611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue J, Peng R. Does CYP2E1 play a major role in the aggravation of isoniazid toxicity by rifampicin in human hepatoctytes? Br J Pharmacol. 2009;157:331–3. doi: 10.1111/j.1476-5381.2009.00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–12. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 29.Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006;45:117–26. doi: 10.1016/j.jhep.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa H, Ohish N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 31.Tang HS, Hu OYP. Assessment of liver function using a novel galactose single point method. Digestion. 1992;52:222–31. doi: 10.1159/000200957. [DOI] [PubMed] [Google Scholar]
- 32.Hu OYP, Hu TM, Tang HS. Determination of galactose in human blood by high-performance liquid chromatograph: comparison with an enzymatic method and application to the pharmacokinetic study of galactose in patients with liver dysfunction. J Pharm Sci. 1995;84:231–5. doi: 10.1002/jps.2600840223. [DOI] [PubMed] [Google Scholar]
- 33.Hu OYP, Tang HS, Chang CL. Novel galactose single point method as a measure of residual liver function: example of cefoperazone kinetics in patients with liver cirrhosis. J Clin Pharmacol. 1995;35:250–8. doi: 10.1002/j.1552-4604.1995.tb04055.x. [DOI] [PubMed] [Google Scholar]
- 34.Hu OYP, Tang HS, Chang CL. The influence of chronic lobular hepatitis on pharmacokinetics of cefoperazone—a novel galactose single-point method as a measure of residual liver function. Biopharm Drug Dispos. 1994;15:563–76. doi: 10.1002/bdd.2510150704. [DOI] [PubMed] [Google Scholar]
- 35.Cuatrecasas P, Segal S. Mammalian galactokinase. Developmental and adaptive characteristics in the rat liver. J Biol Chem. 1965;240:2382–88. [PubMed] [Google Scholar]
- 36.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 37.Kharasch ED, Thummel KE, Mhyre J, Lillibridge JH. Single-dose disulfiram inhibition of chlorzoxazone metabolism: a clinical probe for P450 2E1. Clin Pharmacol Ther. 1993;53:643–50. doi: 10.1038/clpt.1993.85. [DOI] [PubMed] [Google Scholar]
- 38.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–5. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 39.Oliveira EJ, Watson DG, Grant MH. Metabolism of quercetin and kaempferol by rat hepatocytes and the identification of flavonoid glycosides in human plasma. Xenobiotica. 2002;32:279–87. doi: 10.1080/00498250110107886. [DOI] [PubMed] [Google Scholar]
- 40.Jeong HG. Inhibition of cytochrome P450 2E1 expression by oleanolic acid: hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett. 1999;105:215–22. doi: 10.1016/S0378-4274(99)00004-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 339 kb)


