Abstract
Demonstrating bioequivalence (BE) for nasal spray/aerosol products for local action has been very challenging because the relationship between the drug in systemic circulation and the drug reaching the nasal site of action has not been well established. Thus, the current BE standard for these drug/device combination products is based on a weight-of-evidence approach, which contains three major elements: equivalent in vitro performance, equivalent systemic exposure, and equivalent local delivery. In addition, formulation sameness and device similarity are evidences to support BE. This paper presents a comprehensive review of the scientific rationale of the current BE standard and their development history for nasal spray/aerosol products, as well as the Food and Drug Administration’s review and approval status of generic nasal sprays/aerosols with the application of these BE standard.
Key words: bioequivalence, generic, locally acting drug, nasal aerosol, nasal spray
INTRODUCTION
Unlike conventional dosage forms such as tablets, capsules, and injectables, nasal spray/aerosol products deliver a spray containing a metered dose of the active ingredient that is dissolved or suspended in solutions or mixtures of excipients in nonpressurized/pressurized dispensers designed to deliver drug to the nasal cavity. Due to the advantages offered by nasal drug delivery, including non-invasiveness, avoidance of first-pass effect, fast onset of action, and good patient compliance, nasal spray/aerosol products have become increasingly popular over recent decades (1).
The first nasal spray product, Tyzine (tetrahydrozoline hydrochloride) Nasal Solution, a decongestant, was approved by the Food and Drug Administration (FDA) for the treatment of nasal congestion due to colds, hay fever, allergies, or sinusitis in 1954. Since then, a total of 32 nasal spray/aerosol products have been approved for marketing through the new drug application process, as shown in Table I. Among these products, 19 drugs are locally acting nasal sprays/aerosols indicated for the treatment of the symptoms of seasonal allergic rhinitis.
Table I.
Nasal Spray/Aerosol NDA Products Approved by the US FDA
| Product name | Drug name | Sponsor | Strength (mg/spray) | Approval date | Indication |
|---|---|---|---|---|---|
| Astelin Nasal Spray | Azelastine hydrochloride | Meda Pharmaceuticals | 0.125 | 11/01/1996 | Indicated for the treatment of the symptoms of seasonal allergic rhinitis such as rhinorrhea, sneezing, and nasal pruritus in adults and children 5 years and older, and for the treatment of the symptoms of vasomotor rhinitis, such as rhinorrhea, nasal congestion and postnasal drip in adults and children 12 years and older. |
| Astepro Nasal Spray | Azelastine hydrochloride | Meda Pharmaceuticals | 0.1876 | 08/31/2009 | Indicated for the relief of the symptoms of seasonal and perennial allergic rhinitis in patients 12 years of age and older. |
| Atrovent Nasal Spray | Ipratropium bromide | Boehringer Ingelheim | 0.021, 0.042 | 10/20/1995 | Indicated for the symptomatic relief of rhinorrhea associated with the common cold or seasonal allergic rhinitis for adults and children age 5 years and older. |
| Beconase AQ Nasal Spray | Beclomethasone dipropionate | GlaxoSmithKline | 0.042 | 07/27/1987 | Indicated for the relief of the symptoms of seasonal or perennial allergic and nonallergic (vasomotor) rhinitis and for the prevention of recurrence of nasal polyps following surgical removal. |
| Dymista Nasal Spray | Azelastine hydrochloride + fluticasone propionate | Meda Pharmaceuticals | 0.137/0.05 | 05/01/2012 | Indicated for the relief of symptoms of seasonal allergic rhinitis in patients 12 years of age and older who require treatment with both azelastine hydrochloride and fluticasone propionate for symptomatic relief. |
| Flonase Nasal Spray | Fluticasone propionate | GlaxoSmithKline | 0.05 | 10/19/1994 | Indicated for the management of the nasal symptoms of seasonal and perennial allergic and nonallergic rhinitis in adults and pediatric patients 4 years of age and older. |
| Fortical Nasal Spray | Calcitonin salmon | Upsher Smith Laboratory | 200 IU | 08/12/2005 | Indicated for the treatment of postmenopausal osteoporosis in women greater than 5 years postmenopause with low bone mass relative to healthy premenopausal women. |
| Imitrex Nasal Spray | Sumatriptan | GlaxoSmithKline | 5, 20 | 08/26/1997 | Indicated for the acute treatment of migraine attacks with or without aura in adults. |
| Lazanda Nasal Spray | Fentanyl citrate | Archimedes Development | 0.4 | 06/30/2011 | Indicated for the management of breakthrough pain in cancer patients 18 years of age and older who are already receiving and who are tolerant to opioid therapy for their underlying persistent cancer pain. |
| Miacalcin Nasal Spray | Calcitonin salmon | Novartis | 200 IU | 08/17/1995 | Indicated for the treatment of postmenopausal osteoporosis in females greater than 5 years postmenopause with low bone mass relative to healthy premenopausal females. |
| Migranal Nasal Spray | Dihydroergotamine mesylate | Valeant | 0.5 | 12/08/1997 | Indicated for the acute treatment of migraine headaches with or without aura and the acute treatment of cluster headache episodes |
| Minirin Nasal Spray | Desmopressin acetate | Ferring Pharmaceuticals | 0.01 | 09/16/2002 | Indicated for the management of primary nocturnal enuresis. It may be used alone or adjunctive to behavioral conditioning or other nonpharmacological intervention. Also indicated as antidiuretic replacement therapy in the management of central cranial diabetes insipidus and for management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. |
| Nasacort AQ Nasal Spray | Trimacinolone acetonide | Sanofi Aventis | 0.055 | 05/20/1996 | Indicated for treatment of nasal symptoms of seasonal and perennial allergic rhinitis in adults and children 2 years of age and older. |
| Nasarel Nasal Spray | Flunisolide | Teva Branded Pharm | 0.029 | Approved on 03/08/1995; currently discontinued | Indicated for the management of the nasal symptoms of seasonal or perennial rhinitis. |
| Nasalcrom Nasal Spray | Cromolyn sodium | BlackSmith Brands | 5.2 | Approved on 1/03/1997; currently discontinued | Used to prevent and relieve nasal symptoms of hay fever and other nasal allergies including runny/itchy nose, sneezing and allergic stuffy nose. |
| Nasalide Nasal Spray | Flunisolide | Ivax Res | 0.025 | Approved on 9/24/1981; currently discontinued | Indicated for the management of the nasal symptoms of seasonal or perennial rhinitis. |
| Nascobal Nasal Spray | Cyanocobalamin | Par Pharmaceutical | 0.5 | 1/31/2005 | Indicated for the maintenance of normal hematologic status in pernicious anemia patients who are in remission following intramuscular vitamin B12 therapy and who have no nervous system involvement. Also indicated as a supplement for other vitamin B12 deficiencies. |
| Nasonex Nasal Spray | Mometasone furoate | Schering Plough | 0.05 | 10/1/1997 | Indicated for the treatment of nasal symptoms of allergic rhinitis and nasal congestion associated with seasonal allergic rhinitis. Also indicated for prophylaxis of seasonal allergic rhinitis and treatment of nasal polyps. |
| Nicotrol Nasal Spray | Nicotine | Pfizer | 0.5 | 3/22/1996 | Indicated as an aid to smoking cessation for the relief of nicotine withdrawal symptoms. It should be used as a part of a comprehensive behavioral smoking cessation program. |
| Omnaris Nasal Spray | Ciclesonide | Nycomed US | 0.05 | 11/21/2007 | Indicated for treatment of nasal symptoms associated with seasonal allergic rhinitis in adults and children 6 years of age and older and perennial allergic rhinitis in adults and adolescents 12 years of age and older. |
| Patanase Nasal Spray | Olopatadine hydrochloride | Alcon Pharms | 0.665 | 4/15/2008 | Indicated for the relief of the symptoms of seasonal allergic rhinitis in adults and children 6 years of age and older. |
| Rhinocort Aqua Nasal Spray | Budesonide | AstraZeneca | 0.032, 0.064 | Approved on 10/1/1999. The 0.064 mg/spray strength currently discontinued | Indicated for the treatment of seasonal or perennial allergic rhinitis in adults and children ≥6 years. |
| Qnasl Nasal Aerosol | Beclomethasone dipropionate | Teva Branded Pharm | 0.08 | 3/23/2012 | Indicated for the treatment of the nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and adolescents 12 years of age and older. |
| Sprix Nasal Spray | Ketorolac tromethamine | Luitpold | 15.75 | 5/14/2010 Initial USA approval in 1989 | Indicated for short term (up to 5 days) management of moderate to moderately severe pain. |
| Stimate Nasal Spray | Desmopressin acetate | CSL Behring | 1.5 | 3/7/1994 | Indicated for patients with hemophilia A with Factor VIII coagulant activity levels greater than 5% and for patients with mild to moderate classic von Willebrand’s disease (type I) with factor VIII levels greater than 5%. |
| Stadol Nasal Spray | Butorphanol tartrate | BMS | 1 | Approved on 12/12/1991; currently discontinued | Indicated for the management of pain when the use of an opioid analgesic is appropriate. |
| Synarel Nasal Spray | Nafarelin acetate | GD Searle | 0.2 | 2/13/1990 | Indicated for the treatment of central precocious puberty (CPP) (gonadotropin-dependent precocious puberty) in children of both sexes. |
| Vancenase AQ Nasal Spray | Beclomethasone dipropionate monohydrate | Schering | 0.042, 0.084 | Approved on 12/23/1987; currently discontinued. Approved on 6/26/1996; currently discontinued. | Used to treat nasal symptoms such as congestion, sneezing, and runny nose caused by seasonal or year-round allergies. It is also used to keep nasal polyps from coming back after surgery to remove them. |
| Veramyst Nasal Spray | Fluticasone furoate | GlaxoSmithKline | 0.0275 | 4/27/2007 | Indicated for the treatment of symptoms of seasonal and perennial allergic rhinitis in adults and children ≥2 years. |
| Tyzine Nasal Spray | Tetrahydrozoline Hydrochloride | Pfizer | 0.1% | Approved on 10/15/1954; currently discontinued | Used to treat nasal congestion due to colds, hay fever, allergies, or sinusitis. |
| Zetonna Nasal Aerosol | Ciclesonide | Takeda Gmbh | 0.037 | 1/20/2012 | Indicated for the treatment of the nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and adolescents 12 years of age and older. |
| Zomig Nasal Spray | Zolmitriptan | AstraZeneca | 5 | 9/30/2003 | Indicated for the acute treatment of migraine with or without aura in adults. |
Data collected up to October 2012
With the needs of the generic nasal spray/aerosol drug products emerging on the market, demonstration of bioequivalence (BE) for generic nasal spray/aerosol products becomes a challenging task. This paper presents a comprehensive review of the scientific rationale of the current US BE standard and their development history for nasal spray/aerosol products, as well as the FDA’s review and approval status of generic nasal sprays/aerosols based on the application of these BE standard.
CURRENT FDA BIOEQUIVALENCE STANDARD FOR LOCALLY ACTING NASAL SPRAY/AEROSOL PRODUCTS
BE is the absence of a significant difference in the rate and extent to which the active ingredient or active moiety in a pharmaceutical equivalent1 or pharmaceutical alternative2 becomes available at the site of drug action when administered at the same molar dose under similar conditions in an appropriately designed study (2). As noted in the statutory definition, BE focuses on the release of a drug substance from a drug product and subsequent absorption into the site of action. The rate and extent of drug becoming available at the site of action are evaluated to demonstrate BE. According to 21 CFR 320.24 (b), the approaches which can be used to establish BE include in vivo pharmacokinetic (PK) BE studies, in vitro tests, pharmacodynamic (PD) studies, clinical studies and any other approaches deemed adequate by the FDA. The current FDA BE standard for locally acting nasal spray/aerosol products are described below.
BE Standard for Locally Acting Nasal Spray Solution Products
For a locally acting nasal spray product, the relationship between drug delivery to the intended site of action and drug in systemic circulation has not been well established. Thus, it is currently not sufficient to establish BE of nasal spray products for local action by simply comparing the drug absorption in blood or plasma between the test (T) and reference listed product (RLD). According to the 2003 FDA Draft Guidance on Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action (3), bioequivalence of a nasal spray solution product can be established by a number of in vitro tests that demonstrate equivalent product performance, in conjunction to formulation sameness and device comparability of the test and reference products. Specifically, six in vitro tests including single actuation content through container life, droplet size distribution, drug in small particles/droplet, spray pattern, plume geometry, and priming/repriming, which characterize the critical performance of a nasal spray solution product, are considered as necessary to demonstrate bioequivalence of the drug product. In addition, since the in vitro comparative studies alone are not capable of evaluating the effect of a different excipient on the drug action, formulation sameness, meaning the inactive ingredients in the test product formulation be qualitatively (Q1) and quantitatively (Q2) the same, i.e., within ±5%, of the RLD, should be warranted. Furthermore, device comparability warrants that device components such as valve, pump, and actuator designs of the test device be as close as possible in all critical dimensions as the reference device, and further ensures the equivalence of the drug performance.
BE Standard for Locally Acting Nasal Spray Suspension Products
The current FDA recommendations for BE for nasal spray suspension products is based on a weight-of-evidence approach (Fig. 1), which is a composite of following elements: equivalent in vitro performance based on in vitro studies, equivalent systemic exposure based on a PK study, and equivalent local delivery based on a PD or clinical endpoint study. In addition, formulation sameness and device similarity serve as additional evidence to support bioequivalence.
Fig. 1.
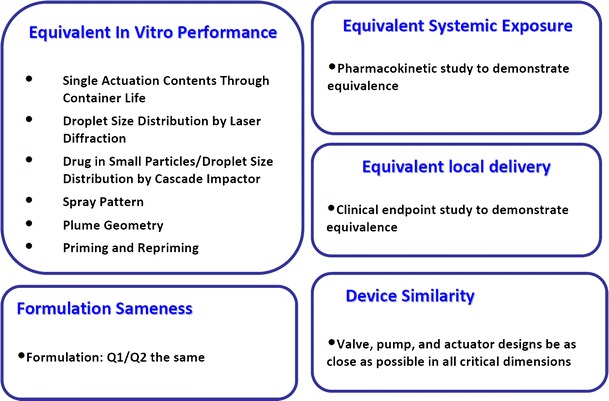
The weight-of-evidence approach to support bioequivalence of locally acting nasal spray suspensions
Scientific Rationale for the Weight-of-Evidence Approach
The scientific rationale for this weight-of-evidence approach is illustrated in Fig. 2. For a nasal spray product that integrates formulation and device, it is important to ensure that its in vitro performance, including critical parameters such as single actuation content, droplet size distribution, and spray pattern are equivalent to that of the RLD. Because such in vitro performance may affect the drug deposition profile including the deposition location, deposition area, and drug residence time in the nose, thus affecting the amount of the drug absorbed in its site of action and to the systemic circulation. Therefore, equivalent in vitro performance is essential to support the equivalence in efficacy as well as safety for a locally acting nasal suspension.
Fig. 2.
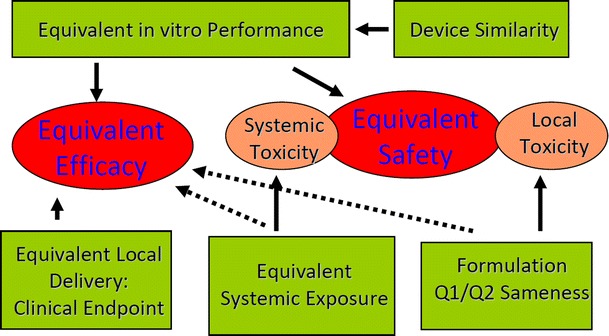
The scientific rationale for the weight-of-evidence approach for the bioequivalence establishment of locally acting nasal spray suspensions
However, the in vitro tests have its limitations. For example, if a suspension formulation contains more than one suspending particles, the current particle sizing technologies are incapable of adequately distinguishing the particles of the active ingredient from the particles of the suspending agent, thus unable to characterize the particle size distribution (PSD) of the active ingredient alone. Meanwhile, drug PSD in suspension formulations has the potential to influence the rate and extent of drug availability to nasal sites of action and to the systemic circulation (4). Furthermore, the in vitro performance is not a direct indicator of clinical efficacy, therefore, a confirmatory clinical endpoint study is recommended to provide direct evidence to assure equivalent local delivery.
On the other hand, measuring the amount of the drug in the blood or plasma is recommended to address the systemic toxicity of the drug. For nasal suspension products that do not produce sufficient blood/plasma concentrations to allow assessment of systemic exposure, a PD or clinical endpoint BE study is recommended to measure systemic absorption to establish equivalent systemic exposure.
The formulation sameness will potentially avoid the unexpected local toxicities resulting from inactive ingredients used in a generic nasal spray suspension formulation. Device similarity is recommended to increase the likelihood of establishing in vitro equivalence as well as to address the interchangeability of generic and RLD products in the patients’ hands.
Above all, the elements in the weight-of-evidence approach either support equivalent efficacy or support equivalent safety; none of them, when standing alone, provides adequate assurance of equivalence in both safety and efficacy. These aggregated evidences, together, build up adequate evidence to support a generic product being equally safe and equally efficacious as the reference product.
BE Standard for Locally Acting Nasal Aerosol Products
Recently, two locally acting nasal aerosol products, Zetonna (Ciclesonide) Nasal Aerosol and Qnasl (Beclomethasone Dipropionate) Nasal Aerosol, were approved and marketed in the US. They are both pressurized, non-aqueous solutions in a metered-dose aerosol device intended only for intranasal use. The scientific rationale for bioequivalence of locally acting nasal spray products, in general, also applies to that of nasal aerosol products. In September 2012, the FDA issued a draft BE Guidance for Ciclesonide Nasal Aerosol, the first drug-specific bioequivalence guidance of nasal aerosol category (5).
Bioequivalence Criteria
The equivalence of the in vitro performance of nasal spray and aerosol products can be evaluated using population bioequivalence (PBE) analysis. The PBE approach assesses total variability of the measure in the population. Recently, FDA developed a stepwise calculation procedure for PBE utilized in the in vitro performance BE evaluation and published it on its public website (6). For systemic exposure, the bioequivalence can be evaluated using average bioequivalence analysis in general.
HISTORY OF BIOEQUIVALENCE STANDARD DEVELOPMENT
Development of the BE Guidance for Nasal Spray/Aerosol Products
The establishment of BE for nasal spray products, especially locally acting nasal spray products has been a long-standing challenge for the generic pharmaceutical industry. In 1978, the FDA received its first nasal spray Abbreviated New Drug Application (ANDA) for generic tetrahydrozoline hydrochloride nasal spray solution. Since then, the FDA has been working on the development of the BE guidance and review standard for generic nasal spray/aerosol products. Figure 3 demonstrates several major milestones during the nasal spray/aerosol products BE standard development process and application review history.
Fig. 3.
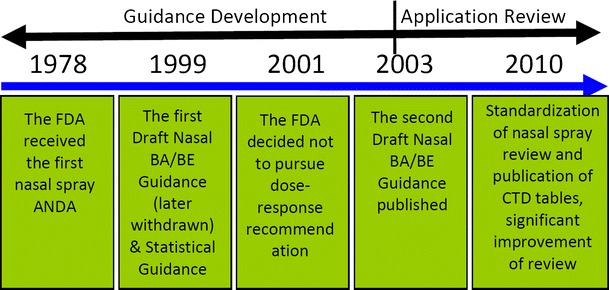
The milestones in the history of BE standard development for nasal spray/aerosol products (CTD “Common Technical Document”)
In June 1999, the first Draft FDA Guidance for Industry: Bioavailability and Bioequivalence Studies for Nasal Aerosols and Nasal Sprays for Local Action (abbreviated as Nasal BA/BE Guidance) was issued by the FDA. Along with this draft guidance, a sister Guidance: FDA Guidance for Industry: Statistical Approaches to Establishing Bioequivalence was published in January 2001. The draft guidance recommends a clinical BE study showing a dose–response to demonstrate equivalent local delivery. In July 2001, an advisory committee meeting was held at FDA. The key issue discussed in the meeting was whether the clinical BE study needs to demonstrate dose–response for locally acting nasal spray suspension products (4). At the meeting, the FDA presented the available data on the therapeutic efficacy of nasal spray suspension products at different doses in patients with allergic rhinitis. Based on the available information, the FDA acknowledged that with current technology and methods, the demonstration of dose–response might not be possible for locally acting drug products for allergic rhinitis. The FDA believed that a showing of dose–response is not necessary for BE studies with a clinical endpoint, as these studies are intended only to confirm the lack of important clinical differences between the test and reference nasal spray suspension formulations. Thus, a placebo-controlled traditional 2-week rhinitis study conducted at the lowest active dose was considered as sufficient to confirm equivalent local delivery of nasal spray suspension products for allergic rhinitis. In April 2003, the FDA published the updated Draft Nasal BA/BE Guidance (3).
DEVELOPMENT OF A STANDARDIZED BE REVIEW PROCEDURE FOR NASAL SPRAY PRODUCTS
Due to the complexity of the nasal spray dosage form, a variety of issues such as missing required data, submission of unnecessary documentation, misplacement of BE information in other sections, data submitted in incorrect format, etc., were found in the nasal spray ANDA submissions prior to 2010 (7).
In order to guide the generic pharmaceutical industry in submitting higher quality ANDAs, standardizing the review process, and improving the review efficiency and quality, a standardized BE review procedure for nasal spray products was established. In this endeavor, a series of data summary tables with standardized format has been developed by the FDA, intending to provide resources to the generic firms to submit and document the data in a concise format consistent with the Common Technical Document. These tables were published on the FDA public website (8) in 2010. The time spent on the reviewing process has been greatly reduced with the improved quality of submissions and implementation of the standardized BE review procedure for nasal spray/aerosol products.
FDA REVIEW AND APPROVALS OF GENERIC NASAL SPRAY/AEROSOL PRODUCTS
As of October 15, 2012, the FDA has approved 31 nasal spray ANDAs; among which, 7 ANDAs were systemically acting nasal spray products and 24 ANDAs were locally acting nasal spray products as shown in Table II. ANDA-074800 for cromolyn sodium nasal spray was approved on July 26, 2001 and was later discontinued based on the information from Drug@FDA (9). There is currently no any generic nasal aerosol products formulated with hydrofluoroalkane propellants approved and marketed in the US.
Table II.
Nasal Spray ANDA Products Approved by the FDA
| Application type–number | Product name | Sponsor | Strength (mg/spray) | Mechanism of action | Approval date |
|---|---|---|---|---|---|
| ANDA–077954 | Azelastine hydrochloride | Apotex | 0.125 | Locally acting | 04/30/2009 |
| ANDA–090423 | Azelastine hydrochloride | Sun Pharma Global | 0.125 | Locally acting | 05/23/2012 |
| ANDA–201846 | Azelastine hydrochloride | Apotex | 0.1876 | Locally acting | 08/31/2012 |
| ANDA–075499 | Butorphanol tartrate | Novex Pharma | 1 | Systemically acting | 12/04/2002 |
| ANDA–075759 | Butorphanol tartrate | Mylan | 1 | Systemically acting | 08/08/2001 |
| ANDA–075824 | Butorphanol tartrate | Roxane | 1 | Systemically acting | 03/12/2002 |
| ANDA–076396 | Calcitonin salmon | Apotex | 200 IU | Systemically acting | 11/17/2008 |
| ANDA–076979 | Calcitonin salmon | Par | 200 IU | Systemically acting | 06/08/2009 |
| ANDA–074800 | Cromolyn sodium | Actavis MID Atlantic LLC | 5.2 | Locally acting | Approved on 07/26/2001; currently discontinued. |
| ANDA–075427 | Cromolyn sodium | Perrigo | 5.2 | Locally acting | 12/12/2001 |
| ANDA–075702 | Cromolyn sodium | Bausch and Lomb Pharmaceuticals | 5.2 | Locally acting | 07/03/2001 |
| ANDA–077976 | Cromolyn sodium | HH and P | 5.2 | Locally acting | 09/07/2007 |
| ANDA–074830 | Desmopressin acetate | Bausch and Lomb Pharmaceuticals | 0.01 | Systemically acting | 01/25/1999 |
| ANDA–077212 | Desmopressin acetate | Sun Pharm Inds | 0.01 | Systemically acting | 04/12/2012 |
| ANDA–074805 | Flunisolide | Bausch and Lomb Pharmaceuticals | 0.025 | Locally acting | 02/20/2002 |
| ANDA–077436 | Flunisolide | Apotex | 0.029 | Locally acting | 08/09/2007 |
| ANDA–077704 | Flunisolide | HH and P | 0.025 | Locally acting | 08/03/2006 |
| ANDA–076504 | Fluticasone propionate | Roxane | 0.05 | Locally acting | 02/22/2006 |
| ANDA–077538 | Fluticasone propionate | Apotex | 0.05 | Locally acting | 09/12/2007 |
| ANDA–077570 | Fluticasone propionate | Hi Tech Pharma | 0.05 | Locally acting | 01/16/2008 |
| ANDA–078492 | Fluticasone propionate | Wockhard EU Operations (SWwiss) AG | 0.05 | Locally acting | 01/09/2012 |
| ANDA–075552 | Ipratropium bromide | Dey LP | 0.021 | Locally acting | 03/31/2003 |
| ANDA–075553 | Ipratropium bromide | Dey LP | 0.042 | Locally acting | 03/31/2003 |
| ANDA–076155 | Ipratropium bromide | Novex Pharma | 0.021 | Locally acting | 04/18/2003 |
| ANDA–076156 | Ipratropium bromide | Novex Pharma | 0.042 | Locally acting | 04/18/2003 |
| ANDA–076598 | Ipratropium bromide | Roxane | 0.042 | Locally acting | 11/05/2003 |
| ANDA–076664 | Ipratropium bromide | Roxane | 0.021 | Locally acting | 11/05/2003 |
| ANDA–076025 | Ipratropium bromide | Bausch and Lomb | 0.021 | Locally acting | 03/31/2003 |
| ANDA–076103 | Ipratropium bromide | Bausch and Lomb | 0.042 | Locally acting | 03/31/2003 |
| ANDA–086576 | Tetrahydrozoline hydrochloride | Fougera Pharms | 0.1% (1 mg in each mL) | Locally acting | 11/30/1979 |
| ANDA–078104 | Triamcinolone acetonide | Teva Branded Pharmaceutical Products R&D | 0.055 | Locally acting | 07/30/2009 |
Data collected up to October 2012
Since the submission of the first nasal spray ANDA in 1978, the FDA has received 87 nasal spray ANDAs as of October 15, 2012, as shown in Table III. There are currently 26 nasal spray ANDAs under review. The numbers of nasal spray ANDAs received each year including and excluding ANDAs withdrawn are shown in Fig. 4. This figure indicates that the number of nasal spray ANDAs submitted increased after the publication of the 2003 Draft FDA Nasal BA/BE Guidance.
Table III.
The Status and Number of Nasal Spray ANDAs Submitted to the FDA
| Status | No. of ANDAs |
|---|---|
| Approved | 31 |
| Pending | 26 |
| Refused to receive | 5 |
| Withdrawn | 25 |
| Total | 87 |
Data collected up to October 2012
Fig. 4.
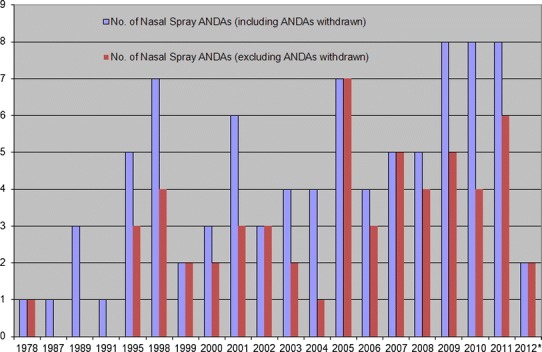
Nasal spray ANDAs received each year (asterisk the time period is from January 01, 2012 to October 15, 2012)
CONCLUSION
This review describes the scientific rationale for the current BE standards for locally acting nasal spray/aerosol products and provides background information on the development of the standard. The application of these scientifically based BE standards has improved the quality of submissions and the review efficiency, and will eventually make high-quality generic nasal spray/aerosol products available to patients.
Footnotes
Per Approved Drug Products with Therapeutic Equivalence Evaluations, 32th Edition, “Drug products are considered pharmaceutical equivalents if they contain the same active ingredient(s), are of the same dosage form, route of administration and are identical in strength or concentration (e.g., chlordiazepoxide hydrochloride, 5mg capsules). Pharmaceutically equivalent drug products are formulated to contain the same amount of active ingredient in the same dosage form and to meet the same or compendial or other applicable standards (i.e., strength, quality, purity, and identity), but they may differ in characteristics such as shape, scoring configuration, release mechanisms, packaging, excipients (including colors, flavors, preservatives), expiration time, and, within certain limits, labeling.” (http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf. Last access: 27 Jan 2013)
Per Approved Drug Products with Therapeutic Equivalence Evaluations, 32th Edition, “Drug products are considered pharmaceutical alternatives if they contain the same therapeutic moiety, but are different salts, esters, or complexes of that moiety, or are different dosage forms or strengths (e.g., tetracycline hydrochloride, 250 mg capsules vs. tetracycline phosphate complex, 250 mg capsules; quinidine sulfate, 200 mg tablets vs. quinidine sulfate, 200 mg capsules). Data are generally not available for FDA to make the determination of tablet to capsule bioequivalence. Different dosage forms and strengths within a product line by a single manufacturer are thus pharmaceutical alternatives, as are extended-release products when compared with immediate-release or standard-release formulations of the same active ingredient.” (http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf. Last access: 27 Jan 2013)
Bing V. Li and Feiyan Jin equally contributed to this work.
Disclaimer
This article reflects the views of the author and should not be construed to represent the Food and Drug Administration’s views or policies.
References
- 1.Pires A, et al. Intranasal drug delivery: how, why and what for? J. Pharm Pharmaceut Sci. 2009;12(3):288–311. doi: 10.18433/j3nc79. [DOI] [PubMed] [Google Scholar]
- 2.Code of Federal Regulations Title 21, 320.1.
- 3.FDA. FDA Guidance for industry: bioavailability and bioequivalence studies for nasal aerosols and nasal sprays for local action. April 2003
- 4.US Food and Drug Administration Advisory Committee for Pharmaceutical Science: Orally Inhaled and Nasal Drug Products. 2001. http://www.fda.gov/ohrms/dockets/ac/01/slides/3764s1.htm. Accessed 14 Mar 2013
- 5.FDA. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM319983.pdf. Accessed 14 March 2013
- 6.FDA. Draft guidance on budesonide inhalation suspension. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm081288.htm. Accessed 26 April 2013
- 7.Li BV (2009) Introduction to a standardized bioequivalence review procedure for nasal spray products: review template and CTD data summary tables. GPhA/FDA 2009 Fall Technical Conference, October 28, 2009
- 8.US Food and Drug Administration, Generic Drugs: information for industry public. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm142112.htm. Accessed 14 Mar 2013
- 9.FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails. Accessed 14 March 2013


