Abstract
Biotherapeutic-reactive antibodies in treatment-naïve subjects (i.e., pre-existing antibodies) have been commonly detected during clinical immunogenicity assessments; however information on pre-existing antibody prevalence, physiological effects, and impact on posttreatment anti-drug antibody (ADA) induction remains limited. In this analysis, pre-existing antibody prevalence and impact on posttreatment ADA induction were determined using ADA data from 12 biotherapeutics analyzed in 32 clinical studies. Approximately half (58%) of the biotherapeutics were associated with some level of pre-existing antibodies and 67% of those were associated with posttreatment ADA induction. Across all studies, 5.6% of study subjects demonstrated presence of pre-existing antibodies, among which, 17% of the individual subjects had posttreatment increases in their ADA titers while 16% had decreased titers and 67% had no change in titers. However, in studies conducted in the rheumatoid arthritis (RA) population, 14.8% of RA patients were associated with pre-existing antibodies and 30% of those had posttreatment titer increases. The results suggest that in most study subjects, pre-existing antibodies pose a low risk for posttreatment ADA induction. That said, the high risk of induction implicated for RA patients, primarily observed in treatments evaluating novel antibody-based constructs, indicates that further understanding of the contribution of product and disease-specific factors is needed. Cross-industry efforts to collect and analyze a larger data set would enhance understanding of the prevalence, nature, and physiological consequences of pre-existing antibodies, better inform the immunogenicity risk profiles of products associated with these antibodies and lead to better fit-for-purpose immunogenicity management and mitigation strategies.
Key words: anti-drug antibodies, immunogenicity risk, pre-existing antibodies
Antibodies that cross-react with a biotherapeutic candidate are often observed during immunogenicity assessment of pre-dose samples from treatment-naïve subjects. These so-called pre-existing antibodies can complicate assessment of true posttreatment anti-drug antibody (ADA) induction, can cause ADA assay interference issues, and in some cases can affect a product’s pharmacokinetics, pharmacodynamics, efficacy, or safety profiles (1,2).
Pre-existing antibodies may be components of the natural antibody population (derived from neonatal B cell components of natural host defenses) or components of adaptive immune responses to environmental antigens or homologous biotherapeutics. Both the specificity of pre-existing antibodies (3,4) and the physiological effects can vary widely. Pre-existing IgE antibodies specific for a posttranslational modification (galactose-α-1,3-galactose carbohydrate) on cetuximab have caused life-threatening infusion hypersensitivity reactions (5), whereas pre-existing antibodies to panitumumab or recombinant human thrombin did not appear to induce posttreatment ADA induction (6,7).
In addition to the potential for pre-existing antibodies to directly affect posttreatment ADA induction, should presence of pre-existing antibodies increase the risk for boosting post treatment ADA induction, there is a potential for even more severe consequences to occur. It is not surprising then that regulatory agencies expect the detection and reporting of pre-existing antibodies and determination of their relevance. Therefore, a more complete understanding of the risk of pre-existing antibodies is needed to develop better immunogenicity management and regulatory strategies.
DATA AND DEFINITIONS USED IN ANALYSIS
Analysis of historical clinical ADA data was performed for 12 biotherapeutics that included humanized monoclonal antibodies (six, all IgG1), novel antibody-based constructs (three), recombinant proteins (two), and antibody conjugate (one). These data were derived from historical clinical studies conducted within a Pfizer division and were pooled from 32 studies in healthy volunteers (N = 499 subjects) and disease populations (N = 1,331 patients) including allergy, hemophilia, neurological diseases, cancer, muscular dystrophy, and inflammatory diseases (Tables I, II, and III).
Table I.
Analysis of Historical Clinical ADA Data for 12 Biotherapeutics
| Product modality | Number of products | Study populations | Study phase | Number of studies | Number of study subjects | Prevalence of pre-existing Abs |
|---|---|---|---|---|---|---|
| Humanized mAb (IgG1) | 6 | Healthy volunteers, neurological disease, allergy, inflammatory disease, muscular dystrophy | Phases I, II | 17 | 1,008 | 0–3.8% |
| Novel antibody-based constructs | 3 | Rheumatoid arthritis | Phase I | 5 | 420 | 5.8–41.7% |
| Recombinant protein | 2 | Hemophilia, muscular dystrophy | Phases I, II | 5 | 246 | 0–16.7% |
| mAb conjugate | 1 | Cancer | Phase I | 4 | 156 | 1.9% |
Table II.
Association of Pre-existing Antibodies (Pre-Ab) with Biotherapeutic Products
| All products in survey | Products studied in RA | Products not studied in RA | |
|---|---|---|---|
| Association with pre-Ab | 58% (7/12) | 100% (3/3) | 44% (4/9) |
| No association with pre-Ab | 42% (5/12) | 0% (0/3) | 56% (5/9) |
Table III.
Prevalence of Pre-existing Antibodies from All Studies
| Percentage | Number of subjects | |
|---|---|---|
| In all study subjects | 5.6% | (103/1830) |
| In healthy volunteers | 0.6% | (3/499) |
| In all disease populations | 7.5% | (100/1331) |
| In disease populations excluding RA | 4.2% | (38/911) |
| In RA patients | 14.8% | (62/420) |
Prevalence was defined as the number of subjects demonstrating the presence of pre-existing antibodies relative to the total number of subjects across all products evaluated and is expressed as a percentage. A subject was positive for posttreatment ADA induction if (a) the pretreatment sample was negative in the ADA assay and at least one posttreatment sample was positive or (b) the pretreatment sample was positive but at least one posttreatment sample had an increase in ADA titer based on the defined ADA increase criteria in the studies.
A biotherapeutic or study was associated with (a) pre-existing antibody if at least one subject had pre-existing antibodies and (b) posttreatment ADA induction if at least one subject was positive for induction.
PRE-EXISTING ANTIBODY PREVALENCE
In this analysis, pre-existing antibodies were associated with 58% (7/12) of the biotherapeutics evaluated, and with 100% of biotherapeutics (3/3) evaluated in rheumatoid arthritis (RA) patients for which pre-existing antibody data were available (Table II). In individual studies, prevalence of pre-existing antibodies ranged from 1 to 42% of the populations evaluated (mean 12.7%, median 10.6%). Notably, the products studied in RA patients were novel antibody-based constructs, 100% of which (3/3 products) were associated with pre-existing antibodies, with prevalence ranging from 5.8 to 42% in individual studies (Table I).
When subjects were pooled from all 32 studies, the overall prevalence of pre-existing antibodies was 5.6%, but dependent on the study population (Table III), 0.6% in healthy volunteers, 14.8% in RA patients, and 4.2% in other disease populations. When subjects were pooled from studies where pre-existing antibodies were observed, prevalence was 3.6% in healthy volunteers, 14.8% in RA patients, and 8.5% in other disease populations (Table IV).
Table IV.
Prevalence of Pre-existing Abs from Studies Associated with Pre-existing Antibodies
| Percentage | Number of Subjects | |
|---|---|---|
| In all study subjects | 10.8% | (103/950) |
| In healthy volunteers | 3.6% | (3/84) |
| In all disease populations | 11.5% | (100/866) |
| In disease populations excluding RA | 8.5% | (38/446) |
| In RA patients | 14.8% | (62/420) |
CORRELATION OF POSTTREATMENT ADA INDUCTION WITH PRE-EXISTING ANTIBODY
Posttreatment ADA induction was observed with 67% of biotherapeutics that were associated with pre-existing antibodies, and 60% of the biotherapeutics that were not associated with pre-existing antibodies. Notably, posttreatment ADA induction was observed in 100% of biotherapeutics studied in RA where pre-existing antibodies were observed (Table V).
Table V.
Association with Posttreatment ADA Induction at Product Level
| Association with ADA induction | No association with ADA induction | |
|---|---|---|
| Products with Pre-Ab | 67% (4/6a) | 33% (2/6) |
| Products without Pre-Ab | 60% (3/5) | 40% (2/5) |
| Products with Pre-Ab and studied in RA | 100% (3/3) | 0% (0/3) |
aOne of the seven products associated with pre-existing antibodies (Pre-Ab) was excluded from comparison with posttreatment ADA induction, as the pre-Ab was only observed in placebo group
In studies where pre-existing antibodies were observed, 13% of subjects with posttreatment ADA were positive for pre-existing antibodies, whereas 87% were negative for pre-existing antibodies (Fig. 1a). Of all subjects with pre-existing antibodies, 17% had elevated titers, 16% decreased titers, and 67% no titer changes posttreatment relative to their pretreatment titers (Fig. 1b). Of all RA patients demonstrating pre-existing antibodies, 30% were positive for posttreatment ADA induction (Fig. 1c).
Fig. 1.
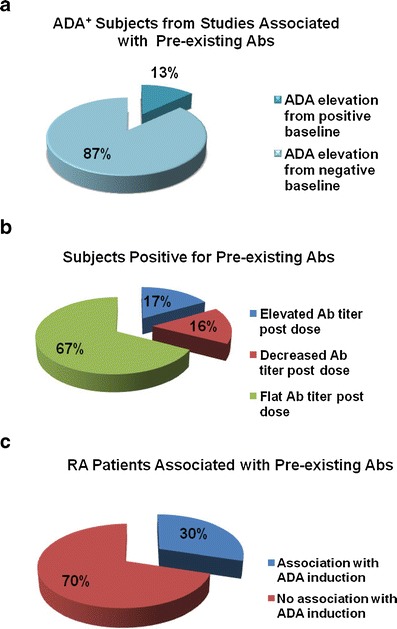
Posttreatment ADA findings for study subjects. a Analysis of all reported ADA positive subjects pooled from studies associated with pre-existing antibodies (Abs). b Analysis of antibody titer changes of subjects positive for pre-existing antibodies in all studies. c Posttreatment ADA findings for RA patients associated with pre-existing antibodies
DISCUSSION
This analysis of historical clinical ADA data suggests some interesting trends. Looking at the pool of all study subjects, the prevalence of pre-existing antibodies was low and infrequently associated with posttreatment ADA induction. However, disease population seemed to be a significant factor in both pre-existing antibodies and posttreatment ADA induction. Even though the size of the current data set would not allow for systematic analysis within all disease populations, among RA patients, prevalence and associated ADA induction rates were higher relative to the total, healthy volunteer subsets, or total disease population studied. It should be noted that criteria for reporting a subject with pre-existing antibody positive for posttreatment induction (significant increase in titer must be observed) is more stringent than the criteria for reporting a baseline negative subject to be positive for posttreatment induction. When evaluated at the product level, 100% of the novel antibody-based constructs studied in RA were associated with posttreatment ADA induction.
Overall, in this analysis, the presence of pre-existing antibodies did not appear to increase risk of posttreatment ADA induction for most biotherapeutics and individual subjects studied. However, high ADA induction risk was implicated for the RA studies. Notably, the present data for this cohort primarily come from studies which evaluated therapeutics with novel antibody-based constructs. The mechanisms underlining increased detection of pre-existing antibodies and association with posttreatment ADA in the RA patients are largely unknown, although presence of rheumatoid factors and higher levels of other autoantibodies in RA patients could both be contributing factors. In addition, modification of normal protein structures may inadvertently reveal epitopes reactive with natural antibodies. Further evaluation is needed to determine the extent to which the disease states or biotherapeutic modalities contribute to the present observations. Evaluation of the epitopes recognized by pre-existing and posttreatment-induced antibodies would also be helpful to understand the contribution of pre-existing antibody to posttreatment immunogenicity risk.
The significance of these results remains to be determined. A larger and more diverse dataset (e.g. disease, modality, prior treatments, consequences of ADA) and additional analysis is needed to better understand the significance and relationships between pre-existing antibodies and posttreatment ADA induction. Results from the recent American Association of Pharmaceutical Scientist pre-existing antibody survey (manuscript accepted (8)) indicated more frequent association of pre-existing antibodies with autoimmune disease indications but identified lack of understanding of the immunogenicity risk as the top gap/barriers to better understanding and dealing with pre-existing antibodies. Cross-industry collaborative efforts to collect more data categorized by product modalities, patient populations, and pre-existing antibody subclasses and specificity are vital to improving our understanding of the biological consequences, level, and scope of immunogenicity risk of pre-existing antibodies.
CONCLUSION
In this analysis, presence of pre-existing antibodies did not appear to correlate with posttreatment ADA induction in many products and individual subjects studied. However, high prevalence of pre-existing antibodies and association with posttreatment ADA were observed for RA patients and the therapeutics with novel antibody-based constructs. Further analysis will enhance our understanding of pre-existing antibodies as an “immunogenicity risk factor” and lead to better and more informed immunogenicity management, mitigation, and regulatory strategies.
ACKNOWLEDGMENTS
The analysis was sponsored by Pfizer Inc. We would like to thank John Nowak for his help collecting clinical study reports. We also thank Christopher Lepsy for reviewing the manuscript and providing constructive suggestions.
REFERENCES
- 1.Ballard JL, Weaver FA, Singla NK, et al. Safety and immunogenicity observations pooled from eight clinical trials of recombinant human thrombin. J Am Coll Surg. 2010;210(2):199–204. doi: 10.1016/j.jamcollsurg.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo T, Suzuki S, Matsuo M, et al. Preexisting antibodies to platelet factor 4-heparin complexes in patients with acute coronary syndrome who have no history of heparin exposure. Pathophysiol Haemost Thromb. 2005;34(1):18–22. doi: 10.1159/000088543. [DOI] [PubMed] [Google Scholar]
- 3.Tatarewicz SM, Juan G, Swanson SJ, et al. Epitope characterization of pre-existing and developing antibodies to an aglycosylated monoclonal antibody therapeutic of G1m17,1 allotype. J Immunol Methods. 2012;382(1–2):93–100. doi: 10.1016/j.jim.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Uchida K, Nakagaki K, et al. High avidity cytokine autoantibodies in health and disease: pathogenesis and mechanisms. Cytokine Growth Factor Rev. 2010;21:263–273. doi: 10.1016/j.cytogfr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Chung CH, Mirakhur B, Chan E, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weeraratne D, Chen A, Pennucci JJ, et al. Immunogenicity of panitumumab in combination chemotherapy clinical trials. BMC Clin Pharmacol. 2011;11:17. doi: 10.1186/1472-6904-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singla NK, Gasparis AP, Ballard JL, et al. Immunogenicity and safety of re-exposure to recombinant human thrombin in surgical hemostasis. J Am Coll Surg. 2011;213(6):722–727. doi: 10.1016/j.jamcollsurg.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Xue L, Fiscella M, Rajadhyaksha M, et al. Pre-existing biotherapeutic-reactive antibodies: survey results within the American Association of Pharmaceutical Scientists. AAPS J. 2013. doi: 10.1208/s12248-013-9492-4 [DOI] [PMC free article] [PubMed]


