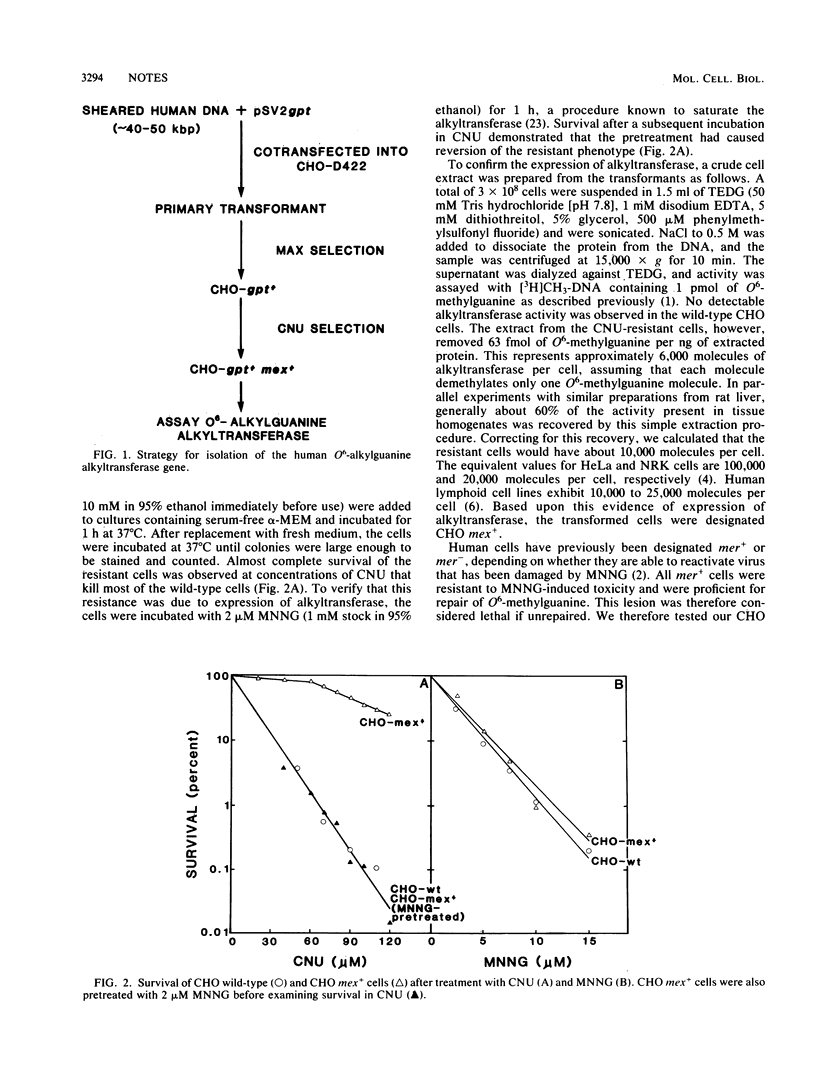
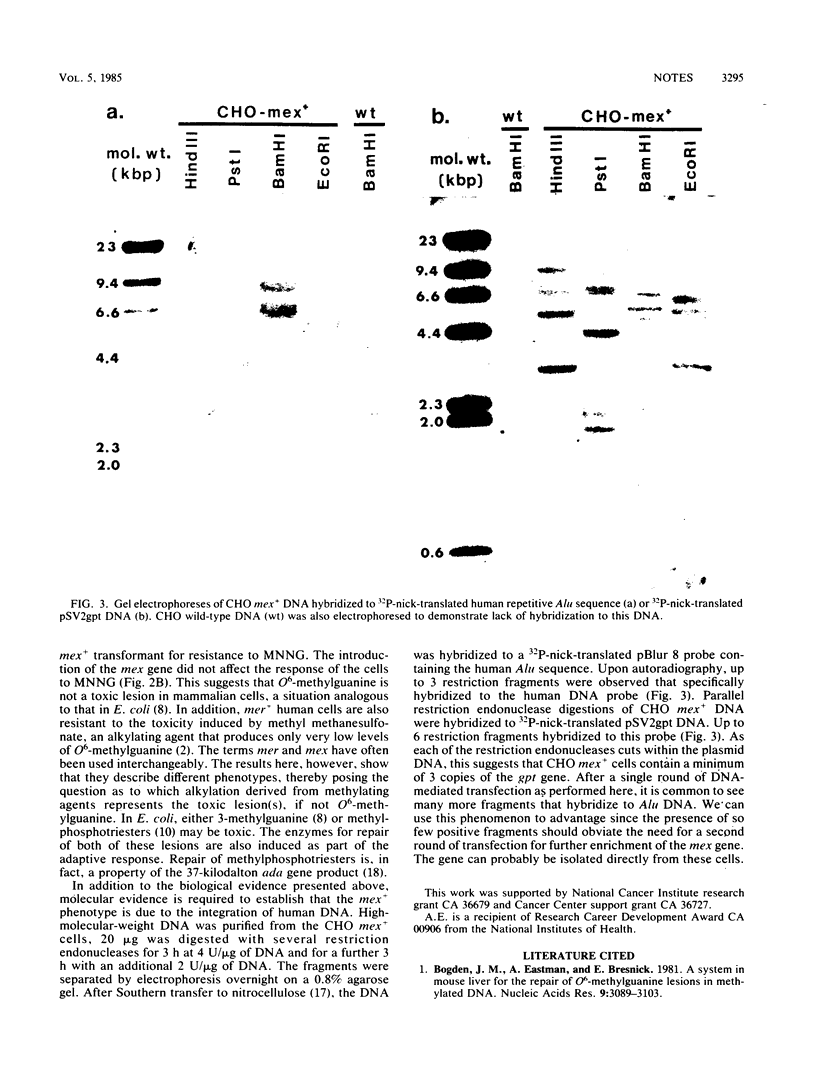
Abstract
Human liver DNA was transfected into CHO cells (mex-) along with pSV2gpt and colonies were selected first for resistance to mycophenolic acid and then to chloroethylnitrosourea. Transformants were obtained that contained approximately 10,000 molecules of O6-alkylguanine alkyltransferase (mex+) per cell. Their genome contained at least three copies of the human Alu sequence.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd, Yarosh D. B., Ziolkowski C. H. Relationship of methyl purines produced by MNNG in adenovirus 5 DNA to viral inactivation in repair-deficient (Mer-) human tumor cell strains. Mutat Res. 1984 Feb;131(2):45–52. doi: 10.1016/0167-8817(84)90010-5. [DOI] [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Foote R. S., Mitra S. Lack of induction of O6-methylguanine-DNA methyltransferase in mammalian cells treated with N-methyl-N'-nitro-N-nitrosoguanidine. Carcinogenesis. 1984 Feb;5(2):277–281. doi: 10.1093/carcin/5.2.277. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Laval F., Laval J. Adaptive response in mammalian cells: crossreactivity of different pretreatments on cytotoxicity as contrasted to mutagenicity. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1062–1066. doi: 10.1073/pnas.81.4.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes M. A., Bingham J. M., Thompson L. H., Strniste G. F. DNA-mediated cotransfer of excision repair capacity and drug resistance into chinese hamster ovary mutant cell line UV-135. Mol Cell Biol. 1984 Jun;4(6):1152–1158. doi: 10.1128/mcb.4.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. G., Edington B. V., Schendel P. F. Inducible repair of phosphotriesters in Escherichia coli. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7380–7384. doi: 10.1073/pnas.80.24.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B., Renard A., Verly W. G. Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6766–6770. doi: 10.1073/pnas.78.11.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Pegg A. E., Roberfroid M., von Bahr C., Foote R. S., Mitra S., Bresil H., Likhachev A., Montesano R. Removal of O6-methylguanine from DNA by human liver fractions. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5162–5165. doi: 10.1073/pnas.79.17.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Joyner A. L., Bernstein A., Whitmore G. F. Molecular identification of a human DNA repair gene following DNA-mediated gene transfer. Nature. 1983 Nov 10;306(5939):206–208. doi: 10.1038/306206a0. [DOI] [PubMed] [Google Scholar]
- Samson L., Schwartz J. L. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980 Oct 30;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teo I., Sedgwick B., Demple B., Li B., Lindahl T. Induction of resistance to alkylating agents in E. coli: the ada+ gene product serves both as a regulatory protein and as an enzyme for repair of mutagenic damage. EMBO J. 1984 Sep;3(9):2151–2157. doi: 10.1002/j.1460-2075.1984.tb02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Mechanism of action of the nitrosoureas--V. Formation of O6-(2-fluoroethyl)guanine and its probable role in the crosslinking of deoxyribonucleic acid. Biochem Pharmacol. 1983 Jul 1;32(13):2011–2015. doi: 10.1016/0006-2952(83)90420-3. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1983;4(6):759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]