Summary
Background
Maternal vitamin D status in pregnancy is a suggested determinant of bone-mineral content (BMC) in offspring, but has been assessed in small studies. We investigated this association in a large prospective study.
Methods
Eligible participants were mother-and-singleton-offspring pairs who had participated in the Avon Longitudinal Study of Parents and Children, and in which the mother had recorded measurements of 25(OH)D concentration in pregnancy and the offspring had undergone dual-energy x-ray absorptiometry at age 9–10 years. 25(OH)D concentrations in pregnancy were assessed per 10·0 nmol/L and classified as sufficient (more than 50·00 nmol/L), insufficient (49·99–27·50 nmol/L), or deficient (lower than 27·50 nmol/L). Associations between maternal serum 25(OH)D concentrations and offspring total body less head (TBLH) and spinal BMC were assessed by trimester.
Results
3960 mother-and-offspring pairs, mainly of white European origin, were assessed (TBLH BMC n=3960, spinal BMC n=3196). Mean offspring age was 9·9 years. 2644 (67%) mothers had sufficient, 1096 (28%) insufficient, and 220 (6%) deficient 25(OH)D concentrations in pregnancy, but TBLH and spinal BMC did not differ between offspring of mothers in the lower two groups versus sufficient 25(OH)D concentration. No associations with offspring BMC were found for any trimester, including the third trimester, which is thought to be most relevant (TBLH BMC confounder-adjusted mean difference −0·03 g per 10·0 nmol/L, 95% CI −1·71 to 1·65; spinal BMC 0·04 g per 10·0 nmol/L, 95% CI −0·12 to 0·21).
Conclusions
We found no relevant association between maternal vitamin D status in pregnancy and offspring BMC in late childhood.
Funding
UK Medical Research Council, Wellcome Trust, and University of Bristol.
Introduction
Vitamin D is essential for the maintenance of calcium homoeostasis and regulation of bone mineralisation.1 Deficiency in children can lead to rickets, and in adults it is related to osteomalacia, osteoporosis, and risk of fractures.2,3 Lowered concentrations of vitamin D during pregnancy are suggested to be related to low bone-mineral content (BMC) in offspring.4 If this association is true, it is relevant to public health, as up to 70% of otherwise healthy pregnant women have insufficient concentrations (most commonly defined as lower than 50·0 nmol/L) of 25-hydroxyvitamin D (25[OH]D).5,6 This association has been assessed in three small (fewer than 200 participants) studies. One suggested a negative association (low maternal 25[OH]D but high offspring BMC),7 another a positive association,4 and a third a null association.8
We did a large prospective study to investigate whether there is an association between maternal 25(OH)D concentrations in pregnancy and offspring BMC. We also aimed to assess whether the association is mediated by the child's own 25(OH)D concentration, which relates directly to BMC.9 Additionally, we tested the hypothesis that the third trimester is a sensitive period for bone mineralisation by 25(OH)D.4,10,11
Methods
Participants
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a prospective population-based study that recruited a cohort of 14 541 pregnant women resident in southwest England with delivery expected between April 1, 1991, and Dec 31, 1992.12,13 13 678 singleton liveborn infants resulted from these pregnancies. For this study, eligible participants were mother-and-singleton-offspring pairs in which the mother had valid results for 25(OH)D concentrations in pregnancy and offspring had undergone dual-energy x-ray absorptiometry (DXA) at age 9–10 years. We obtained ethics approval for this study from the ALSPAC law and ethics committee and the local National Health Service research ethics committee. Participants provided written informed consent.
Measurement of vitamin D concentrations and bone-mineral content
Maternal non-fasting blood samples taken as part of routine antenatal care were collected and stored initially at −20°C, and then at −80°C, with no further freeze–thaw cycles, until the time of 25(OH)D measurement. Serum samples could be from any stage of pregnancy. If a woman had more than one result, we used the latest. The dates of blood sampling were obtained from medical records and verified from the freezer storage data to enable calculation of gestational age at the time of maternal 25(OH)D measurement and adjustment for seasonality. Serum samples from offspring obtained during the clinic visit at age 9–10 years were also assessed. Serum 25(OH)D concentrations for mothers and offspring were measured with high-performance liquid chromatography tandem mass spectrometry with an internal standard (appendix p 1),9,14 in one laboratory, in accordance with the performance target set by the Vitamin D External Quality Assessment Scheme advisory panel. We calculated 25(OH)D concentrations in each trimester of pregnancy and overall. We also calculated a measure of 25(OH)D that was adjusted for seasonality (appendix pp 1–3).
DXA scans were obtained with a Lunar Prodigy scanner (GE Healthcare, London, UK), as described elsewhere.15 We used mean differences (per 10·0 nmol/L or per category of 25[OH]D) in total body less head (TBLH) and spinal BMC as our primary outcomes because maternal pregnancy 25(OH)D concentration is hypothesised to affect BMC. We also investigated associations with bone-mineral density, bone area (with and without adjustment for height), and bone-area-adjusted BMC (appendix p 1).
Statistical analysis
We used medians (IQRs) and proportions to describe all variables for included mothers and offspring, and for those excluded because of missing data (maternal 25(OH)D concentration or offspring BMC results). Differences between included mother-and-offspring pairs and those excluded because of missing data were investigated with t tests for continuously measured variables (with those variables that were right-skewed being logged) and χ2 tests for categorical variables. Pearson's correlation coefficients were used to assess whether maternal 25(OH)D concentrations in different trimesters were associated with each other, and also whether they were associated with offspring 25(OH)D concentrations.
We used linear regression to explore associations between maternal 25(OH)D concentration in pregnancy and offspring BMC: model A was minimally adjusted and included maternal age and offspring age and sex (we do not report results of completely unadjusted associations because they were not notably different from these); model B was the main confounder-adjusted model, and was model A plus adjustment for maternal education, parity, ethnic origin, smoking during pregnancy, and body-mass index before pregnancy; model C was model B plus adjustment for potential mediation by offspring growth and size (birthweight, gestational age, and offspring height, lean mass, and fat mass); and model D was model B plus adjustment for potential mediation by offspring 25(OH)D concentrations (appendix p 3). Mediation was explored by comparison of the confounder-adjusted models with and without the addition of potential mediators. Differences in associations between female and male offspring were tested in all models by the inclusion of interaction terms for sex. Non-linearity was tested with quadratic and cubic polynomials and explored by examination of associations between adult categories of 25(OH)D concentrations (sufficient, 50·00 nmol/L or higher; insufficient, 49·99–27·50 nmol/L; deficient, lower than 27·50 nmol/L), which are based on cutoff points that have been most commonly used in previous studies of pregnant women.5
An important source of 25(OH)D is synthesis in the skin in response to exposure to ultraviolet B, and 25(OH)D concentrations show a strong sinusoidal pattern with date of blood collection—ie, concentrations are lower in participants whose samples were collected during winter months than are those in participants whose samples were collected during summer months (appendix pp 1–3). This pattern was modelled with a sine–cosine regression model. We derived a variable that adjusted each woman's total 25(OH)D concentrations to the date corresponding to her trimester midpoint (first trimester, 6 weeks; second trimester, 20 weeks; third trimester, 34 weeks). To investigate associations between timing of maternal 25(OH)D concentration and offspring BMC, we stratified the participants by trimester. We assessed whether there was statistical evidence for a difference in the associations between trimesters by examining the association in the whole sampleand including an interaction term between trimester of assessment of maternal maternal 25(OH)D concentrations.
We generated predicted third-trimester 25(OH)D concentrations for all women, by adjustment of maternal 25(OH)D concentration to 34 weeks' gestation, irrespective of when the blood samples were taken. A final variable was generated that adjusted for seasonality, to obtain a marker of habitual 25(OH)D concentration, by use of the residuals from the sine–cosine regression models. Similar trigonometric models were fitted to the offspring data to adjust for season, as previously described (appendix pp 1–3).9
Additionally to the main analyses of the relations between maternal pregnancy 25(OH)D concentrations and offspring BMC and other bone outcomes, we investigated whether the previously resported association between estimated third-trimester exposure to ultraviolet B and offspring BMC16 was present in this study sample and whether it was mediated by maternal third-trimester 25(OH)D concentration.
To increase efficiency and keep selection bias to a minimum, we used multivariate multiple imputation (Stata, version 11 MP2) to impute missing values of covariables for eligible participants, according to the method described by Royston.17 The amount of missing data varied from 0 to 16% for any single variable. 72% of had complete data for TBLH BMC and 72% had complete data for spinal BMC (appendix p 4). The distributions of the variables with missing data did not differ substantially between participants with observed data and those with imputed data (appendix p 4). The associations between maternal 25(OH)D concentrations and offspring BMC and other bone outcomes obtained from analyses in those with complete data on all covariables did not differ substantially from the same associations obtained from analyses in the multivariable multiple-imputed datasets (appendix p 5).
Roles of the funding sources
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
3960 eligible mother-and-offspring pairs were assessed in this study (figure 1). The mean age of offspring at the time of BMC assessment was 9·9 years. Maternal 25(OH)D concentrations were measured in the first trimester in 1035 (26%) women, in the second trimester in 879 (22%), and in the third trimester in 2046 (52%). The median 25(OH)D concentration was lowest in the first trimester (55·1 nmol/L, IQR 40·7–74·1), intermediate in the second trimester (60·1 nmol/L, 41·4–83·4), and highest in the third trimester (67·4 nmol/L, 46·8–93·0). For the predicted third-trimester 25(OH)D concentrations, we estimated that 2644 (67%) would be sufficient, 1096 (28%) insufficient, and 220 (6%) deficient.
Figure 1.
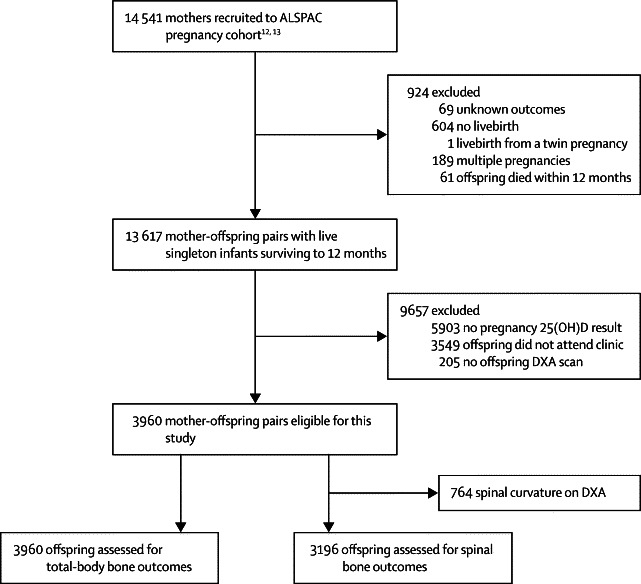
Trial profile
ALSPAC= Avon Longitudinal Study of Parents and Children. 25(OH)D=25-hydroxyvitamin D. DXA=dual-energy x-ray absorptiometry.
Seasonally adjusted and unadjusted 25(OH)D levels correlated strongly. Estimated exposure to ultraviolet B in the third-trimester was marginally positively correlated with third-trimester 25(OH)D concentration, inversely correlated that in the second trimester, and not associated with that in the first trimester (table 1). Maternal 25(OH)D concentration in pregnancy showed a weak association with offspring 25(OH)D concentration, whereas exposure to ultraviolet B showed no association (table 1).
Table 1.
Correlations between maternal 25(OH)D concentrations in pregnancy, exposure to ultraviolet B in the third trimester, and offspring 25(OH)D concentrations
| eUVB (n=3960) |
25(OH)D |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1 (n=1035) | T2 (n=879) | T3 (n=2046) | Cumulative T3 (n=2046)* | Predicted T3 (n=3960) | Maternal SA (n=3960) | Offspring (n=3960) | Offspring SA (n=3960) | |||
| eUVB | 1·00 | .. | .. | .. | .. | .. | .. | .. | .. | |
| 25(OH)D | ||||||||||
| T1 | −0·01 | 1·00 | .. | .. | .. | .. | .. | .. | .. | |
| T2 | −0·38† | NA | 1·00 | .. | .. | .. | .. | .. | .. | |
| T3 | 0·35† | NA | NA | 1·00 | .. | .. | .. | .. | .. | |
| Cumulative T3* | 0·37† | NA | NA | 0·99† | 1·00 | .. | .. | .. | .. | |
| Predicted T3 | 0·07† | 0·91† | 0·93† | 0·99† | 0·99† | 1·00 | .. | .. | .. | |
| Maternal SA | 0·01 | 0·86† | 0·84† | 0·84† | 0·80† | 0·85† | 1·00 | .. | .. | |
| Offspring | −0·08† | 0·01 | 0·22† | 0·16† | 0·16† | 0·17† | 0·14† | 1·00 | .. | |
| Offspring SA | −0·01 | 0·12† | 0·15† | 0·15† | 0·14† | 0·14† | 0·15† | 0·86† | 1·00 | |
eUVB=estimated exposure to ultraviolet B. 25(OH)D=25-hydroxyvitamin D. T1=first trimester. T2=second trimester. T3=third trimester. SA=seasonally adjusted. NA=not available (women only had a measure in one trimester).
Cumulative 25(OH)D exposure over final 10 weeks of T3 for women with a sample taken in third trimester.
p<0·05.
Compared with eligible participants, the mother-and-offspring pairs who were excluded because of missing maternal 25(OH)D or offspring BMC values had mothers who were younger, had lower educational attainment, were more likely to be of non-white ethnic origin, to have already had at least two previous pregnancies, and to have smoked during pregnancy (appendix p 6). Maternal 25(OH)D in the third trimester and offspring gestational age, birthweight, spine BMD, age at outcome measure, and 25(OH)D concentration differed between eligible and excluded maternal-offspring pairs, but the median values were similar (appendix p 7). These small p values have arisen because of the large sample size in this study. The differences are unlikely to be due to chance but are not clinically important.
A linear association was noted between maternal age and maternal 25(OH)D concentration, such that older age was associated with higher 25(OH)D concentrations in the second and third trimesters than was younger age, and greater parity was associated with higher 25(OH)D concentration in the third trimester (appendix p 8). Non-white mothers had lower 25(OH)D levels, particularly from the second trimester onwards, than white mothers, and those who smoked during pregnancy had lower 25(OH)D across all trimesters than non-smokers. Maternal body-mass index before pregnancy and education were not associated with 25(OH)D concentrations in pregnancy.
Maternal 25(OH)D concentration, in any trimester of pregnancy, was not associated with offspring BMC. These null associations were similar in all multivariable models and when 25(OH)D was seasonally adjusted (figure 2, table 2). Mean offspring BMC was similar in the offspring of mothers with insufficient and deficient levels of 25(OH)D in the third trimester, compared with values for offspring of mothers with sufficient levels (table 3). Sensitivity analyses in which the threshold for sufficient 25(OH)D concentration was changed to 75·0 nmol/L also showed no association. Tests of non-linear relationships across the maternal 25(OH)D sufficiency categories and higher-order polynomials were null (table 3).
Figure 2.
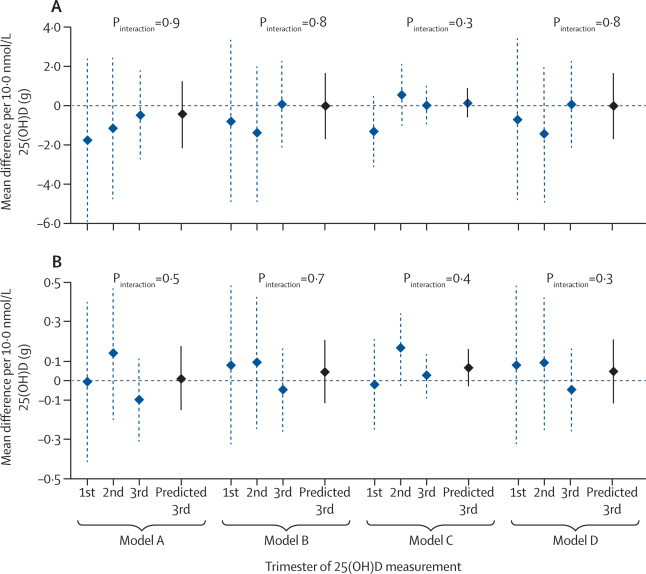
Mean differences in offspring bone-mineral content in relation to maternal 25(OH)D concentrations in pregnancy, by trimester
(A) Offspring total body bone-mineral content (total body less head measured; n=3960). (B) Offspring spine bone-mineral content (n=3196). The p values test whether the association between maternal 25(OH)D concentration and offspring BMC differs by trimester of measurement. Model A adjusted for maternal age and offspring age and sex. Model B, the main confounder-adjusted model, was model A plus adjustment for maternal education, ethnic origin, parity, smoking in pregnancy, and body-mass index before pregnancy. Model C was model B plus adjustment for potential mediation by birthweight, gestational age, and offspring height, lean mass, and fat mass. Model D was model B plus adjustment for potential mediation by offspring 25(OH)D concentrations. Values missing for covariables were calculated by multivariate multiple imputation. 25(OH)D=25-hydroxyvitamin D. Predicted 3rd=all women with 25(OH)D concentrations adjusted to 34 weeks' gestation.
Table 2.
Multivariable associations between predicted maternal third-trimester 25(OH)D concentrations and offspring BMC
|
Model A* |
Model B† |
Model C‡ |
Model D§ |
|||||
|---|---|---|---|---|---|---|---|---|
| Difference in BMC per 10·0 nmol/L 25(OH)D (g) | p value | Difference in BMC per 10·0 nmol/L 25(OH)D (g) | p value | Difference in BMC per 10·0 nmol/L 25(OH)D (g) | p value | Difference in BMC per 10·0 nmol/L 25(OH)D (g) | p value | |
| Predicted third-trimester maternal 25(OH)D concentration | ||||||||
| TBLH BMC (n=3960) | −0·47 (−2·16 to 1·23) | 0·6 | −0·03 (−1·71 to 1·65) | 0·97 | 0·13 (−0·62 to 0·87) | 0·7 | 0·01 (−1·68 to 1·71) | 0·99 |
| Spine BMC (n=3196) | 0·01 (−0·15 to 0·17) | 0·9 | 0·04 (−0·12 to 0·21) | 0·6 | 0·06 (−0·03 to 0·16) | 0·2 | 0·04 (−0·12 to 0·21) | 0·6 |
| Seasonally adjusted predicted-third trimester maternal 25(OH)D concentration | ||||||||
| TBLH BMC (n=3960) | −0·51 (−2·43 to 1·40) | 0·6 | −0·01 (−1·93 to 1·89) | 0·99 | −0·11 (−0·94 to 0·73) | 0·8 | 0·04 (−1·88 to 1·97) | 0·96 |
| Spine BMC (n=3196) | 0·02 (−0·17 to 0·21) | 0·8 | 0·06 (−0·13 to 0·24) | 0·6 | 0·07 (−0·04 to 0·17) | 0·2 | 0·06 (−0·13 to 0·24) | 0·6 |
Data are mean differences (95% CI). Values missing for covariables were calculated by multivariate multiple imputation. BMC=bone-mineral content. 25(OH)D=25-hydroxyvitamin-D. TBLH=total body less head.
Adjusted for maternal age and offspring age and sex.
Main confounder-adjusted model; model A plus adjustment for maternal education, ethnic origin, parity, smoking in pregnancy, and body-mass index before pregnancy.
Model B plus adjustment for potential mediation by birthweight, gestational age, and offspring height, lean mass, and fat mass.
Model B plus adjustment for potential mediation by offspring 25(OH)D concentrations.
Table 3.
Multivariable associations between predicted third-trimester maternal 25(OH)D categories and offspring BMC
|
Model A* |
Model B† |
Model C‡ |
Model D§ |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference in BMC per 25(OH)D category (g) | p value¶ | Difference in BMC per 25(OH)D category (g) | p value¶ | Difference in BMC per 25(OH)D category (g) | p value¶ | Difference in BMC per 25(OH)D category (g) | p value¶ | ||
| TBLH BMC (n=3960) | 0·5 | 0·4 | 0·7 | 0·4 | |||||
| Sufficient (≥50·00 nmol/L; n=2644) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | |||||
| Insufficient (49·99–27·50 nmol/L; n=1096) | −0·9 (−13·4 to 11·5) | −2·1 (−14·4 to 10·2) | 1·0 (−4·5 to 6·6) | −2·4 (−14·8 to 10·0) | |||||
| Deficient (<27·50 nmol/L; n=220) | −10·0 (−34·3 to 14·4) | −11·7 (−35·8 to 12·5) | 1·0 (−9·9 to 11·9) | −12·2 (−36·4 to 12·1) | |||||
| Spine BMC (n=3196) | 0·5 | 0·4 | 0·7 | 0·4 | |||||
| Sufficient (≥50·00 nmol/L; n=2122) | 0 (reference) | 0 (reference) | 0 (reference) | 0 (reference) | |||||
| Insufficient (49·99–27·50 nmol/L; n=884) | −0·1 (−1·3 to 1·1) | −0·3 (−1·5 to 0·9) | −0·1 (−0·8 to 0·6) | −0·3 (−1·5 to 0·9) | |||||
| Deficient (<27·50 nmol/L; n=190) | −0·9 (−3·2 to 1·3) | −0·9 (−3·2 to 1·3) | −0·3 (−1·6 to 1·1) | −0·9 (−3·2 to 1·3) | |||||
Data are mean differences (95% CI). Values missing for covariables were calculated by multivariate multiple imputation. BMC=bone-mineral content. 25(OH)D=25–hydroxyvitamin-D. TBLH=total body less head.
Adjusted for maternal age and offspring age and sex.
Main confounder adjusted model; model A plus adjustment for maternal education, ethnic origin, parity, smoking in pregnancy, and body-mass index before pregnancy.
Model B plus adjustment for potential mediation by birthweight, gestational age and offspring height, lean mass, and fat mass.
Model B plus adjustment for potential mediation by offspring 25(OH)D concentrations.
Testing for linear trend across the 25(OH)D categories; there was no evidence for non-linear associations based on cubic and higher-order polynomials (all p>0·2).
No maternal 25(OH)D measurement in any trimester, with or without seasonal adjustment, was associated with any offspring bone outcomes in any of the multivariable models (appendix pp 9–13). Mean bone outcomes were similar in offspring of mothers with deficient or insufficient 25(OH)D concentrations to those in mothers with sufficient concentrations (appendix pp 10–11). A measure of cumulative 25(OH)D in the last 10 weeks of the 3rd trimester similarly had null associations with all outcomes (results available from authors on request).
Data suggested that associations between maternal 25(OH)D concentrations in the second and third trimesters and offspring BMC differed for girls and boys. In the confounder-adjusted models, higher maternal 25(OH)D concentrations were associated with BMC in girls, but with higher BMC in boys (figure 3). This difference between the sexes, however, was completely explained by mediation by offspring characteristics. Specifically, this interaction was mediated by a difference in the relation between maternal 25(OH)D concentration in pregnancy and offspring height and lean mass for girls and boys. The difference in BMC between the sexes disappeared with adjustment for either of these features alone. Similar patterns of differences between boys and girls were also seen with TBLH adjusted bone area, but there were no other such interactions with maternal 25(OH)D concentrations for offspring bone outcomes in any models.
Figure 3.
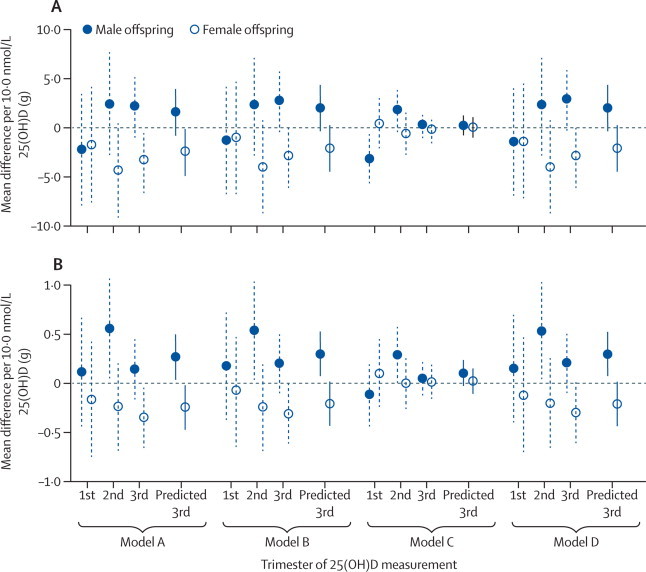
Mean differences in offspring bone-mineral content in relation to maternal 25(OH)D concentrations in pregnancy, by trimester and sex of offspring
(A) Offspring total body bone-mineral content (total body less head measured; n=3960). (B) Offspring spine bone-mineral content (n=3196). Model A adjusted for maternal age and offspring age and sex. Model B, the main confounder-adjusted model was model A plus adjustment for maternal education, ethnic origin, parity, smoking in pregnancy, and body-mass index before pregnancy. Model C was model B plus adjustment for potential mediation by birthweight, gestational age, and offspring height, lean mass, and fat mass. Model D was model B plus adjustment for potential mediation by offspring 25(OH)D. Values missing for covariables were calculated by multivariate multiple imputation. 25(OH)D=25-hydroxyvitamin D. Predicted 3rd=all women with 25(OH)D concentrations adjusted to 34 weeks' gestation.
Estimated exposure to ultraviolet B in the third trimester was positively associated with TBLH BMC (table 4). When we adjusted for offspring age at the time of bone measurements, however, these associations became null. Associations between estimated exposure to ultraviolet B and all other bone outcomes were also null after age was taken into account (appendix p 14). Reassessment of the previously reported sample16 showed that adjustment for offspring age completely attenuated all the associations to null. This removal of the association with adjustment for age occurred because estimated third-trimester exposure to ultraviolet B in the ALSPAC cohort is associated with offspring age at the time of BMC assessment (mean difference in age 1·2 months per 1 SD increase in estimated exposure to ultraviolet B (95% CI 1·1–1·3, p<0·0001).
Table 4.
Multivariable associations between maternal estimated exposure to ultraviolet B in the third trimester and offspring BMC
|
Unadjusted eUVB |
eUVB adjusted for age of offspring at DXA |
eUVB adjusted for age of offspring at DXA and maternal 25(OH)D |
||||
|---|---|---|---|---|---|---|
| Difference per SD (g) | p value | Difference per SD (g) | p value | Difference per SD (g) | p value | |
| TBLH BMC (n=3960) | 13·8 (8·26 to 19·34) | <0·0001 | 0·95 (−4·71 to 6·61) | 0·7 | 1·04 (−4·65 to 6·72) | 0·7 |
| Spine BMC (n=3196) | 0·33 (−0·20 to 0·86) | 0·2 | −0·64 (−1·18 to −0·10) | 0·02 | −0·66 (−1·20 to −0·11) | 0·02 |
Data are mean differences (95% CI). eUVB=estimated exposure to ultraviolet B. DXA=dual x-ray absorptiometry. 25(OH)D=25-hydroxyvitamin D. TBLH=total body less head. BMC=bone-mineral content.
Discussion
In this large prospective study, we found no evidence that maternal 25(OH)D concentration in any trimester of pregnancy was associated with offspring BMC or other bone outcomes at a mean offspring age of 9·9 years. The positive association we reported previously between estimated ambient exposure to ultraviolet B in the third trimester of pregnancy and offspring BMC and BMD at age 9·9 years16 seems to be fully explained by offspring age at the time of DXA. Ordinarily, we would not expect differences between children's ages to be associated with the mothers' potential exposure to sunlight towards the end of pregnancy. For this reason, in our previous report we did not judge the children's ages at the time of BMC assessment to be a confounder and, therefore, did not control for this feature. We now realise that offspring age in this study is associated with mothers' estimated exposure to ultraviolet B in the third trimester, owing to a quirk in the dates when offspring had their BMC assessed. Those who had been born in the late summer or early autumn (and, therefore, whose mothers were in their third-trimester in the summer months) were more likely to undergo DXA scanning at older average age than children born at other times of year. Childhood age is positively associated with BMC,8 which is confirmed by our results from this study: each year of age was associated with 132·0 g (95% CI 115·5–148·8) greater total BMC. As a result of the strong associations between offspring age and maternal estimated exposure to ultraviolet B in the third trimester and offspring BMC, our findings show that age confounds the previously reported associations between these two features. Thus, our findings show that there is no strong evidence that maternal 25(OH)D concentrations in pregnancy are related to childhood BMC.
An important strength of this study is its large sample size, as previous studies included fewer than 200 mother-and-offspring pairs (panel). We were able to adjust for several potential confounding factors and to compare associations with maternal 25(OH)D concentrations in different trimesters. Some previous studies have only reported associations for logged maternal 25(OH)D concentration;4,8 our results were essentially the same after log transformation (results available from authors on request).
Panel. Research in context.
Systematic review
We systematically searched PubMed with the term “pregn* [AND] (vitamin D [OR] 25(OH)D) [AND] (bone mineral content [OR] BMC [OR] bone density [OR] bone mineral density [OR] BMD)”, and also identified relevant articles from the reference lists of any retrieved papers. We only considered studies in human beings. We identified four reports from three independent cohort studies that had assessed the association between pregnancy 25(OH)D concentration with offspring BMC. In the first study of 50 mother-and-offspring pairs, lower concentrations of maternal 25(OH)D in pregnancy were associated with higher whole-body BMC in offspring at age 2 weeks (mean difference −0·139 g per 1 nmol/L increase in 25(OH)D, p<0·05).7 In a later and larger study of 198 mother-and-offspring pairs, where the mean age of offspring was 9 years, serum 25(OH)D concentration in the third trimester of pregnancy was weakly positively associated with whole-body BMC (Pearson's correlation coefficient r=0·21, p=0·0088) and lumbar spinal BMC (r=0·17, p=0·03).4 Lastly, in a study of 60–125 mother-and-offspring pairs (numbers varied for different outcomes at different ages), maternal 25(OH)D concentration in pregnancy above the median was positively associated with offspring tibial BMC at birth (mean difference 0·047 g/cm, 95% CI 0·011–0·082) compared with maternal 25(OH)D concentrations below the median.18 However, postnatally, the age-related increase in tibial BMC was greater in those whose mothers had 25(OH)D concentrations below the median than in those whose mothers had values higher than the median (by 0·062 g/cm, SE 0·029). By 14 months the BMC no longer differed between the lower and higher maternal 25(OH)D groups (mean value around 0·44 g/cm, obtained by use of a ruler on the figure provided in the report).8 Despite inconsistencies, these results have been interpreted as evidence that variation in maternal vitamin D status in pregnancy is causally related to offspring bone health via intrauterine mechanisms. In particular, the findings of the second study4 have been frequently cited as evidence for the importance of vitamin D supplementation in pregnancy. If vitamin D status in pregnancy is causally related to offspring bone outcomes, the issue is important for public health, as high proportions of otherwise healthy pregnant women are reported to be deficient of vitamin D or have insufficient vitamin D. Although correlation in the second study was weak, it was linear across the whole distribution of maternal 25(OH)D concentrations,4 which suggests that if the association is causal, increases in pregnancy vitamin D status in all women could lead to important population-level improvements in BMC.
Interpretation
We did a large observational study and found no evidence of any association between maternal 25(OH)D concentrations in pregnancy and offspring total-body (less head) or spinal BMC. The third trimester is thought to be most relevant to offspring BMC, but our findings did not differ when assessed by timing of 25(OH)D measurement. Null associations were found in all statistical models and in a series of sensitivity analyses. The pooling of results from previous studies and our study was not feasible because of the different ways in which the association has been modelled. Nevertheless, our study was more than ten times larger than previous studies combined. In view of the differing directions of previous results (positive, null, and negative associations) and the sample sizes relative to that in our study, if we were able to pool all the previous study results, including ours, the pooled results would be consistent with those from our study alone (presented here) and would not be notably more precisely estimated (confirmed by a simulation analysis, unpublished). Thus, our results challenge the assertion that vitamin D supplementation should be provided to pregnant women to prevent low BMC in offspring in later life.
25(OH)D=25-hydroxyvitamin D. BMC=bone-mineral content.
As with other prospective studies, there was loss to follow-up and not all mothers had samples available for assessment of 25(OH)D levels. Children who were excluded mainly had mothers who were younger, less well educated, non-white ethnic origin, and higher parity and were likely to smoke in pregnancy than did included children. Maternal 25(OH)D concentrations and offspring BMC and most other bone outcomes, however, were similar for included and excluded offspring. Around two-thirds (67%) of women in this study had sufficient concentrations of 25(OH)D in pregnancy. In populations with lower maternal 25(OH)D concentrations, offspring BMC might also be reduced. Nevertheless, we found no difference in mean BMC between offspring of mothers who had sufficient or deficient 25(OH)D concentrations in pregnancy. A study that reported a positive association between maternal 25(OH)D and offspring BMC found a linear dose-response relation across the whole 25(OH)D distribution.4 Furthermore, the definitions of 25(OH)D insufficiency and deficiency are strongly debated, particularly in pregnancy.18
In one study of 50 mother-and-offspring pairs, lower concentrations of maternal 25(OH)D were associated with higher infant whole-body BMC at age 2 weeks (panel).7 In a later study of 198 mother-and-offspring pairs, a linear dose-response association was seen between lower maternal 25(OH)D concentrations in the third trimester and lower whole-body and lumbar-spine BMC (panel).4 In a third study, a positive association was shown between maternal 25(OH)D levels in pregnancy above the median value and offspring tibial BMC at birth (panel).19 Of note, though, tibial BMC increased at a greater rate postnatally in the offspring of mothers with 25(OH)D concentrations below the median (by 0·062 g/cm, SE 0·029) than did BMC in those whose mothers had 25(OH)D levels above the median; by age 14 months, the BMC values were identical in the two subgroups.8
The inconsistency in findings might reflect chance variation around a null value in small studies, as is supported by the null findings in our much larger study. The ages at which outcomes were assessed differed between studies, which might also have contributed to inconsistencies. That maternal 25(OH)D concentrations in pregnancy would affect BMC at birth and in early infancy seems more plausible than later in childhood. Maternal 25(OH)D concentration in pregnancy is positively correlated with concentrations in cord blood,20 and maternal 25(OH)D concentration in the third trimester has been suggested to affect bone mineralisation in utero.4,10,11 A positive association has been shown between maternal 25(OH)D concentration and BMC at birth,19 but is not sustained.8 By contrast, a positive association between maternal 25(OH)D concentration in pregnancy with offspring BMC was found by Javaid and colleagues in children assessed at age 9–10 years.4
Javaid and colleagues’ study and our study have many features in common: both involved cohorts from the south of England, largely of white European origin; levels of maternal 25(OH)D were similar; both measured outcomes when offspring were aged 9–10 years; both used DXA to assess BMC; and both adjusted for age as well as a range of potential confounding characteristics. The studies differ for timing of 25(OH)D measurement: in Javaid and colleagues’ study all measurements were made in the third trimester, whereas we included women with measurements from different trimesters. Nevertheless, our sample of 2046 women with third-trimester values is more than ten times larger than that of Javaid and colleagues (n=198), and we found no evidence of association in this subgroup.
International guidelines have cited the Javaid and colleagues’ findings as evidence for the importance of vitamin D supplementation in pregnant women. The magnitude of association in that study (r=0·17–0·21), however, was weak and unlikely to be clinically important for individuals. Assessment of our substantially larger sample suggested no association. We believe, therefore, that there is no strong evidence that pregnant women should receive vitamin D supplementation to prevent low BMC in their offspring, although we cannot comment on other possible effects of vitamin D in pregnant women. Furthermore, we have previously shown that offspring 25(OH)D concentrations at a mean age of 9·9 years are prospectively associated with BMC and other bone outcomes assessed at a mean age of 15·5 years in this cohort.9 Our results should not be interpreted as suggesting that individual 25(OH)D concentrations are not an important determinants of bone health.
This online publication has been corrected. The corrected version first appeared at thelancet.com on June 21, 2013
Acknowledgments
Acknowledgments
We thank the families who took part in this study and the midwives for recruiting them, and thank the whole Avon Longitudinal Study of Parents and Children team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. Funding for this study was largely from a UK Medical Research Council Grant (G0701603), which provided funds for all 25(OH)D concentration assays, AKW's salary, and the time spent on this study by DAL, WDF, and JHT. AF is supported by a UK Medical Research Council postdoctoral research fellowship. The UK Medical Research Council, the Wellcome Trust (grant 092731), and the University of Bristol, provide core support for the Avon Longitudinal Study of Parents and Children study. DAL, AKW, and AF work in a centre that receives infrastructure funding from the UK Medical Research Council (grant G0600705).
Contributors
DAL obtained funds, designed the study, had overall responsibility for managing the study, completed the background literature search, contributed to the analysis protocol, contributed to data management, wrote the first draft of the paper, and collated comments from other authors. AKW contributed to the analysis protocol and completed analyses. AF contributed to data management. AS contributed to deriving the offspring 25(OH)D variables used in this study. WDF managed laboratory assays of 25(OH)D. JHT managed data collection for all bone measurements. All authors commented on drafts of the paper.
Conflicts of interest
We declare that we have no conflicts of interest.
Supplementary Material
Supplementary Material
Professor Debbie Lawlor reports on the association of maternal vitamin D status in pregnancy with bone-mineral content in offspring, and discusses the implications for current clinical guidelines.
References
- 1.Weaver CM. Vitamin D, calcium homeostasis, and skeleton accretion in children. J Bone Miner Res. 2007;22:V45–V49. doi: 10.1359/jbmr.07s201. [DOI] [PubMed] [Google Scholar]
- 2.Gartner LM, Greer FR. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111:908–910. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- 3.Chung M, Lee J, Terasawa T, Lau J, Trikalinos TA. Vitamin D with or without calcium supplementation for prevention of cancer and fractures: an updated meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:827–838. doi: 10.7326/0003-4819-155-12-201112200-00005. [DOI] [PubMed] [Google Scholar]
- 4.Javaid MK, Crozier SR, Harvey NC. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet. 2006;367:36–43. doi: 10.1016/S0140-6736(06)67922-1. [DOI] [PubMed] [Google Scholar]
- 5.Dror DK, Allen LH. Vitamin F inadequacy in pregnancy: biology, outcomes and interventions. Nutrition Rev. 2012;68:465–477. doi: 10.1111/j.1753-4887.2010.00306.x. [DOI] [PubMed] [Google Scholar]
- 6.Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child. 2007;92:737–740. doi: 10.1136/adc.2007.122689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiler H, Fitzpatrick-Wong S, Veitch R. Vitamin D deficiency and whole-body and femur bone mass relative to weight in healthy newborns. CMAJ. 2005;172:757–761. doi: 10.1503/cmaj.1040508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viljakainen HT, Korhonen T, Hytinantti T. Maternal vitamin D status affects bone growth in early childhood—a prospective cohort study. Osteoporos Int. 2011;22:883–891. doi: 10.1007/s00198-010-1499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayers A, Fraser WD, Lawlor DA, Tobias JH. 25-Hydroxyvitamin-D3 levels are positively related to subsequent cortical bone development in childhood: findings from a large prospective cohort study. Osteoporos Int. 2012;23:2117–2128. doi: 10.1007/s00198-011-1813-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salle BL, Delvin EE, Lapillonne A, Bishop NJ, Glorieux FH. Perinatal metabolism of vitamin D. Am J Clin Nutr. 2000;71:1317S–1324S. doi: 10.1093/ajcn/71.5.1317s. [DOI] [PubMed] [Google Scholar]
- 11.Lapillonne A, Braillon P, Claris O, Chatelain PG, Delmas PD, Salle BL. Body composition in appropriate and in small for gestational age infants. Acta Paediatr. 1997;86:196–200. doi: 10.1111/j.1651-2227.1997.tb08868.x. [DOI] [PubMed] [Google Scholar]
- 12.Fraser A, Macdonald-wallis C, Tilling K. Cohort profile: the Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int J Epidemiol. 2012 doi: 10.1093/ije/dys066. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd A, Golding J, Macleod J. Cohort Profile: The ‘Children of the 90s'—the index offspring of the Avon Longitudinal Study of Parents and Children (ALSPAC) Int J Epidemiol. 2012 doi: 10.1093/ije/dys064. published online April 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolppanen AM, Fraser A, Fraser WD, Lawlor DA. Risk factors for variation in 25-Hydroxyvitamin D3 and D2 concentrations and vitamin D deficiency in children. J Clin Endocrinol Metab. 2012;97:1202–1210. doi: 10.1210/jc.2011-2516. [DOI] [PubMed] [Google Scholar]
- 15.Macdonald-Wallis C, Tobias JH, Davey Smith G, Lawlor DA. Relation of maternal prepregnancy body mass index with offspring bone mass in childhood: is there evidence for an intrauterine effect? Am J Clin Nutr. 2010;92:872–880. doi: 10.3945/ajcn.2010.29501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sayers A, Tobias JH. Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J Clin Endocrinol Metab. 2009;94:765–771. doi: 10.1210/jc.2008-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 18.Yu CKH, Ertl R, Samaha R, Akolekar R, Nicolaides KH. Normal range of maternal serum vitamin D at 11–13 weeks gestation. Fetal Diagn Ther. 2011;30:94–99. doi: 10.1159/000324340. [DOI] [PubMed] [Google Scholar]
- 19.Viljakainen HT, Saarnio E, Hytinantti T. Maternal vitamin D status determines bone variables in the newborn. J Clin Endocrinol Metab. 2010;95:1749–1757. doi: 10.1210/jc.2009-1391. [DOI] [PubMed] [Google Scholar]
- 20.Thomas SD, Fudge AN, Whiting M, Coates PS. The correlation between third-trimester maternal and newborn-serum 25-hydroxy-vitamin D in a selected South Australian group of newborn samples. BMJ Open. 2011;1:e000236. doi: 10.1136/bmjopen-2011-000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Professor Debbie Lawlor reports on the association of maternal vitamin D status in pregnancy with bone-mineral content in offspring, and discusses the implications for current clinical guidelines.


