Abstract
BACKGROUND AND OBJECTIVE:
Vesicoureteral reflux (VUR) is diagnosed in ∼30% to 40% of children who have imaging studies after urinary tract infections (UTIs). Our goal is to characterize children enrolled in the Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial and to compare our study cohort with those from previously published studies.
METHODS:
RIVUR investigators from 19 pediatric sites in the United States recruited 607 children with grade I through IV VUR. Children were enrolled after a first or second UTI. This cross-sectional report of baseline data includes extensive clinical, parental report, and imaging study results.
RESULTS:
RIVUR recruited 607 children (558 girls, 49 boys) with grade I (11%), II (42%), III (38%), or IV (8%) reflux. The median age was 12 months, and most children (91%) were enrolled after their first UTI. The UTI leading to enrollment was both febrile and symptomatic for 323 children, febrile only in 197 children, and symptomatic only in 86. Renal involvement at baseline as documented by a 99mTc dimercaptosuccinic acid scan was uncommon with cortical defects identified in 89 (15%) children. Bladder and bowel dysfunction was identified in 71 (56%) of 126 toilet-trained subjects assessed.
CONCLUSIONS:
RIVUR is the largest prospective, randomized trial for children with primary VUR to date, comparing prophylaxis with placebo. The study sample comprises patients from 19 pediatric clinical sites in the United States, whose demographic and clinical characteristics may differ from those of children enrolled in previous trials from other countries.
Keywords: vesicoureteral reflux (VUR), urinary tract infections, renal scarring, clinical research/trials
What’s Known on This Subject:
The ideal management of children with vesicoureteral reflux (VUR) remains a source of debate. There is little evidence to support many of the current management practices for children with VUR who have had 1 or 2 urinary tract infections.
What This Study Adds:
Baseline associations, including bladder and bowel dysfunction and imaging studies, from the largest randomized, controlled trial conducted to date aimed at assessing the value of antimicrobial prophylaxis in children with urinary tract infection and VUR are presented.
Approximately one-third of children who have a urinary tract infection (UTI) have vesicoureteral reflux (VUR), which is associated with a higher risk of renal scarring.1 However, VUR is neither necessary nor sufficient for renal damage to occur; scars are noted in children who do not have VUR, and many children with higher-grade VUR never develop scars. The Randomized Intervention for Children with Vesicoureteral Reflux (RIVUR) trial was designed to address limitations of previous studies, mostly the lack of a placebo or observation arm, and provide higher-quality evidence about the efficacy of antimicrobial prophylaxis for preventing recurrent UTI in children with VUR.2–4
Recent trials evaluated the efficacy of antimicrobial prophylaxis among children with UTI and VUR and showed either no or slight decrease in the incidence of recurrent UTI and renal scarring.5 Despite methodologic limitations of these trials addressed in recent editorials5,6 and the trials’ lack of evaluation of the impact of bladder and bowel dysfunction (BBD) on recurrent UTI, recent management guidelines have used these data to question the role of antimicrobial prophylaxis or even the need to obtain imaging studies to detect VUR in children after an initial episode of UTI.7–10
This report provides cross-sectional baseline data from the ongoing RIVUR trial at 19 clinical sites in the United States. We provide demographic and clinical characteristics of RIVUR participants as well as study design parameters that enable placement of our sample in context with previous US and international cohort studies of children with UTI and VUR.
Methods
Study Design
The RIVUR trial is a randomized, double-blind, placebo-controlled trial of prophylactic trimethoprim-sulfamethoxazole in children with VUR. The rationale and trial design have been described previously.2–4 Briefly, we enrolled infants and children aged 2 to 71 months with grade I through IV VUR after a first or second febrile or symptomatic UTI. Primary exclusion criteria included index UTI diagnosis >112 days before enrollment, comorbid urologic anomalies (Society of Fetal Ultrasonography grade 4 hydronephrosis with renal parenchyma atrophy, ureterocele, urethral valve, solitary kidney, profoundly small [>2 SD below the mean] kidney, multicystic dysplastic kidney, neurogenic bladder, pelvic kidney, or fused kidney), contraindications for use of trimethoprim-sulfamethoxazole, and selected other medical conditions. Children are followed for 2 years to ascertain recurrent febrile or symptomatic UTI, renal scarring, prophylaxis failure, antimicrobial resistance, medication adherence, and quality of life measures. A Data and Safety Monitoring Board and institutional review boards governing each site approved the protocol, and parents or legal guardians provided written informed consent for each participant before any study procedures.
Data Collection
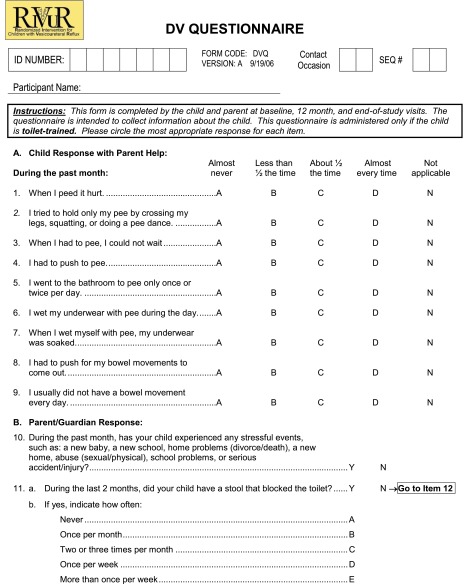
Screening and enrollment of children into the study took place from June 2007 through May 2011. Screening data are incomplete because the study did not begin data capture for screening until November 2007. Baseline data collection included medical history, physical examination, and a parent-administered quality-of-life questionnaire. In toilet-trained children, a parent-administered questionnaire that was based on a modified version of the scoring system reported by Farhat and colleagues11–13 (see Appendix 1) captured urination and bowel movement characteristics. Data were also collected on 5 of the 6 conditions that define chronic constipation according to the Paris Consensus on Childhood Constipation Terminology Group14 including frequency of bowel movements as <3 per week, >1 fecal incontinence event per week, passing of large stool that obstructed the toilet, retentive posture and behavior, and pain during defecation. Chronic constipation was defined by the presence of ≥2 conditions being met during the preceding 2 months.
Child’s race and ethnicity were self-reported by parents; mixed race is analyzed as nonwhite. The index UTI was the child’s first or second UTI that was used to qualify the child for study enrollment. To be eligible, the index UTI met stringent criteria4 including documented evidence of (1) pyuria on urinalysis, (2) culture-proven infection, and (3) fever (≥38°C) or symptoms within 24 hours of urine collection. We required a urine culture yielding a single organism that was neither lactobacillus nor candida, at ≥5 × 104 colony forming units per milliliter for catheterized or suprapubic aspiration urine specimens or ≥105 colony forming units per milliliter for clean voided specimens. Qualifying symptoms for the index UTI included suprapubic, abdominal, or flank pain or tenderness; urinary urgency, frequency or hesitancy; dysuria; and malodorous urine or in infants ≤4 months of age, failure to thrive, dehydration, or hypothermia. Blood pressure, height (or length), and weight were measured using available equipment and local procedures. Age is reported at the time of randomization.
Radiographic procedures included renal ultrasound, voiding cystourethrogram (VCUG), and 99mTc dimercaptosuccinic acid (DMSA) scintigraphy. Renal ultrasound and VCUG were completed within 112 days after the index UTI and before enrollment; most DMSA scans were obtained within 2 weeks of enrollment but no more than 112 days after the index UTI. Although eligibility was based on local reports for ultrasound and VCUG, images were also assessed by 2 pediatric radiologists using a standardized reading protocol.4,15 We report these data from the central radiologists. VUR was graded according to the system of the International Reflux Study Group.16 Worst VUR was defined as the highest grade of reflux17 from either the left or right side. Similarly, 2 pediatric nuclear medicine physicians centrally interpreted DMSA scans for the presence of cortical defects.4,18 Scans with decreased uptake of DMSA tracer were differentiated by (1) retention of renal contours (consistent with acute pyelonephritis) or (2) loss of contours or cortical thinning (consistent with renal scarring) as demonstrated by Majd and Rushton.19 The extent of cortical defects was assessed semiquantitatively by dividing the renal cortex into 12 segments and counting the number of renal parenchyma segments affected.4,18 Severity was determined by the number of segments affected: mild (1–2), moderate (3–4), or severe (≥5) or as global atrophy characterized by a diffusely scarred and shrunken kidney.
Statistical Analyses
We used Centers for Disease Control and Prevention growth standards for children ≥2 years of age and World Health Organization standards for children <2 years of age to convert height and weight measurements to z scores and percentiles.20–22 We calculated blood pressure percentiles for gender, age, and height for children ≥1 year of age using regression models specified in the fourth report from the National High Blood Pressure Education Program Working Group on Children and Adolescents.23 For children <1 year of age, we dichotomized blood pressure at the 90th percentile based on cut points from the Second Task Force.24 VUR and renal cortical defects are reported if present on either kidney, with severity reported as the worst of both kidneys. The presence of BBD was defined by a score of ≥6 for females and ≥9 for males, using the scoring weights from Farhat and colleagues.11 BBD and chronic constipation were evaluated only for children who were toilet-trained for both urine and bowel movements.
With the exception of age, for which a Kruskal-Wallis test was performed due to the skewed distribution, comparison of continuous characteristics by VUR grade was by analysis of variance; χ2 test was used for comparisons of categorical variables unless an expected cell count of <5 was present in which case Fisher’s exact test was used. SAS version 9.2 software (SAS Institute, Cary, NC) was used for all statistical analyses.
Results
Screening data are incomplete because of delayed onset of screening data capture but include 492 (81%) of the 607 enrolled participants. Of 10 753 children with screening data available, only 12% met all eligibility criteria. Almost 60% of screened children did not meet study criteria for a first or second UTI; more than half of the screened children either did not have VCUG results (24%) or did not have VUR (30%) or had grade V VUR (1%); 14% failed other eligibility criteria. Of the 1311 eligible screened children, 62% refused and 38% were enrolled. Those eligible but not enrolled did not differ from those enrolled in the distributions of gender, race, or ethnicity (data not shown).
The 19 clinical sites (Table 1) enrolled 558 girls and 49 boys (18 or 37%, circumcised). Most had grade II (42%) or grade III (38%) VUR; 11% had grade I (including 7 children with no VUR based on the central assessment) and 8% had grade IV (including 1 child with grade V based on the central assessment). The distribution of VUR grade by gender is provided in Table 2. VCUGs were not available for central assessment for 5 children. Approximately half of the children had unilateral VUR and half had bilateral VUR. Demographic and clinical characteristics of participants in the RIVUR study, overall and by VUR grade, are summarized in Table 3. Children were aged 2 to 71 months, with a median age of 12 months. White race predominated (82% of girls and 65% of boys); 40 (7%) children were mixed race, 27 (5%) were African American, and 48 (8%) reported other races. Race was not recorded for 10 children. Seventy-seven children (13%) were of Hispanic ethnicity.
TABLE 1.
RIVUR Clinical Sites
| Hospital | City, State | Principal Investigator | Recruitment Sites | Number Randomized | ||
|---|---|---|---|---|---|---|
| Female | Male | Overall | ||||
| Akron Children’s Hospital | Akron, OH | Dan McMahon, MD | PCP, ED, UP, NP, IS | 14 | 0 | 14 |
| Johns Hopkins School of Medicine | Baltimore, MD | Ranjiv Mathews, MD | PCP, UP, NP, IS | 49 | 7 | 56 |
| University of Alabama Birmingham | Birmingham, AL | Sahar Fathallah, MD | PCP, UP, NP | 3 | 1 | 4 |
| Children’s Hospital of Boston | Boston, MA | Caleb Nelson, MD | PCP, ED, UP, NP | 25 | 5 | 30 |
| Women and Children’s Hospital of Buffalo | Buffalo, NY | Saul Greenfield, MD | PCP, ED, UP, IS | 46 | 2 | 48 |
| Ann & Robert H. Lurie Children’s Hospital of Chicago | Chicago, IL | Earl Cheng, MD | PCP, ED, UP, NP, IS | 13 | 2 | 15 |
| Cincinnati Children’s Hospital | Cincinnati, OH | William R. DeFoor, Jr., MD | PCP, UP, IS | 9 | 0 | 9 |
| Children’s Hospital of Michigan | Detroit, MI | Tej Mattoo, MD | IS | 57 | 3 | 60 |
| Penn State Hershey Medical Center | Hershey, PA | Ross Decter, MD | PCP, ED, UP, NP, IS | 2 | 0 | 2 |
| Texas Children’s Hospital | Houston, TX | Eileen Brewer, MD | UP, NP | 7 | 2 | 9 |
| Children’s Mercy Hospital | Kansas City, MO | Mary Ann Queen, MD | UP, NP, IS | 6 | 0 | 6 |
| University of Wisconsin Children’s Hospital | Madison, WI | Sharon Bartosh, MD | PCP, ED, UP, NP, IS | 2 | 0 | 2 |
| University of Oklahoma | Oklahoma City, OK | Brad Kropp, MD | UP | 36 | 1 | 37 |
| Children’s Hospital of Philadelphia | Philadelphia, PA | Ron Keren, MD | PCP, ED, UP, IS | 60 | 6 | 66 |
| Children’s Hospital of Pittsburgh | Pittsburgh, PA | Alejandro Hoberman, MD | PCP, ED, UP, IS | 146 | 8 | 154 |
| Oregon Health & Science University | Portland, OR | Steven Skoog, MD | PCP, ED, UP, IS | 28 | 5 | 33 |
| Children’s National Medical Center | Washington, DC | H. Gil Rushton, MD | PCP, ED, UP, NP, IS | 35 | 5 | 40 |
| Alfred I. duPont Hospital for Children | Wilmington, DE | Amy Renwick, MD | PCP, UP, IS | 7 | 1 | 8 |
| Wake Forest University Baptist Medical Center | Winston-Salem, NC | Milan Nadkarni, MD | PCP, ED, UP, NP, IS | 13 | 1 | 14 |
ED, emergency department; IP, inpatient service; NP, nephrology practice; PCP, primary care practice; UP, urology practice.
TABLE 2.
Distribution of VUR Grade by Gender, n (%)
| Worst VUR Gradea | Girls | Boys | Overall |
|---|---|---|---|
| I | 65 (12) | 3 (6) | 68 (11) |
| II | 232 (42) | 22 (45) | 254 (42) |
| III | 212 (38) | 18 (37) | 230 (38) |
| IV | 44 (8) | 6 (12) | 50 (8) |
Seven children with a central assessment of ‘no VUR’ are included in grade I and one child with a central assessment of grade V is included in grade IV.
TABLE 3.
Demographic and Clinical Characteristics of RIVUR Participants
| Characteristics | Overall (n = 607) | VUR Grade I (n = 68) | VUR Grade II (n = 254) | VUR Grade III (n = 230) | VUR Grade IV (n = 50) | Pa |
|---|---|---|---|---|---|---|
| Age in mo, median (IQR) | 12 (6–31) | 17 (11–44) | 12.5 (6–32) | 11 (5–27) | 8 (4–18) | <.001 |
| Female gender | 558 (92%) | 65 (96%) | 232 (91%) | 212 (92%) | 44 (88%) | NS |
| Nonwhite race | 115 (19%) | 14 (21%) | 51 (20%) | 45 (20%) | 4 (9%) | NS |
| Height-for-age percentile | 56 ± 31.6 | 60 ± 30.1 | 54 ± 31.4 | 54 ± 32.5 | 62 ± 31.5 | NS |
| Weight-for-age percentile | 61 ± 28.0 | 65 ± 27.1 | 62 ± 27.5 | 59 ± 29.0 | 63 ± 26.4 | NS |
| Blood pressure ≥90th percentile | ||||||
| Systolic | 155 (31%) | 14 (26%) | 61 (29%) | 64 (34%) | 14 (34%) | NS |
| Diastolic | 186 (38%) | 22 (41%) | 81 (39%) | 69 (38%) | 13 (32%) | NS |
| Hematocrit (%) | 35 ± 3.0 | 36 ± 2.4 | 35 ± 3.1 | 35 ± 3.1 | 33 ±3 .0 | .001 |
| Platelet count (× 10⁹/L) | 385 ± 121.6 | 371 ± 131.0 | 382 ± 108.1 | 384 ± 117.7 | 424 ± 182.6 | NS |
| Toilet trained for urine and bowel | 134 (22%) | 22 (33%) | 62 (25%) | 39 (17%) | 11 (22%) | .032 |
| BBDb | 71 (56%) | 10 (50%) | 34 (57%) | 23 (64%) | 4 (40%) | NS |
| Decreased 99mTc DMSA uptake with loss of renal contours or cortical thinningc | 21 (4%) | 2 (3%) | 4 (2%) | 9 (4%) | 6 (13%) | .008 |
| Decreased 99mTc DMSA uptake with retention of renal contoursc | 71 (12%) | 6 (9%) | 31 (12%) | 24 (10%) | 10 (20%) | NS |
| Ureter duplication | 26 (4%) | 0 (0%) | 9 (4%) | 11 (5%) | 6 (12%) | .017 |
| Hydronephrosisd | 32 (5%) | 4 (6%) | 5 (2%) | 12 (5%) | 11 (22%) | <.001 |
Statistics are reported as number (%) or mean ± SD unless otherwise indicated; the VCUGs for 5 participants were not available for centralized evaluation. IQR, interquartile range; NS, not significant.
NS P > .10; tests for association between characteristic and VUR grade based on Kruskal-Wallis test for age, analysis of variance for other continuous variables, and χ2 or Fisher’s exact test for categorical variables.
Assessed among 126 toilet-trained children; based on modification of the Dysfunctional Voiding Scoring System.11
Worst of left and right kidney; decreased uptake with loss of renal contour or cortical thinning is consistent with renal scarring; decreased update with retention of renal contours is consistent with acute pyelonephritis.
Hydronephrosis less than Society of Fetal Ultrasonography grade 4.
Of the 607 children enrolled, 554 (91%) were recruited after their first UTI; 53 (9%) had a second UTI before enrollment. The index UTI was febrile and symptomatic in 323 (53%) of the children, febrile only in 197 (32%), symptomatic only in 86 (14%), and with fever and symptoms outside of the 24-hour window of urine collection for 1 child. Escherichia coli was the predominant infective organism in 538 (89%) index UTIs with Klebsiella pneumoniae (n = 16, 3%) and Proteus mirabilis (n = 14, 2%) the next most frequent. Children with index UTIs from non–E coli infections were more likely to have a higher grade of VUR (14 of 66 [21%] vs 35 of 535 [7%] had grade IV VUR, P = .0003), a nonfebrile index UTI (16 of 68 [24%] vs 71 of 538 [13%], P = .02), and a higher proportion of loss of renal contour (5 of 61 [8%] vs 16 of 520 [3%], P = .06) than children with E coli infections.
Twenty-one (4%) of the 582 children with DMSA scan results had decreased tracer uptake with loss of renal contour (scarring) at baseline (Table 4) with severity classified as mild (n = 1), moderate (n = 5), severe (n = 6), or global atrophy (n = 9). Loss of renal contour was noted in 17 of 535 (3%) girls and in 4 of 47 boys (9%) assessed. Decreased tracer uptake in conjunction with retention of renal contour (acute pyelonephritis) was identified in 71 (12%) children, including 3 children who also had loss of contour. In 100 children, the DMSA scan was performed within 30 days of the index UTI; timing of the scan was not statistically associated with the index UTI being febrile, but there was a nonsignificant higher proportion of loss of renal contour present in scans performed ≥31 days after the index UTI (20 of 21 children with loss of renal contour had the DMSA scan ≥1 month after the index UTI, P = .23). Twenty-seven percent of children with decreased uptake associated with no loss of renal contour underwent the DMSA scan within 30 days of the index UTI diagnosis. Compared with children with normal DMSA scans, more of those having any cortical defect were aged ≥12 months at enrollment (62% vs 49%, P = .03), had a febrile index UTI (93% vs 84%, P = .02), had a UTI before the index UTI (16% vs 7%, P = .007), and had grade IV VUR (17% vs 7%, P = .02; Cochran-Armitage trend test P = .03).
TABLE 4.
Participant Characteristics by DMSA Scan Results
| Characteristics | Decreased Uptake With Loss of Contoursa (n = 21) | Decreased Uptake With Contour Retentiona (n = 71) | Any Cortical Defect | ||
|---|---|---|---|---|---|
| Yes (n = 89) | No (n = 493) | Pb | |||
| DMSA timingc | |||||
| <31 d | 1 (5%) | 19 (27%) | 20 (22%) | 78 (16%) | NS |
| ≥31 d | 20 (95%) | 52 (73%) | 69 (78%) | 415 (84%) | |
| Age (mo) | |||||
| <12 | 8 (38%) | 27 (38%) | 34 (38%) | 250 (51%) | 0.030 |
| ≥ 12 | 13 (62%) | 44 (62%) | 55 (62%) | 243 (49%) | |
| Gender | |||||
| Females | 17 (81%) | 69 (97%) | 84 (94%) | 451 (91%) | NS |
| Males | 4 (19%) | 2 (3%) | 5 (6%) | 42 (9%) | |
| Index UTI | |||||
| Febrile | 20 (95%) | 66 (93%) | 83 (93%) | 415 (84%) | 0.025 |
| Not febrile | 1 (5%) | 5 (7%) | 6 (7%) | 78 (16%) | |
| Previous UTI | |||||
| None | 17 (81%) | 61 (86%) | 75 (84%) | 458 (93%) | 0.007 |
| 1 | 4 (19%) | 10 (14%) | 14 (16%) | 35 (7%) | |
| VUR grade | |||||
| I | 2 (10%) | 6 (8%) | 8 (9%) | 55 (11%) | 0.017 |
| II | 4 (19%) | 31 (44%) | 34 (38%) | 211 (43%) | |
| III | 9 (43%) | 24 (34%) | 32 (36%) | 189 (39%) | |
| IV | 6 (29%) | 10 (14%) | 15 (17%) | 33 (7%) | |
NS, not significant.
Includes 3 children who had decreased DMSA uptake with both loss of contour or cortical thinning (consistent with renal scarring) and retention of contour (consistent with acute pyelonephritis) based on DMSA scan.
NS: P value > .10; tests for association between characteristic and any cortical defect based on χ2 or Fisher’s exact test.
Number of days between the Index UTI diagnosis and DMSA scan.
BBD was evident in 71 (56%) of 126 toilet-trained children, generally reflecting a mixture of both bladder and bowel symptoms; the assessment was not completed for 8 toilet-trained children. All 71 children with BBD were girls (only 2 boys were both toilet-trained and completed the BBD assessment), and BBD was not associated with race, ethnicity, VUR grade, or presence of any renal cortical defect in this small subgroup. Sixteen toilet-trained children met criteria for chronic constipation, of whom 14 also met criteria for BBD.
Boys were significantly younger than girls at baseline; 84% of males vs 46% of females were <12 months of age (P < .0001). Thirteen of 41 boys <1 year of age were circumcised (32%) compared with 5 (63%) of 8 older boys. Of 17 Hispanic boys, 14 were <1 year of age and uncircumcised; of the 3 older Hispanic boys, 1 was circumcised. Six boys had grade IV VUR, of whom 2 were circumcised. Among the male children, 71% of index UTIs were E coli, and 71% of these boys were uncircumcised compared with 43% of those with non–E coli infections.
Table 5 profiles children enrolled in RIVUR relative to cohort studies of VUR in children following UTI that were considered in the American Academy of Pediatrics technical report.25 Although design and patient characteristics vary across studies, children with intermediate grades of VUR (ie, grades II and III) predominate.
TABLE 5.
Selected Studies of VUR and UTI in Children
| Study: first author (year) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RIVUR | Camacho (2004)a | Chand (2003)b | Craig (1997)c | Fernandez-Menendez (2003)d | Hansson (2004)e | Mahant (2002)f | McDonald (2000)g | Oostenbrink (2000)h | Pinto (2004)i | Sargent (1995)j | Zamir (2004)k | |
| Country | USA | Spain | USA | Australia | Spain | Sweden | Canada | USA | Netherlands | USA | Canada | Israel |
| Recruitment setting | Urology, nephr, PC, ED, inpatient, radiology | Radiology | ED | ED | ED | Inpatient | Inpatient | PC, ED | Radiology | Radiology, urology, nephr, PC | Inpatient | |
| N | 607 | 152 | 15 504 | 272 | 158 | 303 | 162 | 139 | 140 | 141 | 309 | 255 |
| No. previous UTIs | 0 or 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||
| No. with VUR | 602 | 32 | 3771 | 77 | 33 | 80 | 35 | 33 | 37 | 38 | 91 | 45 |
| Grade I | 68 | 461 | 14 | 13 | 2 | 1 | 2 | 13 | ||||
| Grade II | 254 | 2284 | 28 | 31 | 20 | 9 | 13 | 18 | ||||
| Grade III | 230 | 747 | 19 | 27 | 6 | 16 | 14 | 13 | ||||
| Grade IV | 49 | 182 | 12 | 8 | 7 | 5 | 5 | 1 | ||||
| Grade V | 1 | 97 | 4 | 1 | 0 | 2 | 4 | |||||
| Age limits | 2 mo to <6 y | 1 mo to 12 y | 0 to 21 y | <5 y | 1 mo to 14 y | <2 y | <5 y | <10 y | 1 mo to 12 y | |||
| Age, median | 12 mo | 8.3 mo | 85 d | |||||||||
| Age, mean | 19.6 mo | 20 mo | ||||||||||
| Male | 49 | 608 | 149 | 64 | 163 | 91 | 47 | 117 | 63 | |||
| Female | 558 | 3163 | 123 | 94 | 140 | 71 | 94 | 192 | 192 | |||
| Median age boys | 5 mo | 3.1 m | ||||||||||
| Median age girls | 12 mo | 8.5 m | ||||||||||
| White | 482 | 3520 | ||||||||||
| Black | 27 | 141 | ||||||||||
| Asian | 17 | |||||||||||
| Other | 81 | 110 | ||||||||||
| Hispanic | 77 | 141 | ||||||||||
| Non-Hispanic | 527 | 0 | ||||||||||
ED, emergency department; nephr, nephrology; PC, primary care.
Prospective cohort study of children with first febrile UTI.49
Retrospective review of VCUG or radionuclide cystogram.28
Cross-sectional study of children with first UTI.50
Prospective cohort study of children with first UTI.51
Retrospective cross-sectional study of children with first UTI.52
Retrospective chart review of children with VCUG after UTI.54
Cross-sectional study of children with first UTI.55
Retrospective chart review of first VCUG for UTI.56
Retrospective study of first VCUG for UTI.57
Prospective cohort study of children hospitalized with first UTI.58
Discussion
The distribution of RIVUR study participants by gender and within racial and ethnic groups is consistent with previous studies in the United States. In American cohorts of children with VUR identified after UTI, boys represent a minority,26,27 as they do in VCUG-based studies.28,29 This was also reflected in the RIVUR cohort. When race is reported in studies of VUR in the United States, white race predominates.28–33 Approximately 80% of children in the RIVUR study were white and 5% were African American. Thirteen percent of participants self-reported as being of Hispanic ethnicity. Low rates of neonatal circumcision among Hispanics may account for uncircumcised Hispanic males representing approximately one-third of all male subjects in the RIVUR study.30,34 More than 90% of children with VUR in 2 previous American series had grade III or less reflux.26,27 Similarly, in the RIVUR cohort, 92% of children had grades III or less, the majority being grade II (42%) or grade III (38%). Grade IV accounted for only 8% of children. While children with Grade V VUR were excluded in the RIVUR study, this grade of VUR is seen in a small fraction of children that have VUR identified after a UTI.35,36 More than 1 in 5 children in the RIVUR study were toilet trained, and >50% of these children exhibited BBD documented using the Farhat instrument11 modified for US vernacular. More children in the trial will experience BBD during the 2-year observation period. This is a particularly interesting subgroup of children, given the known increased likelihood of breakthrough UTIs in children with BBD receiving antimicrobial prophylaxis.37,38
An established renal scar as defined by decreased uptake of tracer in conjunction with loss of renal contour or cortical thinning at baseline was infrequent in the RIVUR cohort (4%), a proportion substantially lower than previously reported.39,40 Possible explanations include that participants had experienced only 1 or 2 UTIs, the acceptance of children into the trial after a nonfebrile (although symptomatic) UTI, a relatively low proportion of children with Grade IV VUR, scans were obtained relatively close to the time of the index UTI so defects consistent with scars may not have had sufficient time to become visible, and adjudication review by 2 pediatric nuclear medicine specialists. Scarring characterized by decreased tracer uptake with loss of cortical margin can be due to either an episode of acute pyelonephritis more than a few weeks before scanning or congenital dysplasia. Decreased uptake of tracer with retention of renal contours, consistent with acute pyelonephritis, was more common (12%), and most were after febrile index UTIs. Interestingly, more than half of those with decreased uptake and contour retention had grades I and II VUR. A number of those patients with acute pyelonephritis will be left with permanent scars. Renal scarring represents a secondary outcome of the RIVUR study, and it remains to be seen how many additional scars will be identified at completion of the 2-year observation period, but baseline presence of any cortical defect is consistent with the more recent studies of children after first UTI.41
Consistent with previously reported results of renal ultrasound performed in children after UTI,1 most were interpreted as normal; hydronephrosis was rarely identified (5%), and in most instances (23/32, or 72%), it was associated with dilated VUR (grades III and IV). Conversely, and as indicated by others,42–44 ultrasonography was not particularly useful in identifying dilated VUR. Among children with grade III or IV VUR, hydronephrosis was diagnosed in only 5% and 22% of children, respectively, a low sensitivity consistent with previous reports.1 This is particularly relevant given recently published UTI guidelines7–10,25 that generally recommend not performing a VCUG after an initial febrile UTI unless ultrasonographic findings are abnormal or other risk factors are present.
Although there appears to be reasonably high agreement between pediatric radiologists in the grading of VUR,15 some discrepancy is expected. RIVUR eligibility was based on VUR grade assigned at the enrollment site (local reading), whereas the results being reported herein are from the standardized and adjudicated grades determined by 2 RIVUR radiologists (central reading). Differences in interpretation of the scans are evident as our data include 7 children with no VUR and 1 child with grade V VUR based on the central assessment but who were considered to have VUR grade I through IV based on the local report.
In recently published series of children with VUR from Europe and Australia, boys with VUR accounted for 31% to 50% of participants, almost all of whom were uncircumcised.39,45–48 In contrast, 92% of subjects in the RIVUR study were girls. Of all subjects enrolled, ∼50% were aged <1 year, particularly male subjects (84%), a third of whom were circumcised. A more routine practice of circumcision may explain the relative paucity of boys presenting with febrile UTI and VUR in the US population.
E coli was the most frequently isolated organism from the index UTI. According to some, non–E coli UTIs have been associated with more clinically significant VUR.10 Our data suggest that there may be a correlation between non–E coli index UTI and higher reflux grade and a higher proportion of children with loss of renal contour, despite a lower rate of febrile index UTI.
Retrospective or prospective studies of children with primary VUR all differ in the demographics of the population studied. They reflect a variety of study designs with differences in inclusion and exclusion criteria (eg, number of previous UTIs and grade of VUR) and with differences in numbers of patients, patient age, health care delivery systems, recruitment settings, sociocultural characteristics (including those driven by geographic location), and race and ethnic distributions that can influence the results obtained, reducing the ability to generalize to all populations. These venue and demographic differences may explain disparate outcomes across studies. Although screening data are limited and selection bias can never be ruled out completely, the RIVUR trial enrolled a large number of children from a variety of clinical settings reflecting the medical and cultural milieu in the United States from 2007 to 2011.
Conclusions
The evaluation and management of children with UTI and VUR remains controversial and unsettled. The RIVUR study is the largest prospective, randomized trial for children with primary VUR comparing prophylaxis with placebo to date. It provides a large patient population from the United States, the demographic composition of which may differ from those reported elsewhere. In addition, because of the use of adjudicated radiographic interpretations, stringent definition of UTI, consistent assessment of BBD, and close clinical monitoring, it is anticipated that the outcomes will enable improved evidence-based evaluation and treatment algorithms.
Acknowledgments
We thank the RIVUR participants, their families and participating physicians, investigators, and staffs for making this research possible. Principal investigators of the RIVUR clinical sites are Sharon M. Bartosh, MD (University of Wisconsin Hospital and Clinics, Madison, WI); Eileen Brewer, MD (Texas Children’s Hospital, Houston, TX); Earl Y. Cheng, MD (Ann & Robert H. Lurie Children’s Hospital, Chicago, IL); Ross Decter, MD (Penn State Milton S. Hershey Medical Center, Hershey, PA); William Robert DeFoor, Jr, MD, MPH (Cincinnati Children’s Hospital, Cincinnati, OH); Sahar Fathallah-Shaykh, MD (University of Alabama at Birmingham School of Medicine, Birmingham, AL); Saul P. Greenfield, MD (Women and Children’s Hospital of Buffalo, Buffalo, NY); Alejandro Hoberman, MD (Children’s Hospital of Pittsburgh, Pittsburgh, PA); Ron Keren, MD, MPH (Children’s Hospital of Philadelphia, Philadelphia, PA); Bradley P. Kropp, MD, FAAP, FACS (University of Oklahoma Health Sciences Center, Oklahoma City, OK); Ranjiv Mathews, MD (Johns Hopkins School of Medicine, Baltimore, MD); Tej K. Mattoo, MD, DCH, FRCP (UK) (Wayne State University School of Medicine, Detroit, MI); Daniel R. McMahon, MD (Akron Children’s Hospital, Akron, OH); Milan Nadkarni, MD (Wake Forest University Baptist Medical Center, Winston-Salem, NC); Caleb P. Nelson, MD, MPH (Boston Children’s Hospital, Boston, MA); Mary Ann Queen, MD (Children’s Mercy Hospital of Kansas City, Kansas City, MO); Amy Renwick, MD (Alfred I. duPont Hospital for Children, Wilmington, DE); H. Gil Rushton, Jr., MD, FAAP (Children’s National Medical Center, Washington, DC); Steven J. Skoog, MD, FACS, FAAP (Oregon Health and Science University, Portland, OR); Ming-Hsien Wang, MD (Johns Hopkins School of Medicine, Baltimore, MD).
Glossary
- BBD
bladder and bowel dysfunction
- DMSA
99mTc dimercaptosuccinic acid
- RIVUR
Randomized Intervention for Children with Vesicoureteral Reflux
- UTI
urinary tract infection
- VCUG
voiding cystourethrogram
- VUR
vesicoureteral reflux
Appendix Dysfunctional Voiding (DV) Questionnaire
Note: Items 1–10 on the questionnaire are used in the evaluation of BBD.
Footnotes
Dr Carpenter directs the RIVUR Data Coordinating Center and carried out the statistical analyses, participated in conceptualization and design of the study, and drafted the initial abstract and Methods and Results sections; Dr Hoberman is a core clinical site investigator and was responsible for enrolling study participants, participated in conceptualization and design of the study, and drafted the initial Introduction section; Drs Mattoo and Mathews are core clinical site investigators and were responsible for enrolling study participants, and participated in conceptualization and design of the study; Dr Keren is a core clinical site investigator and was responsible for enrolling study participants, participated in the conceptualization and design of the study, and prepared the initial draft of Table 4; Dr Chesney is chair of the Steering Committee and he participated in the conceptualization and design of the study; Dr Moxey-Mims conceptualized and participated in the design of the study; Dr Greenfield is a core clinical site investigator and was responsible for enrolling study participants, drafted the initial Discussion and Conclusions sections, and participated in the conceptualization and design of the study; and all authors reviewed and revised the manuscript, and approved the final manuscript as submitted.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00405704).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by grants U01 DK074059, U01 DK074053, U01 DK074082, U01 DK074064, U01 DK074062, and U01 DK074063 from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services. The trial was also supported by the Children’s Hospital of Philadelphia Clinical and Translational Science Award (grant UL1TR000003) from the National Center for Research Resources, now at the National Center for Advancing Translational Sciences, National Institutes of Health. Funded by the National Institutes of Health (NIH).
References
- 1.Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348(3):195–202 [DOI] [PubMed] [Google Scholar]
- 2.Chesney RW, Carpenter MA, Moxey-Mims M, et al. members of the RIVUR Steering Committee . Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR): background commentary of RIVUR investigators. Pediatrics. 2008;122(suppl 5):S233–S239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keren R. Pediatrics. RIVUR trial. Introduction. Pediatrics. 2008;122(suppl 5):S231–S232 [DOI] [PubMed] [Google Scholar]
- 4.Keren R, Carpenter MA, Hoberman A, et al. Rationale and design issues of the Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR) study. Pediatrics. 2008;122(suppl 5):S240–S250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoberman A, Keren R. Antimicrobial prophylaxis for urinary tract infection in children. N Engl J Med. 2009;361(18):1804–1806 [DOI] [PubMed] [Google Scholar]
- 6.Wan J, Skoog SJ, Hulbert WC, et al. Executive Committee, Section on Urology, American Academy of Pediatrics . Section on Urology response to new Guidelines for the Diagnosis and Management of UTI. Pediatrics. 2012;129(4). Available at: www.pediatrics.org/cgi/content/full/129/4/e1051 [DOI] [PubMed] [Google Scholar]
- 7.Ammenti A, Cataldi L, Chimenz R, et al. Italian Society of Pediatric Nephrology . Febrile urinary tract infections in young children: recommendations for the diagnosis, treatment and follow-up. Acta Paediatr. 2012;101(5):451–457 [DOI] [PubMed] [Google Scholar]
- 8.Riccabona M, Avni FE, Blickman JG, et al. Imaging recommendations in paediatric uroradiology: minutes of the ESPR workgroup session on urinary tract infection, fetal hydronephrosis, urinary tract ultrasonography and voiding cystourethrography, Barcelona, Spain, June 2007. Pediatr Radiol. 2008;38(2):138–145 [DOI] [PubMed] [Google Scholar]
- 9.Roberts KB, Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610 [DOI] [PubMed] [Google Scholar]
- 10.Mori R, Lakhanpaul M, Verrier-Jones K. Diagnosis and management of urinary tract infection in children: summary of NICE guidance. BMJ. 2007;335(7616):395–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhat W, Bägli DJ, Capolicchio G, et al. The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol. 2000;164(3 pt 2):1011–1015 [DOI] [PubMed] [Google Scholar]
- 12.Farhat W, McLorie GA, O’Reilly S, Khoury A, Bägli DJ. Reliability of the pediatric dysfunctional voiding symptom score in monitoring response to behavioral modification. Can J Urol. 2001;8(6):1401–1405 [PubMed] [Google Scholar]
- 13.Shaikh N, Hoberman A, Wise B, et al. Dysfunctional elimination syndrome: is it related to urinary tract infection or vesicoureteral reflux diagnosed early in life? Pediatrics. 2003;112(5):1134–1137 [DOI] [PubMed] [Google Scholar]
- 14.Benninga M, Candy DC, Catto-Smith AG, et al. The Paris Consensus on Childhood Constipation Terminology (PACCT) Group . The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. J Pediatr Gastroenterol Nutr. 2005;40(3):273–275 [DOI] [PubMed] [Google Scholar]
- 15.Greenfield SP, Carpenter MA, Chesney RW, Zerin JM, Chow J. The RIVUR voiding cystourethrogram pilot study: experience with radiologic reading concordance. J Urol. 2012;188(4 suppl):1608–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981;67(3):392–400 [PubMed] [Google Scholar]
- 17.Lebowitz RL, Olbing H, Parkkulainen KV, Smellie JM, Tamminen-Möbius TE, International Reflux Study in Children . International system of radiographic grading of vesicoureteric reflux. Pediatr Radiol. 1985;15(2):105–109 [DOI] [PubMed] [Google Scholar]
- 18.Ziessman HA, Majd M. Importance of methodology on (99m)technetium dimercapto-succinic acid scintigraphic image quality: imaging pilot study for RIVUR (Randomized Intervention for Children With Vesicoureteral Reflux) multicenter investigation. J Urol. 2009;182(1):272–279 [DOI] [PubMed] [Google Scholar]
- 19.Majd M, Rushton HG. Renal cortical scintigraphy in the diagnosis of acute pyelonephritis. Semin Nucl Med. 1992;22(2):98–111 [DOI] [PubMed] [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group . WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85 [DOI] [PubMed] [Google Scholar]
- 21.Grummer-Strawn LM, Reinold CM, Krebs NF, National Center for Chronic Disease Prevention and Health Promotion. Use of World Health Organization and CDC Growth Charts for Children Aged 0–59 Months in the United States. Atlanta, GA: Deptartment of Health and Human Services, Centers for Disease Control and Prevention; 2010 [Google Scholar]
- 22.World Health Organization. WHO Anthro (version 3.2.2, January 2011) and macros. Available at: www.who.int/childgrowth/software/en. Accessed September 2, 2012
- 23.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 suppl 4th report):555–576 [PubMed]
- 24.Report of the Second Task Force on Blood Pressure Control in Children—1987. Task Force on Blood Pressure Control in Children. National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79(1):1–25 [PubMed] [Google Scholar]
- 25.Finnell SM, Carroll AE, Downs SM, Subcommittee on Urinary Tract Infection . Technical report—diagnosis and management of an initial UTI in febrile infants and young children. Pediatrics. 2011;128(3). Available at: www.pediatrics.org/cgi/content/full/128/3/e749 [DOI] [PubMed] [Google Scholar]
- 26.Greenfield SP, Ng M, Wan J. Experience with vesicoureteral reflux in children: clinical characteristics. J Urol. 1997;158(2):574–577 [PubMed] [Google Scholar]
- 27.Skoog SJ, Belman AB, Majd M. A nonsurgical approach to the management of primary vesicoureteral reflux. J Urol. 1987;138(4 pt 2):941–946 [DOI] [PubMed] [Google Scholar]
- 28.Chand DH, Rhoades T, Poe SA, Kraus S, Strife CF. Incidence and severity of vesicoureteral reflux in children related to age, gender, race and diagnosis. J Urol. 2003;170(4 pt 2):1548–1550 [DOI] [PubMed] [Google Scholar]
- 29.Melhem RE, Harpen MD. Ethnic factors in the variability of primary vesico-ureteral reflux with age. Pediatr Radiol. 1997;27(9):750–751 [DOI] [PubMed] [Google Scholar]
- 30.Arant BS, Jr. Medical management of mild and moderate vesicoureteral reflux: followup studies of infants and young children. A preliminary report of the Southwest Pediatric Nephrology Study Group. J Urol. 1992;148(5 Pt 2):1683–1687 [DOI] [PubMed] [Google Scholar]
- 31.Askari A, Belman AB. Vesicoureteral reflux in black girls. J Urol. 1982;127(4):747–748 [DOI] [PubMed] [Google Scholar]
- 32.Horowitz M, Gershbein AB, Glassberg KI. Vesicoureteral reflux in infants with prenatal hydronephrosis confirmed at birth: racial differences. J Urol. 1999;161(1):248–250 [PubMed] [Google Scholar]
- 33.Skoog SJ, Belman AB. Primary vesicoureteral reflux in the black child. Pediatrics. 1991;87(4):538–543 [PubMed] [Google Scholar]
- 34.West W, Venugopal S. The low frequency of reflux in Jamaican children. Pediatr Radiol. 1993;23(8):591–593 [DOI] [PubMed] [Google Scholar]
- 35.Anderson PA, Rickwood AM. Features of primary vesicoureteric reflux detected by prenatal sonography. Br J Urol. 1991;67(3):267–271 [DOI] [PubMed] [Google Scholar]
- 36.Oliveira EA, Diniz JS, Silva JM, Rabelo EA, Pontes AK, Souza MF. Features of primary vesicoureteric reflux detected by investigation of foetal hydronephrosis. Int Urol Nephrol. 1998;30(5):535–541 [DOI] [PubMed] [Google Scholar]
- 37.Koff SA, Wagner TT, Jayanthi VR. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J Urol. 1998;160(3 Pt 2):1019–1022 [DOI] [PubMed] [Google Scholar]
- 38.Snodgrass W. The impact of treated dysfunctional voiding on the nonsurgical management of vesicoureteral reflux. J Urol. 1998;160(5):1823–1825 [PubMed] [Google Scholar]
- 39.Brandström P, Esbjörner E, Herthelius M, Swerkersson S, Jodal U, Hansson S. The Swedish reflux trial in children: III. Urinary tract infection pattern. J Urol. 2010;184(1):286–291 [DOI] [PubMed] [Google Scholar]
- 40.Olbing H, Claësson I, Ebel KD, et al. Renal scars and parenchymal thinning in children with vesicoureteral reflux: a 5-year report of the International Reflux Study in Children (European branch). J Urol. 1992;148(5 Pt 2):1653–1656 [DOI] [PubMed] [Google Scholar]
- 41.Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics. 2010;126(6):1084–1091 [DOI] [PubMed] [Google Scholar]
- 42.Blane CE, DiPietro MA, Zerin JM, Sedman AB, Bloom DA. Renal sonography is not a reliable screening examination for vesicoureteral reflux. J Urol. 1993;150(2 Pt 2):752–755 [DOI] [PubMed] [Google Scholar]
- 43.DiPietro MA, Blane CE, Zerin JM. Vesicoureteral reflux in older children: concordance of US and voiding cystourethrographic findings. Radiology. 1997;205(3):821–822 [DOI] [PubMed] [Google Scholar]
- 44.Mahant S, Friedman J, MacArthur C. Renal ultrasound findings and vesicoureteral reflux in children hospitalised with urinary tract infection. Arch Dis Child. 2002;86(6):419–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Craig JC, Simpson JM, Williams GJ, et al. Prevention of Recurrent Urinary Tract Infection in Children with Vesicoureteric Reflux and Normal Renal Tracts (PRIVENT) Investigators . Antibiotic prophylaxis and recurrent urinary tract infection in children. N Engl J Med. 2009;361(18):1748–1759 [DOI] [PubMed] [Google Scholar]
- 46.Montini G, Rigon L, Zucchetta P, et al. IRIS Group . Prophylaxis after first febrile urinary tract infection in children? A multicenter, randomized, controlled, noninferiority trial. Pediatrics. 2008;122(5):1064–1071 [DOI] [PubMed] [Google Scholar]
- 47.Pennesi M, Travan L, Peratoner L, et al. North East Italy Prophylaxis in VUR study group . Is antibiotic prophylaxis in children with vesicoureteral reflux effective in preventing pyelonephritis and renal scars? A randomized, controlled trial. Pediatrics. 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1489 [DOI] [PubMed] [Google Scholar]
- 48.Roussey-Kesler G, Gadjos V, Idres N, et al. Antibiotic prophylaxis for the prevention of recurrent urinary tract infection in children with low grade vesicoureteral reflux: results from a prospective randomized study. J Urol. 2008;179(2):674–679, discussion 679 [DOI] [PubMed] [Google Scholar]
- 49.Camacho V, Estorch M, Fraga G, et al. DMSA study performed during febrile urinary tract infection: a predictor of patient outcome? Eur J Nucl Med Mol Imaging. 2004;31(6):862–866 [DOI] [PubMed] [Google Scholar]
- 50.Craig JC, Irwig LM, Christie J, et al. Variation in the diagnosis of vesicoureteric reflux using micturating cystourethrography. Pediatr Nephrol. 1997;11(4):455–459 [DOI] [PubMed] [Google Scholar]
- 51.Fernández-Menéndez JM, Málaga S, Matesanz JL, Solís G, Alonso S, Pérez-Méndez C. Risk factors in the development of early technetium-99m dimercaptosuccinic acid renal scintigraphy lesions during first urinary tract infection in children. Acta Paediatr. 2003;92(1):21–26 [DOI] [PubMed] [Google Scholar]
- 52.Hansson S, Dhamey M, Sigström O, et al. Dimercapto-succinic acid scintigraphy instead of voiding cystourethrography for infants with urinary tract infection. J Urol. 2004;172(3):1071–1073, discussion 1073–1074 [DOI] [PubMed] [Google Scholar]
- 53.Mahant S, To T, Friedman J. Timing of voiding cystourethrogram in the investigation of urinary tract infections in children. J Pediatr. 2001;139(4):568–571 [DOI] [PubMed] [Google Scholar]
- 54.McDonald A, Scranton M, Gillespie R, Mahajan V, Edwards GA. Voiding cystourethrograms and urinary tract infections: how long to wait? Pediatrics. 2000;105(4):E50. [DOI] [PubMed] [Google Scholar]
- 55.Oostenbrink R, van der Heijden AJ, Moons KG, Moll HA. Prediction of vesico-ureteric reflux in childhood urinary tract infection: a multivariate approach. Acta Paediatr. 2000;89(7):806–810 [PubMed] [Google Scholar]
- 56.Pinto KJ. Vesicoureteral reflux in the Hispanic child with urinary tract infection. J Urol. 2004;171(3):1266–1267 [DOI] [PubMed] [Google Scholar]
- 57.Sargent MA, Stringer DA. Voiding cystourethrography in children with urinary tract infection: the frequency of vesicoureteric reflux is independent of the specialty of the physician requesting the study. AJR Am J Roentgenol. 1995;164(5):1237–1241 [DOI] [PubMed] [Google Scholar]
- 58.Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89(5):466–468 [DOI] [PMC free article] [PubMed] [Google Scholar]