Abstract
OBJECTIVE:
To examine factors affecting center differences in mortality for extremely low birth weight (ELBW) infants.
METHODS:
We analyzed data for 5418 ELBW infants born at 16 Neonatal Research Network centers during 2006–2009. The primary outcomes of early mortality (≤12 hours after birth) and in-hospital mortality were assessed by using multilevel hierarchical models. Models were developed to investigate associations of center rates of selected interventions with mortality while adjusting for patient-level risk factors. These analyses were performed for all gestational ages (GAs) and separately for GAs <25 weeks and ≥25 weeks.
RESULTS:
Early and in-hospital mortality rates among centers were 5% to 36% and 11% to 53% for all GAs, 13% to 73% and 28% to 90% for GAs <25 weeks, and 1% to 11% and 7% to 26% for GAs ≥25 weeks, respectively. Center intervention rates significantly predicted both early and in-hospital mortality for infants <25 weeks. For infants ≥25 weeks, intervention rates did not predict mortality. The variance in mortality among centers was significant for all GAs and outcomes. Center use of interventions and patient risk factors explained some but not all of the center variation in mortality rates.
CONCLUSIONS:
Center intervention rates explain a portion of the center variation in mortality, especially for infants born at <25 weeks’ GA. This finding suggests that deaths may be prevented by standardizing care for very early GA infants. However, differences in patient characteristics and center intervention rates do not account for all of the observed variability in mortality; and for infants with GA ≥25 weeks these differences account for only a small part of the variation in mortality.
Keywords: mortality rates, outcome, NICU, preterm infants, extremely preterm infants
What’s Known on This Subject:
Significant variation in the mortality of preterm infants has been observed among NICUs. Factors explaining this variation have been difficult to identify.
What This Study Adds:
Sizable center differences in mortality exist, even among similarly sized NICUs in academic centers. Patient characteristics and center treatment rates explain some of the center effect, especially for the youngest infants, but a significant portion of these differences remains unexplained.
Both patient factors (eg, gestational age [GA]) and preventive or therapeutic interventions (eg, antenatal corticosteroids) are known to affect the mortality risk of very preterm infants.1 The hospital in which care is provided also affects mortality risk. Variability in mortality rate by center has been observed in several populations and networks.2–7 Two factors may contribute to these center differences: (1) the inherent risk of the patient population served by a center and (2) the patient care delivered at the center. Existing models to predict neonatal mortality at birth are based on individual patient characteristics surrounding birth.1,8–11 These models are useful in providing outcome benchmarks and have been modified as clinical care has improved. They do not, however, adequately address the center effect on outcome. Variation in the patient populations accounts for some but not all of the differences in mortality among centers.12,13 Examining the effect of center on the mortality of extremely low birth weight (ELBW; ≤1000 g) infants may yield insights into ways to improve neonatal care.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN), a consortium of university-affiliated NICUs, has maintained a data registry of extremely premature infants using standard data collection and follow-up assessment methods since 1986. This data set allows examination of the variation in neonatal practice and outcomes among academic medical centers. It provides an opportunity to examine possible explanations for the center effect on mortality while controlling for infant and maternal characteristics. In this study, we estimated the variability of ELBW mortality among NRN centers. We assessed the effect of the rates at which centers use certain interventions on this variability in mortality, while adjusting for the effects of patient risk factors known to influence mortality.
Methods
Eligible infants from all NRN centers were identified, and patient risk factors and center intervention rates were extracted from records of neonatal and maternal characteristics and interventions available in the NRN registry. These variables were used to build models designed to predict mortality outcomes. Early mortality (≤12 hours) and in-hospital mortality were analyzed separately, first for all infants and then for 2 subgroups of infants, those with GA <25 weeks and those with GA ≥25 weeks.
Data Source
Infants born at NRN centers in 2006–2009 with birth weight of 401 to 1000 g and GA of 22 (0/7) through 28 (6/7) weeks were included. Infants with syndromes or major malformations were excluded. The final data set consisted of 5418 infants from 16 centers. NRN membership did not change during the period of study, 2006–2009.
The NRN ELBW patient registry was approved by the institutional review board at each participating site. Trained research personnel collected maternal, pregnancy, delivery, and infant data as well as morbidity and intervention data from birth until hospital discharge, death, or 120 days of age if still hospitalized. Hospital death after 120 days or survival to discharge was obtained for infants who were hospitalized for longer periods. Definitions for maternal and infant characteristics and interventions were predetermined and described in the manual of operations. GA was determined as the best obstetric estimate on the basis of the mother’s last menstrual period and fetal ultrasonography. Patient risk factors and interventions were obtained from individual patient records. Center intervention rates were calculated from the patient data within a given center.
Outcomes
The 2 main outcomes examined in this study were mortality in the first 12 hours after birth (early mortality) and mortality before discharge (in-hospital mortality). These outcomes were not mutually exclusive, because in-hospital mortality included those infants who died early. Deaths that occurred in the delivery room were included in these mortality outcomes. These outcomes were additionally examined in 2 subgroups of patients, those with GA <25 weeks and those with GA ≥25 weeks. The rationale behind testing in these subgroups was that centers or individual physicians within centers may have different policies regarding the resuscitation of infants born at <25 weeks. We assumed that infants born at ≥25 weeks’ GA were likely to receive aggressive obstetric and neonatal care, whereas infants born before 25 weeks might have had less aggressive care before and after birth.14
Patient Risk Factors
Patient risk factors used in this study included mother’s educational level (less than versus high school diploma or higher), private health insurance coverage, prenatal care utilization, gender of the infant, race, ethnicity, birth weight, gestational age, 5-minute Apgar score (<5 vs ≥5), and multiple birth (versus single birth). Individual birth weights and gestational ages were expressed as differences from local NICU means.
Center Intervention Rates
Interventions available in the NRN registry that may be related to mortality were included in the analysis. These variables were as follows: provision of antenatal corticosteroids, mode of delivery, support given to the infant at delivery (continuous positive airway pressure [CPAP], tracheal intubation, epinephrine), NICU admission, prophylactic indomethacin, early antibiotics (started in the first 72 hours and given for ≥5 days), respiratory support in the NICU (supplemental oxygen, CPAP or assisted ventilation, surfactant, inhaled nitric oxide), postnatal corticosteroids, and any human milk feeding. Center rates of specific interventions (ie, percentage of mothers or infants receiving an intervention) were calculated by aggregating individual patient data for eligible infants over the period of the years 2006–2009 at each center.
Statistical Analysis
Multilevel hierarchical models were used to evaluate center differences in mortality and to evaluate 2 major sources of variation that might explain differences in mortality among centers: patient risk factors and center intervention rates. The assessment of center intervention rates by the presence or absence of patient-level risk factors in a hierarchical model was conducted by using 4 models (Table 1). The derivation and underlying assumptions of the models are described in Appendix 1.
TABLE 1.
Components of Multilevel Hierarchical Models
| Patient-Level Factors | Center Intervention Rates | |
|---|---|---|
| No | Yes | |
| No | Model 1 | Model 2 |
| Yes | Model 3 | Model 4 |
Each model yields an estimated parameter that measures the average center mortality (in log odds terms) and the mortality variance, an estimate of the magnitude of variation or dispersion in center mortality. An estimated mortality variance significantly greater than zero indicates variation in mortality among centers that is unlikely to have occurred by chance alone. A decrease in the variance estimates from models with, to those without, center intervention rates indicates the degree to which these interventions explain the center difference in mortality. By using principal components analysis, all center intervention rates were assigned appropriate weights and combined into a single center intervention score for inclusion in the final models. Principal components analysis allowed this to be accomplished despite the collinearity (ie, high correlation between independent variables) among intervention rates. A technical description of the principal components analysis is provided in Appendix 2 and Appendix Table. The clinical implications of these models, ie, their ability to explain center variation in mortality rate, are described below.
Model 1: Intercept Only
This baseline reference model includes center mortality data and is used to assess the level of and variation in overall center mortality but does not include patient risk factors or the intervention score.
Model 2: Center Intervention Rates
This model extends Model 1 by adding the center intervention score as a predictor of mortality to assess the extent to which variation of center mortality can be explained by center intervention rates. Compared with Model 1, the decrease in the estimated mortality variance provides a measure of overall center mortality that can be explained by intervention rates, without correcting for patient population.
Model 3: Patient-Level Risk Factors
This model includes only infant and maternal characteristics. It is used to assess the magnitude of center variation in mortality while controlling for infant and maternal characteristics. Models 3 and 4 can be compared directly only with each other and not with Model 1 or 2.
Model 4: Patient Risk Factors and Center Intervention Rates
This model extends Model 3 by adding the center intervention score as a predictor of center mortality and includes covariates of the infant and maternal characteristics. This model is used to assess the effect of the intervention rates on center mortality after controlling for patient-level risk factors. A decrease in the mortality variance provides a measure of the center variation in mortality explained by center intervention rates after controlling for patient risk factors.
These models were derived for all infants and for the subgroups with GAs <25 weeks and ≥25 weeks. All modeling analyses were performed by using the Xtmelogit command in Stata 12 (StataCorp, College Station, TX).
Results
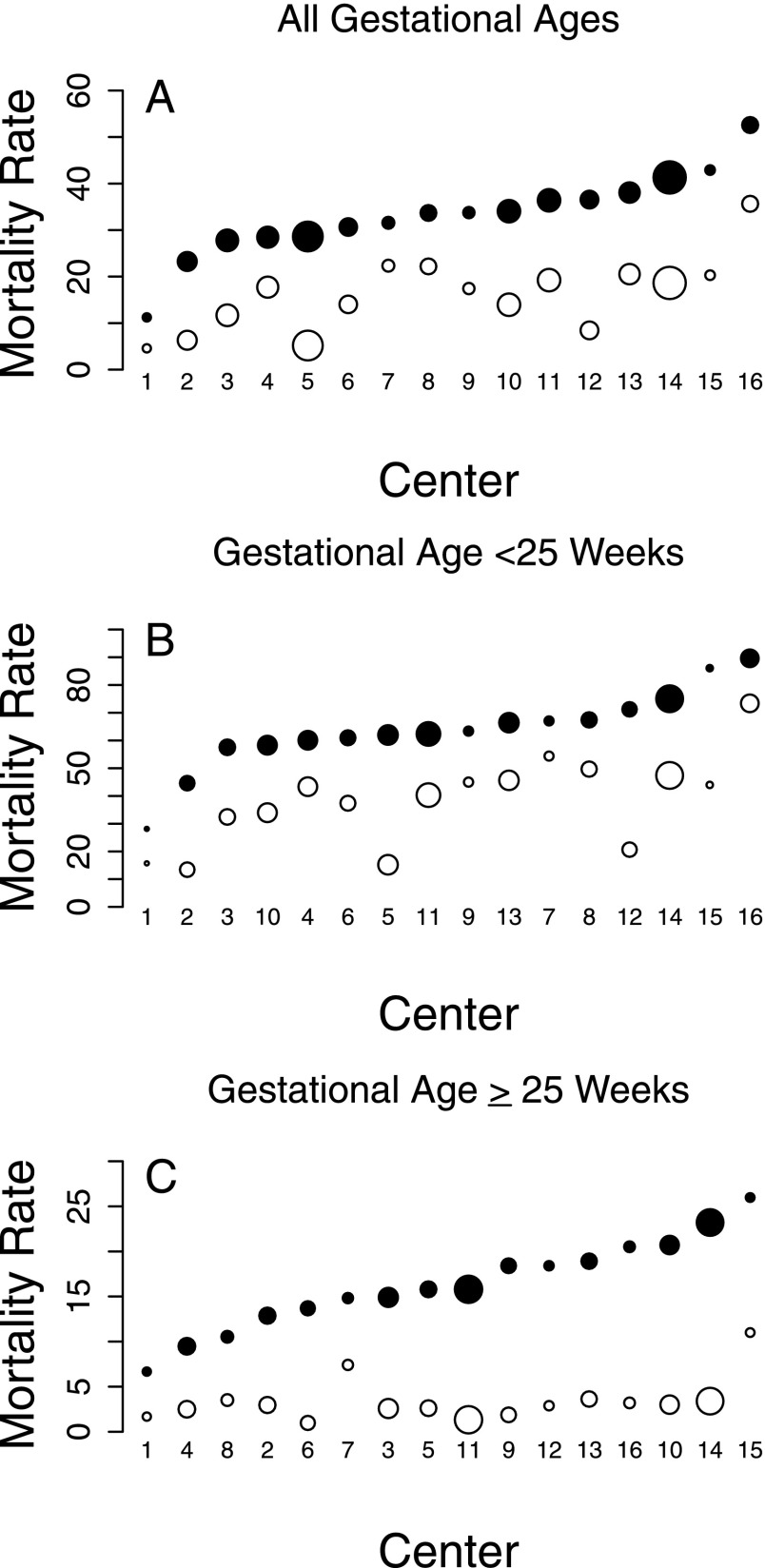
Wide ranges in intervention rates and mortality were observed among NRN centers for infants of all GAs as well as for those with GAs <25 weeks and ≥25 weeks (Table 2, Fig 1). The ranges of the intervention rates for infants with GA <25 weeks were typically wider than those for infants with GA ≥25 weeks.
TABLE 2.
Medians and Ranges of Center Intervention Rates and Outcomes: 2006–2009
| Percentage of Infants in Centers | ||||||
|---|---|---|---|---|---|---|
| All Infants (N = 152–588) | <25 Weeks’ GA (n = 32–205) | ≥25 Weeks’ GA (n = 120–392) | ||||
| Median | Range | Median | Range | Median | Range | |
| Intervention | ||||||
| Antenatal corticosteroids | 82 | 35–93 | 58 | 21–81 | 92 | 47–99 |
| Vaginal vertex delivery | 30 | 22–41 | 46 | 31–61 | 21 | 16–26 |
| Cesarean delivery | 63 | 45–76 | 36 | 10–63 | 77 | 68–83 |
| Tracheal intubation | 63 | 41–82 | 63 | 24–94 | 62 | 37–88 |
| CPAP | 29 | 4–53 | 9 | 0–31 | 39 | 6–65 |
| NICU admission | 86 | 74–98 | 65 | 45–95 | 99 | 95–100 |
| Epinephrine | 4 | 1–12 | 5 | 0–14 | 3 | 1–11 |
| Respiratory support excluding high-frequency ventilation | 80 | 61–91 | 54 | 23–87 | 95 | 83–98 |
| Supplemental oxygen | 82 | 61–95 | 61 | 26–89 | 95 | 87–98 |
| High-frequency ventilation | 38 | 23–76 | 44 | 20–84 | 33 | 15-–79 |
| Surfactant | 70 | 50–88 | 61 | 25–92 | 77 | 65–91 |
| Nitric oxide | 5 | 1–22 | 6 | 0–44 | 6 | 1–20 |
| Postnatal corticosteroids | 10 | 1–29 | 11 | 0–50 | 8 | 1–23 |
| Prophylactic indomethacin | 12 | 0–80 | 13 | 0–75 | 11 | 0–94 |
| Early antibiotics | 39 | 23–92 | 37 | 16–84 | 41 | 22–94 |
| Human milk feeding | 64 | 50–95 | 41 | 13–81 | 81 | 59–98 |
| Outcome | ||||||
| Early mortality (≤12 hours) | 18 | 5–36 | 42 | 13–73 | 3 | 1–11 |
| In-hospital mortality | 34 | 11–53 | 63 | 28–90 | 16 | 7–26 |
The range in the number of infants per center (n) is shown for each GA category.
FIGURE 1.
Mortality rates by center. A, Results for infants of all GAs (22 [0/7] through 28 [6/7] weeks). B, Results for infants with GA of 22 [0/7] through 24 [6/7] weeks. C, Results for infants with GA of 25 [0/7] through 28 [6/7] weeks. Closed circles represent in-hospital mortality and open circles early (≤12 hours) mortality. The size of the circles represents the number of infants treated at a given center over the study period. The centers are ordered by in-hospital mortality for each graph individually, which means that a center may not have the same number in each panel.
Our major estimation results from the multilevel models are summarized in the following paragraphs (Table 3).
TABLE 3.
Estimation Results from Multilevel Models
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| All GAs: early mortality | ||||
| Variance in mortality | 0.38 (0.18–0.83) | 0.07 (0.03–0.19) | 0.64 (0.29–1.42) | 0.27 (0.11–0.64) |
| OR of intervention | 0.79 (0.74–0.84) | 0.76 (0.67–0.86) | ||
| All GAs: in-hospital mortality | ||||
| Variance in mortality | 0.16 (0.07–0.37) | 0.03 (0.01–0.09) | 0.31 (0.14–0.67) | 0.14 (0.06–0.32) |
| OR of intervention | 0.87 (0.83–0.90) | 0.84 (0.77–0.92) | ||
| GA <25 weeks: early mortality | ||||
| Variance in mortality | 0.50 (0.23–1.10) | 0a | 1.08 (0.49–2.40) | 0.08 (0.02–0.38) |
| OR of intervention | 0.76 (0.72–0.79) | 0.67 (0.61–0.74) | ||
| GA <25 weeks: in-hospital mortality | ||||
| Variance in mortality | 0.35 (0.14– 0.85) | 0.05 (0.01–0.19) | 0.62 (0.27–1.46) | 0.20 (0.08–0.52) |
| OR of intervention | 0.83 (0.78–0.88) | 0.79 (0.71–0.87) | ||
| GA ≥25 weeks: early mortality | ||||
| Variance in mortality | 0.21 (0.06–0.71) | 0.11 (0.02–0.52) | 0.25 (0.07–0.92) | 0.17 (0.04–0.74) |
| OR of intervention | 0.84 (0.73– 0.96) | 0.86 (0.73–1.01) | ||
| GA ≥25 weeks: in-hospital mortality | ||||
| Variance in mortality | 0.09 (0.03–0.25) | 0.07 (0.03–0.21) | 0.14 (0.05–0.37) | 0.13 (0.05–0.35) |
| OR of intervention | 0.92 (0.84–1.01) | 0.94 (0.84–1.05) | ||
Variance in center mortality (log odds of mortality) and ORs for intervention scores of center intervention rates are shown (95% CI in parentheses).
Not statistically significant.
Infants of All GAs: Early Mortality
Model 2 indicates the intervention score had a significant association with center mortality (odds ratio [OR]: 0.79; 95% confidence interval [CI]: 0.74–0.84) and reduced the center mortality variance from 0.38 to 0.07. After controlling for patient risk factors in Models 3 and 4, the association of the intervention score remained significant (OR: 0.76; 95% CI: 0.67–0.86) and the mortality variance was reduced by from 0.64 to 0.27.
Infants of All GAs: In-Hospital Mortality
Before controlling for individual risk factors, the intervention score significantly reduced the odds of the overall center in-hospital mortality (OR: 0.87; 95% CI: 0.83–0.90) and reduced its variance from 0.16 to 0.03. After controlling for patient characteristics, the effect of the intervention score also remained significant (OR: 0.84; 95% CI: 0.77–0.91) and reduced the variance of the center mortality rate from 0.31 to 0.14.
Infants With GA <25 Weeks: Early Mortality
The intervention score was significantly related to mortality (OR: 0.76; 95% CI: 0.72–0.79) and accounted for all variation in mortality among centers as evidenced by the variance estimate of 0 for Model 2. After accounting for patient risk factors, the intervention score was still significant (OR: 0.67; 95% CI: 0.61–0.74) and reduced the mortality variance from 1.08 to 0.08.
Infants With GA <25 Weeks: In-Hospital Mortality
Before correcting for patient characteristics, the intervention score was significantly related to odds of mortality (OR: 0.83; 95% CI: 0.78–0.88) and reduced the mortality variance from 0.35 to 0.05. After controlling for individual risk factors, the intervention score remained significant (OR: 0.79; 95% CI: 0.71–0.87) and reduced the mortality variance from 0.62 to 0.20 among centers.
Infants With GA ≥25 Weeks: Early Mortality
The association of the intervention score with early mortality was significant for infants with GA ≥25 weeks but had a wider CI than that among all infants or the <25-week subgroup (OR: 0.84; 95% CI: 0.73–0.96). Including the intervention score reduced the center mortality variance from 0.21 to 0.11. After controlling for patient risk factors, the association of the intervention score became borderline significant (OR: 0.86; 95% CI: 0.73–1.01) and reduced the variance of the center mortality rate from 0.25 to 0.17.
Infants With GA ≥25 Weeks: In-Hospital Mortality
The association of the intervention score was not statistically significant either before or after accounting for patient risk factors.
The mortality variance estimates from all models, in all subgroups of each outcome, after accounting for patient risk factors were significantly greater than zero. Thus, center variation in early and in-hospital mortality exists even after controlling for intervention rates and patient risk factors.
Discussion
Our study yields a number of interesting points for consideration. First, it reveals that in the NRN, there was significant variation among centers in ELBW mortality. This result was true for both early and in-hospital mortality for each GA subset; it is shown in the observed center death rates and also by the significant center mortality variance estimates from Model 1, which did not include center interventions or patient risk factors, for each outcome and subgroup. Second, our modeling results cast doubt on the commonly held concept that variation in outcomes is due largely to differences in patient population. The center mortality variance estimates after correcting for patient risk factors (Model 3) were still significant for all outcomes and subgroups. Finally, center intervention rates predicted mortality outcomes and reduced differences in the outcomes among the centers. In deriving the intervention scores, interventions not generally given in the first 12 hours of life were excluded for the early mortality outcome. As indicated by the weighting factors used in the principal components analysis (Appendix 2), the intervention scores were primarily composed of the averages of the center intervention rates of NICU admission, respiratory support, supplemental oxygen, surfactant, and, for infants of <25 weeks’ GA, antenatal corticosteroids, cesarean delivery, and tracheal intubation. The intervention scores showed significantly positive association with both early and in-hospital mortality in the all-GA population and in the <25-week GA subgroup, but they showed only a trend toward significance in the ≥25-week GA subgroup. In addition, the center mortality variance was greatly reduced when the intervention score was included in the models. These intervention scores reduced the mortality variance estimates substantially for both early and in-hospital mortality for the all-GA group, and in the <25-week GA subgroup the reductions were even greater. This finding was true even after correction for patient risk factors. Therefore, a substantial number of additional infants would be predicted to survive both past 12 hours and until discharge should the utilization of the examined interventions be increased for infants at the earliest gestational ages (<25 weeks). One potential explanation for the finding that increased intervention is especially beneficial for infants with GA <25 weeks concerns the wider range of the centers’ use of supportive therapies in the intrapartum period and the first hours of life. In particular, there was large practice variation among centers in the use of antenatal corticosteroids, cesarean delivery, respiratory support, and NICU admission for these most premature infants. Our derived intervention score more effectively represents the specific intervention therapies examined among infants with GA <25 weeks than among infants with GA ≥25 weeks. More research is needed to identify and determine other factors associated with mortality among infants with GA ≥25 weeks.
This study’s strengths include the use of high-quality data from the centers of the NRN collected by trained research staff applying standardized methods. The use of prospectively collected data has presumably limited the probability of selection bias and misclassification of covariates and outcomes. The use of the hierarchical modeling method allowed us to separate the effects of patient- and center-level variables and to assess the impacts of center-level variables both on mortality and on its variability among centers. Limitations of the study include the relatively small number of centers. Also, capturing all variability in care is unlikely given the limited number of elements in the NRN database, even after correcting for patient-level differences. Thus, some interventions or approaches not available in the NRN database may affect center mortality rates. This observational study reveals an association between interventions and survival, but intervention trials are required to demonstrate a true causal link between specific interventions and patient survival. Finally, our study was not able to examine variability in physician-level care within centers or the quality with which interventions were implemented, which are additional possible contributors to variation in outcome.
Our results show that even though aggressive intervention with more infants would increase survival, the use of the interventions we studied does not fully explain the center variation in mortality, which implies that there are unaccounted-for variables that affect the center differences in mortality. Previous studies have postulated what these factors may be. Horbar et al12 found that patient volume and the presence of a residency program were not predictive of outcomes. Rogowski et al13 found that the percentage of Medicaid beneficiaries, type of hospital ownership, and presence of a teaching hospital were not significant predictors of outcomes. These investigators also found that very low volume centers had worse outcomes and that the outcome was related to the level of NICU. These findings do not explain the variation in mortality among NRN centers, which are all high-volume, high-level centers.
Our results suggest that decisions of whether to resuscitate very preterm infants affect mortality and are detectable at the center level. This observation raises ethical issues. However, creating clear policies on the appropriateness of resuscitating ELBW infants is extremely difficult,15,16 and a thorough examination of this topic is beyond the scope of this study. This report simply highlights that increased survival is possible, and the authors believe that decision makers (parents and physicians) should be aware of this fact. More aggressive intervention of these neonates would increase their likelihood of survival. The current study does not address the impact of more aggressive intervention on the likelihood of “intact” survival, ie, survival without major neurodevelopmental impairment, but this association has been shown in this network.17 More active intervention of extremely preterm infants may benefit not only these infants but also more mature infants; centers in which intensive care is more frequently provided to extremely preterm infants have been shown to have better outcomes, too, for preterm infants of later gestation.18
Neonatal care has been advanced by the development and testing of beneficial therapies.19 It is important to note that the magnitude of the center effect on ELBW mortality is larger than the intervention effects of even the most potent therapies, such as antenatal corticosteroids and surfactant replacement therapy. This fact underscores the importance of stratification by center in all multicenter clinical trials in neonatology. The findings of our study support a role for comprehensive, well-designed, quality-improvement trials focusing on the care of very early GA infants.
Conclusions
There are large differences among centers in the mortality rate of extremely preterm infants. Patient characteristics do not adequately account for this variability. Center use of certain interventions predicts survival for the lowest GA infants, but it explains little of the observed variability in in-hospital mortality for infants born at ≥25 weeks. Some as-yet-unmeasured characteristics of the centers account for the remainder of the observed variability. The variation in mortality among centers may be reduced through standardization of care for very early GA infants. However, other center differences may explain the residual differences in mortality.
Acknowledgments
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Drs Abhik Das (DCC Principal Investigator) and Lei Li (DCC Statistician) had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine.
Alpert Medical School of Brown University and Women and Infants Hospital of Rhode Island (U10 HD27904): William Oh, MD; Angelita M. Hensman, RN, BSN; Dawn Andrews, RN MS; Kristen Angela, RN.
Case Western Reserve University, Rainbow Babies and Children's Hospital (U10 HD21364, M01 RR80): Avroy A. Fanaroff, MD; Bonnie S. Siner, RN.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084): Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Holly L. Mincey, RN, BSN; Jody Hessling, RN; Lenora Jackson.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30): Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Katherine A. Foy, RN.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39): David P. Carlton, MD; Ann M. Blackwelder, RNC, BS, MS.
Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750): Brenda B. Poindexter, MD, MS; Dianne E. Herron, RN; Leslie Dawn Wilson, BSN, CCRC.
RTI International (U10 HD36790): W. Kenneth Poole, PhD; Jeanette O’Donnell Auman, BS; Margaret Cunningham, BS; Carolyn M. Petrie Huitema, MS; James W. Pickett II, BS; Scott E. Schaefer, MS; Kristin M. Zaterka-Baxter, RN, BSN.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70): Krisa P. Van Meurs, MD; David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS, CCRC; Melinda S. Proud, RCP; Andrew W. Palmquist, RN, BSN.
Tufts Medical Center (U10 HD53119, M01 RR54): Ivan D. Frantz III, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN, BSN.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32): Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN.
University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461): Neil N. Finer, MD; Maynard R. Rasmussen MD; Paul R. Wozniak, MD; Kathy Arnell, RNC; Renee Bridge, RN; Clarence Demetrio, RN; Wade Rich, BSHS, RRT.
University of Iowa Children’s Hospital (U10 HD53109, M01 RR59): John A. Widness, MD; Karen J. Johnson, RN, BSN; Nancy J. Krutzfield, RN, MA.
University of Miami Holtz Children’s Hospital (U10 HD21397): Shahnaz Duara, MD; Ruth Everett-Thomas, RN, MSN.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997): Kristi L. Watterberg, MD; Lu-Ann Papile, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Rebecca Montman, BSN; Carol Hartenberger, BSN, MPH.
University of Rochester Medical Center, Golisano Children’s Hospital (U10 HD40521, M01 RR44): Dale L. Phelps, MD; Linda J. Reubens, RN, CCRC; Erica Burnell, RN; Cassandra Horihan, MS; Rosemary Jensen.
University of Texas Southwestern Medical Center at Dallas, Parkland Health and Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633): Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Luc P. Brion, MD; Alicia Guzman; Gaynelle Hensley, RN; Melissa H. Leps, RN; Diana M. Vasil, RNC-NIC; Nancy A. Miller, RN; Lizette E. Torres, RN.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373): Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Beverly Foley Harris, RN, BSN; Anna E. Lis, RN, BSN; Sarah Martin, RN, BSN; Georgia E. McDavid, RN; Patti L. Pierce Tate, RCP; Maegan C. Simmons, RN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64): Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN, BSN, CCRC; Karie Bird, RN; Jill Burnett, RNC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN, BSN; Karen Zanetti, RN.
Wake Forest University, Baptist Medical Center, Brenner Children's Hospital, and Forsyth Medical Center (U10 HD40498, M01 RR7122): T. Michael O’Shea, MD, MPH; Nancy J. Peters, RN, CCRP.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385): Rebecca Bara, RN, BSN; Mary E. Johnson, RN, BSN; Elizabeth Billian, RN, MBA.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, M01 RR125): Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Patricia Cervone, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Glossary
- CI
confidence interval
- CPAP
continuous positive airway pressure
- ELBW
extremely low birth weight
- GA
gestational age
- NRN
National Institute of Child Health and Human Development Neonatal Research Network
- OR
odds ratio
Appendix 1: Specifications of Multilevel Hierarchical Models
Denote the outcome variable by an indicator yij, such that patient i in center j will die (yij = 1) or survive (yij = 0). Let aj be a random variable and uj be a normally distributed random-error term with a mean of 0 and an SD of σ for center j. Let PCJ be the principal components score from the center intervention rates with coefficients γ1 and x1ij … x9ij representing the 9 patient and maternal characteristics with corresponding coefficients (β1 … β0). The 4 model forms used for the probability of death, P(yij = 1), are as follows.
Model 1
Patient-Level Model
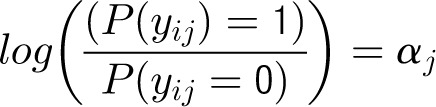
Center-Level Model
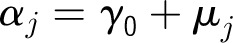
Model 2
Patient-Level Model
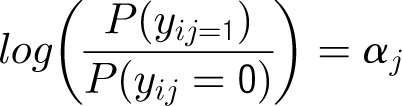
Center-Level Model
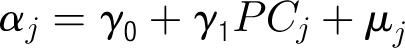
Model 3
Patient-Level Model
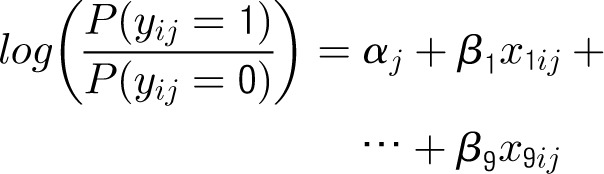
Center-Level Model
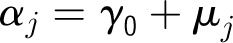
Model 4
Patient-Level Model
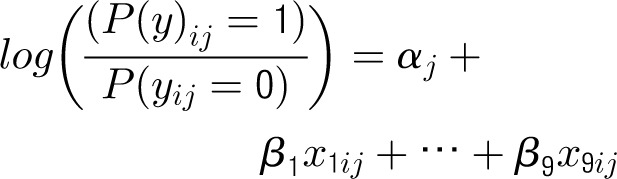
Center-Level Model
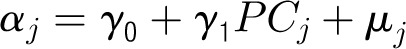
On the basis of the above models, the average center mortality rate and its range within 2 SDs (presented in Tables 2 and 3) can be computed as
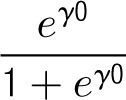
and the range as
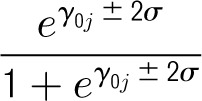
Appendix 2: Composition of First Principal Components of Center Intervention Rates
Principal components analysis is a method to reduce the number of variables under consideration. We have included as many as 15 types of interventions in this study. It is unrealistic to include all of these interventions simultaneously in the multilevel models, but we can try to derive an index from these intervention rates and use it as a predictor. The principal components generated from the intervention rates are such indices in the form of linear combinations of the intervention rates and can best represent the variation of these intervention rates among centers. A brief description of the method follows.
Let X represent a matrix in which each column contains the intervention rates for a center and β represent a standardized vector of the weighting factors in the linear combination of the intervention rates such that β′β = 1. For simplicity of notation, the intervention rates are deducted by their means so that they have a mean of 0. Then, linear combinations of the intervention rates can be represented for all centers as β′X.
A variance estimate of linear combination of the intervention rates will be as follows:
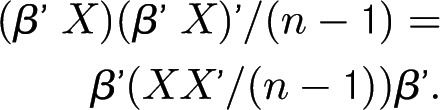
Note that XX′/(n − 1) is a variance estimate of the intervention rates and often denoted by Σ. Principal components analysis produces a set of uncorrelated linear combinations whose variances, added together, are identical to that in Σ. Usually, they are ordered by their variances, and the proportion of variance to total variance indicates their relative importance. The first principal component that has the maximum variance is often of particular interest Those principal components with a small variance may be ignored.
The proportion of variance and the weighting factor β for the first principal component of the intervention rates for predicting early mortality and in-hospital mortality are shown below. It can be seen that the proportion of variance is higher for the intervention rates for early mortality and much higher among infants with GA <25 weeks. Also, these principal components are essentially an average of all center rates except for CPAP and epinephrine. The weighting factors for the center rates are nearly equal, but those for CPAP and epinephrine are close to zero. Similarly, the principal components score for in-hospital mortality is an average of all center rates except for CPAP, epinephrine, and prophylactic indomethacin.
APPENDIX TABLE.
Proportion of Variance and Weighting Factors (β) for the First Principal Component of the Intervention Rates for Predicting Early Mortality and In-Hospital Mortality
| Early Mortality | In-Hospital Mortality | |||||
|---|---|---|---|---|---|---|
| All Infants | GA <25 Weeks | GA ≥25 Weeks | All Infants | GA <25 Weeks | GA ≥25 Weeks | |
| Variance accounted for by first principal component, % | 53.2 | 69.8 | 31.2 | 44.7 | 60.5 | 25.1 |
| Principal component (weighting factors) | ||||||
| Antenatal corticosteroids | 0.34 | 0.33 | −0.01 | 0.31 | 0.30 | 0.07 |
| Cesarean delivery | 0.36 | 0.33 | −0.24 | 0.33 | 0.30 | −0.09 |
| Tracheal intubation | 0.29 | 0.35 | 0.40 | 0.26 | 0.32 | 0.35 |
| CPAP | −0.05 | 0.10 | −0.16 | 0.00 | 0.12 | 0.01 |
| NICU admission | 0.38 | 0.34 | 0.32 | 0.33 | 0.30 | 0.16 |
| Epinephrine | 0.00 | 0.09 | −0.30 | 0.02 | 0.09 | −0.09 |
| Respiratory support excluding high-frequency ventilation | 0.34 | 0.31 | 0.34 | 0.30 | 0.28 | 0.12 |
| Supplemental oxygen | 0.39 | 0.35 | 0.44 | 0.36 | 0.31 | 0.36 |
| High-frequency ventilation | 0.23 | 0.28 | 0.13 | 0.24 | 0.26 | 0.35 |
| Surfactant | 0.38 | 0.36 | 0.46 | 0.34 | 0.32 | 0.42 |
| Early antibiotics | 0.25 | 0.32 | 0.15 | 0.27 | 0.30 | 0.34 |
| Postnatal corticosteroids | NA | NA | NA | 0.23 | 0.20 | 0.30 |
| Prophylactic indomethacin | NA | NA | NA | −0.02 | 0.08 | −0.21 |
| Nitric oxide | NA | NA | NA | 0.15 | 0.17 | 0.30 |
| Human milk feeding | NA | NA | NA | 0.28 | 0.31 | 0.22 |
NA, not applicable.
Footnotes
Mr Alleman participated in the conception and design of the study including the analysis plan and in the interpretation of the data and wrote the first and subsequent drafts of the manuscript and helped to revise it critically for important intellectual content; Dr Bell conceived and helped to design the study including the analysis plan, participated in the interpretation of the data, and revised the manuscript critically for important intellectual content; Dr Li helped to design the analysis plan and was responsible for the data management and analysis, performed the analysis with guidance from Mr Alleman and Dr Bell and with advice from Drs Das and Wallace, and helped to revise the manuscript critically for important intellectual content; Drs Dagle and Murray participated in the conception and design of the study, participated in the interpretation of the data, and helped to revise the manuscript critically for important intellectual content; Drs Smith, Ambalavanan, Laughon, Cotton, Shankaran, Walsh, Laptook, Ellsbury, and Higgins participated in the design of the study, participated in the interpretation of the data, and helped to revise the manuscript critically for important intellectual content; Dr Stoll participated in the design of the study, chaired the committee responsible for designing and managing the study from which the data were drawn, participated in the interpretation of the data, and helped to revise the manuscript critically for important intellectual content; Dr Goldberg and Carlo participated in the conception of the study, participated in the interpretation of the data, and reviewed the manuscript critically for important intellectual content; Ms Hale and Ms Newman participated in the design of the study, contributed to the data collection, and helped to revise the manuscript critically for important intellectual content; and Drs Wallace and Das participated in the design of the study and the plan for data analysis, were responsible for the data management, participated in the analysis, and helped to revise the manuscript critically for important intellectual content.
FINANCIAL DISCLOSURE: The authors have indicated that they have no financial relationships relevant to this article to disclose.
FUNDING: The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network’s Generic Database Study. Dr Higgins is employed by the NICHD. The institutions of the other authors (except for Dr Ellsbury) received grant funding from the NICHD in support of this study. Funded by the National Institutes for Health.
References
- 1.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD, National Institute of Child Health and Human Development Neonatal Research Network . Intensive care for extreme prematurity—moving beyond gestational age. N Engl J Med. 2008;358(16):1672–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SK, McMillan DD, Ohlsson A, et al. Variations in practice and outcomes in the Canadian NICU network: 1996-1997. Pediatrics. 2000;106(5):1070–1079 [DOI] [PubMed] [Google Scholar]
- 3.Simpson JM, Evans N, Gibberd RW, Heuchan AM, Henderson-Smart DJ, Australian and New Zealand Neonatal Network . Analysing differences in clinical outcomes between hospitals. Qual Saf Health Care. 2003;12(4):257–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vohr BR, Wright LL, Dusick AM, et al. Neonatal Research Network . Center differences and outcomes of extremely low birth weight infants. Pediatrics. 2004;113(4):781–789 [DOI] [PubMed] [Google Scholar]
- 5.Cotten CM, Oh W, McDonald S, et al. NICHD Neonatal Research Network . Prolonged hospital stay for extremely premature infants: risk factors, center differences, and the impact of mortality on selecting a best-performing center. J Perinatol. 2005;25(10):650–655 [DOI] [PubMed] [Google Scholar]
- 6.Kusuda S, Fujimura M, Sakuma I, et al. Neonatal Research Network, Japan . Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 2006;118(4). Available at: www.pediatrics.org/cgi/content/full/118/4/e1130 [DOI] [PubMed] [Google Scholar]
- 7.Almeida MFB, Guinsburg R, Martinez FE, et al. Perinatal factors associated with early deaths of preterm infants born in Brazilian Network on Neonatal Research centers. J Pediatr (Rio J). 2008;84(4):300–307 [DOI] [PubMed] [Google Scholar]
- 8.Horbar JD, Onstad L, Wright E. Predicting mortality risk for infants weighing 501 to 1500 grams at birth: a National Institutes of Health Neonatal Research Network report. Crit Care Med. 1993;21(1):12–18 [DOI] [PubMed] [Google Scholar]
- 9.Richardson DK, Gray JE, McCormick MC, Workman K, Goldmann DA. Score for Neonatal Acute Physiology: a physiologic severity index for neonatal intensive care. Pediatrics. 1993;91(3):617–623 [PubMed] [Google Scholar]
- 10.Parry G, Tucker J, Tarnow-Mordi W, UK Neonatal Staffing Study Collaborative Group . CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361(9371):1789–1791 [DOI] [PubMed] [Google Scholar]
- 11.Ambalavanan N, Carlo WA, Bobashev G, et al. National Institute of Child Health and Human Development Neonatal Research Network . Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–1373 [DOI] [PubMed] [Google Scholar]
- 12.Horbar JD, Badger GJ, Lewit EM, Rogowski J, Shiono PH, Vermont Oxford Network . Hospital and patient characteristics associated with variation in 28-day mortality rates for very low birth weight infants. Pediatrics. 1997;99(2):149–156 [DOI] [PubMed] [Google Scholar]
- 13.Rogowski JA, Horbar JD, Staiger DO, Kenny M, Carpenter J, Geppert J. Indirect vs direct hospital quality indicators for very low-birth-weight infants. JAMA. 2004;291(2):202–209 [DOI] [PubMed] [Google Scholar]
- 14.Kaempf JW, Tomlinson MW, Campbell B, Ferguson L, Stewart VT. Counseling pregnant women who may deliver extremely premature infants: medical care guidelines, family choices, and neonatal outcomes. Pediatrics. 2009;123(6):1509–1515 [DOI] [PubMed] [Google Scholar]
- 15.Nuffield Council on Bioethics Critical Care Decisions in Fetal and Neonatal Medicine: Ethical Issues. London, United Kingdom: Nuffield Council on Bioethics; 2006 [Google Scholar]
- 16.Batton DG, Committee on Fetus and Newborn . Clinical report—antenatal counseling regarding resuscitation at an extremely low gestational age. Pediatrics. 2009;124(1):422–427 [DOI] [PubMed] [Google Scholar]
- 17.Carlo WA, McDonald SA, Fanaroff AA, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Association of antenatal corticosteroids with mortality and neurodevelopmental outcomes among infants born at 22 to 25 weeks’ gestation. JAMA. 2011;306(21):2348–2358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PB, Ambalavanan N, Li L, et al. Approach to infants born at 22–24 weeks’ gestation: relationship to outcome of more-mature infants. Pediatrics 2012;129(6). Available at: www.pediatrics.org/cgi/content/full/129/6/e1508 [DOI] [PMC free article] [PubMed]
- 19.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27(4):281–287 [DOI] [PubMed] [Google Scholar]