Abstract
BACKGROUND:
A novel erythropoiesis stimulating agent (ESA), darbepoetin alfa (Darbe), increases hematocrit in anemic adults when administered every 1 to 3 weeks. Weekly Darbe dosing has not been evaluated in preterm infants. We hypothesized that infants would respond to Darbe by decreasing transfusion needs compared with placebo, with less-frequent dosing than erythropoietin (Epo).
METHODS:
Preterm infants 500 to 1250 g birth weight and ≤48 hours of age were randomized to Darbe (10 μg/kg, 1 time per week subcutaneously), Epo (400 U/kg, 3 times per week subcutaneously) or placebo (sham dosing) through 35 weeks’ gestation. All received supplemental iron, folate, and vitamin E, and were transfused according to protocol. Transfusions (primary outcome), complete blood counts, absolute reticulocyte counts (ARCs), phlebotomy losses, and adverse events were recorded.
RESULTS:
A total of 102 infants (946 ± 196 g, 27.7 ± 1.8 weeks’ gestation, 51 ± 25 hours of age at first dose) were enrolled. Infants in the Darbe and Epo groups received significantly fewer transfusions (P = .015) and were exposed to fewer donors (P = .044) than the placebo group (Darbe: 1.2 ± 2.4 transfusions and 0.7 ± 1.2 donors per infant; Epo: 1.2 ± 1.6 transfusions and 0.8 ± 1.0 donors per infant; placebo: 2.4 ± 2.9 transfusions and 1.2 ± 1.3 donors per infant). Hematocrit and ARC were higher in the Darbe and Epo groups compared with placebo (P = .001, Darbe and Epo versus placebo for both hematocrit and ARCs). Morbidities were similar among groups, including the incidence of retinopathy of prematurity.
CONCLUSIONS:
Infants receiving Darbe or Epo received fewer transfusions and fewer donor exposures, and fewer injections were given to Darbe recipients. Darbepoetin and Epo successfully serve as adjuncts to transfusions in maintaining red cell mass in preterm infants.
Keywords: anemia, transfusions, prematurity, erythropoietin
What’s Known on This Subject:
Preterm infants in the NICU receive the greatest number of transfusions of any patient population. The administration of the long-acting erythropoiesis stimulating agent (ESA) darbepoetin to reduce or eliminate transfusions in preterm infants has not been evaluated.
What This Study Adds:
Infants receiving ESAs received half the number of transfusions and were exposed to approximately half the donors compared with the placebo group. More than half of the ESA recipients (59% darbepoetin recipients, 52% erythropoietin recipients) remained untransfused during their hospitalization.
Erythropoiesis stimulating agents (ESAs) have been used clinically for more than 20 years to stimulate red blood cell production. Erythropoietin (Epo) successfully stimulates erythropoiesis and decreases transfusion requirements in adults and children with anemia attributable to end-stage renal disease or cancer. In preterm infants, ESAs have shown varied success in decreasing the total number and volume of transfusions.1,2 Darbepoetin alfa (Darbe) is a biologically modified long-acting ESA.3 The increased half-life of Darbe allows for dosing every 1 to 4 weeks in adults with anemia attributable to end-stage renal disease or cancer. Single-dose pharmacokinetics in preterm infants revealed a similar prolonged half-life4,5; however, weekly dosing of Darbe has not been evaluated.
Transfusion guidelines have been adapted by many US neonatal units; yet, few studies have evaluated outcomes of restrictive transfusion guidelines,6–8 and transfusion studies have not been performed at high-altitude centers (>4800 feet above sea level) where oxygen use (a neonatal transfusion trigger in previous studies6,7) is generally increased in NICU populations. After publication of the Iowa transfusion study,6 many centers considered using more liberal transfusion guidelines. However, long-term neurodevelopmental outcomes among those randomized to be liberally transfused were considerably worse.9,10 Recent studies suggest an association between preterm infant morbidities, such as necrotizing enterocolitis (NEC) and significant intracranial hemorrhage (ICH), with transfusions11–13; therefore, using ESAs to avoid these possible risks might prove clinically important.
We designed a study to assess whether Darbe would decrease transfusions in preterm infants, using a restrictive transfusion protocol applied at 4 high-altitude centers (the University of New Mexico in Albuquerque, NM; McKay Dee Hospital in Ogden, UT; LDS Hospital/Intermountain Medical Center in Murray, UT; and the University of Colorado in Denver, CO). Although not designed as an equivalence trial, we hypothesized that similar to Epo, the administration of Darbe to preterm infants would result in increased reticulocyte counts and decreased transfusions compared with placebo. If successful, Darbe might be more beneficial to preterm infants in that fewer injections would be required to achieve the same benefit.
Methods
Subjects
Infants of 500 to 1250 g birth weight, ≤48 hours of age, and who were expected to survive the first days of life (as determined by the attending neonatologist) were eligible. Infants transfused before enrollment were not excluded. Infants with trisomies, significant congenital anomalies (including known neurologic anomalies), hypertension, seizures, thromboses, hemolytic disease, or receiving Epo clinically were ineligible for study. The study was approved by the institutional review boards at the Universities of New Mexico and Colorado, and Intermountain Health Care. Parental consent was obtained. An investigational new drug application was approved by the Food and Drug Drug Administration (IND 100138). The study was registered on ClinicalTrials.gov (NCT 00334737).
Randomization was stratified by center using a computer-generated permuted block method. Multiples were randomized to the same treatment group. All caregivers and investigators (except research pharmacists and coordinators) were masked to treatment assignment.
Dosing of Study Drug
Infants were randomized in masked fashion to 1 of 3 groups: Epo, 400 U/kg, given subcutaneously 3 times a week (Monday, Wednesday, and Friday); Darbe, 10 μg/kg, given subcutaneously once a week,14,15 with sham dosing 2 other times per week; or placebo, consisting of 3 sham doses per week. The study drug was brought to the bedside in a closed container, and injections were shielded behind screens and out of earshot from caregivers and parents. An adhesive bandage covered the true and sham injection sites. Dosing continued until 35 completed weeks’ gestation, discharge, transfer to another hospital, or death. Doses of Darbe and Epo were initially based on study entry weight and adjusted weekly. Study drug concentrations were chosen to give equivalent volumes (0.1 mL/kg body weight) of Darbe or Epo.
Criteria for Withholding or Stopping the Study Drug
Criteria for withholding the study drug included absolute neutrophil count <500/µL; hematocrit >50% (not due to transfusion) associated with an absolute reticulocyte count (ARC) ≥200 000 cells/µL; thrombocytopenia (<50 000/ μL) or thrombocytosis (>700 000/µL); elevated creatinine (>2.7 mg/dL); a systemic adverse reaction to injection (hypotension, respiratory compromise, or systemic rash); or hypertension, defined as mean blood pressure >2 SD above gestational age average. The study drug was restarted when these conditions resolved. Treatment was stopped if clinical seizures occurred, if thromboses were diagnosed, or if hypertension or neutropenia recurred.
Supplements
All infants (regardless of treatment arm) received supplemental iron, folate (50 µg per day oral), and vitamin E (15 IU per day oral).16 Iron dextran, 3 mg/kg once a week was added to parenteral nutrition while infants were receiving <60 mL/kg per day enteral feedings. Oral iron 3 mg/kg per day was started when feedings were ≥60 mL/kg per day, and increased to 6 mg/kg per day when feedings reached 120 mL/kg per day. Serum ferritin concentrations were used to adjust iron dosing. For infants in whom ferritin concentrations were >400 ng/mL, the parenteral or enteral dose of iron was decreased by 50%; for infants in whom ferritin concentrations were <50 ng/mL, the parenteral or enteral dose was doubled.
Laboratory Evaluations
Complete blood counts and ARC were obtained at day 1 (before study drug) and every 2 weeks during the study period. Treating clinicians were unaware of study laboratory results unless values met criteria for holding/stopping the study drug or met transfusion criteria. Ferritin concentrations were evaluated at 14 and 42 days of study. All infants (regardless of treatment arm) were evaluated for anti-ESA antibodies.
Data Collection
Blood pressure was recorded daily. The number and volume of transfusions, donor exposures, phlebotomy losses (determined by laboratory-specified blood volumes and bedside recording), and adverse events were recorded from birth through 36 weeks’ gestation, transfer, or death. The number of ventilator days, length of hospital stay, and incidence of the following were recorded: bronchopulmonary dysplasia (oxygen administration at 36 weeks corrected gestational age17), retinopathy of prematurity (ROP; all stages18), ICH (including periventricular leukomalacia19), patent ductus arteriosus and treatment (surgery or indomethacin), NEC,20 late-onset sepsis (a positive blood or cerebral spinal fluid culture obtained in the presence of compatible signs of septicemia after 72 hours of age, or culture-negative clinical infection after 72 hours of age for which the infant received antibiotics for ≥5 days), and any incidence of neutropenia or thrombocytopenia. A data safety monitoring board reviewed adverse and serious adverse events every 6 months.
Transfusion Guidelines
Infants were transfused based on clinical or research laboratory results according to a standardized, restrictive transfusion protocol used clinically at the University of New Mexico (Table 1). Each infant was assigned a matched, leuko-reduced, citrate-phosphate-dextrose adenine anticoagulant-preserved donor unit, made available in a sterile docking device capable of 50-mL aliquots of packed red blood cells (PRBCs) with ≥4 transfusions per unit, and a shelf life of 28 days. PRBCs were irradiated just before administration over 2 to 4 hours.
TABLE 1.
Transfusion Protocol
| Hct/Hgb | Respiratory Support and/or Symptoms | PRBC Volume |
|---|---|---|
| ≤30/≤10 | Moderate/significant ventilation (MAP >8 cm H2O and FiO2 >0.4) | 15–20 mL/kg |
| ≤25/≤8 | Minimal respiratory support (any mechanical ventilation with FiO2≤0.4, OR CPAP >6 cm H2O and FiO2 ≥0.4) | 20 mL/kg |
| ≤20/≤7 | Supplemental oxygen or CPAP with an FiO2≤0.4, and at least 1 of the following: | 20 mL/kg |
| • ≥24 h of tachycardia (heart rate >180) or tachypnea (RR >60); | ||
| • a doubling of the oxygen requirement from the previous 48 h | ||
| • lactate ≥2.5 mEq/L or an acute metabolic acidosis (pH<7.20); | ||
| • weight gain <10 g/kg/d over the previous 4 d while receiving ≥120 kcal/kg/d; | ||
| • undergoing surgery within 24 h | ||
| ≤18/≤6 | Asymptomatic and an ARC <100 000 cells/µL | 20 mL/kg |
CPAP, continuous positive airway pressure; Hct, hematocrit; Hgb, hemoglobin; MAP, mean airway pressure; RR, respiratory rate. Transfusions could be administered in 2 10-mL/kg aliquots, as determined by attending clinician.
Sample Size Estimate
The primary outcome was number of transfusions. Secondary outcomes included donor exposure, ARC, hematocrit, hospital stay, and morbidities associated with preterm birth, specifically ROP. A total of 27 infants in each group were required to identify a difference of 2 transfusions between Darbe and placebo, using an α of 0.05 and 80% power. Two-year follow-up was planned; to account for deaths (15%) and loss to follow-up (10%), 34 infants per group were enrolled.
Statistical Analysis
The number of transfusions, donors, and transfusion violations were compared using Poisson regression. For univariate variables, differences among groups were compared by using 2-tailed Fisher’s exact tests for categorical variables and 1-way analysis of variance or Kruskal-Wallis test (depending on distributional characteristics) for continuous variables. For repeated measures data, groups were compared by using linear mixed models (random coefficient regression).
Results
A total of 905 infants were screened for the study. Of those, 513 were eligible. Reasons for nonrandomization included parent refusal (49%), consent not requested (8%), or enrollment in other studies (43%). A total of 102 infants were enrolled in the study between July 2006 and May 2010. Three subjects (1 who had the study drug mistakenly held at the start of the study and subsequently never received any study drug; 1 who was found to be ineligible based on congenital neurologic anomaly on head ultrasound noted before receiving study drug; and 1 who died of a pulmonary hemorrhage before receiving study drug) were excluded from analysis. One infant had the study drug stopped at 34 weeks’ corrected gestation at the request of parents. All infants who received at least 1 dose of study drug were included in analysis.
Maternal and infant characteristics are shown in Table 2. By chance, a greater number of female infants were enrolled in the Darbe group (P = .039), and more mothers were diagnosed with abruption in the placebo group (P = .024). There were no other significant differences among the 3 groups in maternal or neonatal characteristics. Prestudy hematocrit, transfusions, and phlebotomy losses were similar among groups.
TABLE 2.
Baseline Characteristics
| Darbe, n = 33 | Epo, n = 33 | Placebo, n = 33 | P = | |
|---|---|---|---|---|
| Maternal information | ||||
| Antenatal steroids, % (n) | 94 (31) | 88 (29) | 85 (28) | .614 |
| Chorio, % (n) | 18 (6) | 9 (3) | 12 (4) | .820 |
| Antibiotics, % (n) | 61 (20) | 58 (19) | 67 (22) | .813 |
| Preeclampsia, % (n) | 48 (16) | 36 (12) | 36 (12) | .543 |
| Abruption, % (n) | 0 | 9 (3) | 18 (6) | .039 |
| Previa, % (n) | 3 (1) | 3 (1) | 6 (2) | 1.000 |
| Diabetes, % (n) | 9 (3) | 15 (5) | 12 (4) | .926 |
| C/S, % (n) | 76 (25) | 73 (24) | 79 (26) | .956 |
| Black, % (n) | 9 (3) | 6 (2) | 3 (1) | .869 |
| White, % (n) | 39 (13) | 55 (18) | 36 (12) | .301 |
| Hispanic, % (n) | 42 (14) | 30 (10) | 52 (17) | .239 |
| Native American, % (n) | 3 (1) | 9 (3) | 9 (3) | .694 |
| Pacific Islander, % (n) | 6 (2) | 0 | 0 | .327 |
| Neonatal information | ||||
| Gestation, wk | 27.9 ± 1.8 | 27.8 ± 1.9 | 27.3 ± 1.8 | .528 |
| 28.0 [26.4, 29.0] | 28.0 [26.3, 29.0] | 27.6 [26.0, 28.6] | ||
| Birth weight, g | 941 ± 164 | 957 ± 212 | 933 ± 221 | .816 |
| 940 [855, 1090] | 942 [781, 1150] | 995 [740, 1141] | ||
| Head circumference, cm | 25.7 ± 2.9 | 25.5 ± 2.1 | 25.1 ± 2.8 | .655 |
| 25.5 [24.0, 26.5] | 25.5 [24.5, 26.5] | 25.0 [23.0, 26.5] | ||
| Length, cm | 33.4 ± 6.3 | 35.5 ± 3.5 | 34.7 ± 3.6 | .192 |
| 35.3 [33.0, 36.0] | 36.0 [34.0, 37.5] | 36.0 [33.0, 37.5] | ||
| Female, % (n) | 64 (21) | 33 (11) | 39 (13) | .039 |
| SGA, % (n) | 21 (7) | 18 (6) | 12 (4) | .368 |
| Singleton % (n) | 76 (25) | 82 (27) | 70 (23) | .564 |
| Twin sets, n | 3 | 2 | 4 | .559 |
| Inborn, % (n) | 94 (31) | 88 (29) | 91 (30) | 1.00 |
| Fetal distress, % (n) | 27 (9) | 44 (14) | 48 (16) | .188 |
| 1-min Apgar | 5.0 ± 2.3 | 4.7 ± 2.6 | 4.8 ± 2.4 | .913 |
| 5 [3, 7] | 5 [2, 7] | 4.5 [3, 7] | ||
| 5-min Apgar | 7.4 ± 1.9 | 7.3 ± 1.8 | 7.3 ± 1.4 | .742 |
| 8 [7, 9] | 8 [7, 8] | 8 [6.5, 8] | ||
| Hematocrit, % | 44.5 ± 7.4 | 44.9 ± 7.3 | 44.5 ± 5.4 | .870 |
| 46.2 [41, 50] | 45.7 [39.9, 49.5] | 45 [43.0, 47.7] | ||
| MCV, fL | 116 ± 9 | 117 ± 8 | 117 ± 7 | .835 |
| 116 [112, 123] | 117.5 [112, 124] | 116 [111, 118] | ||
| WBC, × 106/μL | 10.1 ± 8.8 | 9.3 ± 6.5 | 7.2 ± 3.3 | .182 |
| 80.0 [5.6, 11.2] | 6.9 [5.3,11.5] | 6.4 [4.4, 9.3] | ||
| Absolute neutrophil count, × 103/μL | 4.3 ± 6.9 | 4.2 ± 5.1 | 2.9 ± 2.8 | .272 |
| 3.0 [1.7, 4.3] | 2.5 [1.4, 5.1] | 1.9 [1.1, 3.7] | ||
| Platelets, × 103/μL | 203 ± 71 | 210 ± 61 | 203 ± 77 | .724 |
| 190 [146, 235] | 217 [166, 254] | 201 [147, 239] | ||
| Transfused before study, % (n) | 18 (6) | 15 (5) | 18 (6) | 1.00 |
| PRBC volume, mL/kg | 13.2 ± 5.2 | 14.2 ± 2.3 | 27.2 ± 14.5 | .413 |
| 15.2 [14.6, 15.5] | 14.8 [14.8, 15.1] | 30.5 [10.0, 35.9] | ||
| Prestudy phlebotomy loss, mL/kg | 5.0 ± 4.5 | 5.5 ± 3.5 | 6.7 ± 5.1 | .189 |
| 3.9 [1.6, 7.1] | 5.5 [3, 7.8] | 5.4 [3.5, 9.3] | ||
| Age at first dose, h | 46 ± 27 | 54 ± 25 | 62 ± 65 | .414 |
| 47 [21, 61] | 59 [34, 73] | 48 [36, 65] |
C/S, cesarean section; fL, femtoliter; MCV, mean corpuscular volume (fL); SGA, small for gestational age; WBC, white blood cells. P values represent comparison among 3 groups. Under Neonatal information, values in the top line of each section are mean ± SD, and values in the second line of each section are median (first and third quartile).
Infants in the Darbe and Epo groups (both separately and combined) required significantly fewer transfusions (P = .015, combined comparison) and were exposed to significantly fewer donors during the study (P = .044, combined comparison) than those in the placebo group (Table 3). Fifty-nine percent of Darbe, 52% of Epo, and 38% of the placebo group did not receive any transfusions during their hospitalization. Transfusion protocol violations occurred at similar rates for transfused subjects, either because the hematocrit exceeded the value specified by the transfusion guidelines or because less than the prescribed transfusion volume was administered (Table 3). There were no significant differences in phlebotomy losses among groups during the study (Table 3).
TABLE 3.
Transfusions and Phlebotomy Losses
| Darbe, n = 33 | Epo, n = 33 | Placebo, n = 33 | P1 = | P2 = | |
|---|---|---|---|---|---|
| Transfusions/subject | 1.2 ± 2.4 | 1.2 ± 1.6 | 2.4 ± 2.9 | .015 | .949 |
| 0 [0, 1] | 0 [0, 2] | 1 [0, 4] | |||
| Donors/subject | 0.7 ± 1.2 | 0.8 ± 1.0 | 1.2 ± 1.3 | .044 | .558 |
| 0 [0, 1] | 0 [0, 2] | 1 [0, 2] | |||
| Volume, mL/kg | 30 ± 58 | 23 ± 33 | 51 ± 65 | .041 | .600 |
| 0 [0, 29.2] | 0 [0, 36.6] | 20.0 [0, 87.9] | |||
| Volume, mL/kg, transfused subjects only | 75.6 ± 73.2 | 48.0 ± 32.7 | 80.7 ± 65.3 | .36 | .61 |
| 45 [20.0, 102.4] | 39.8 [21.5, 65.7] | 83.3 [20.0, 97.8] | |||
| Subjects not transfused, n (%) | 20 (59%) | 17 (52%) | 12 (38%) | .088 | .620 |
| Transfusion protocol violations/transfused subject, total | 0.8 ± 1.7 | 1.1 ± 1.1 | 0.9 ± 1.4 | .805 | .661 |
| 0 [0, 1] | 1 [0, 1] | 0 [0, 1] | |||
| Due to high hematocrit | 0.7 ± 1.7 | 0.8 ± 1.1 | 0.5 ± 1.3 | .435 | .895 |
| 0 [0, 1] | 0.5 [0, 1] | 0 [0, 0] | |||
| Due to low transfusion volume | 0.2 ± 0.4 | 0.3 ± 0.8 | 0.4 ± 0.6 | .300 | .381 |
| 0 [0, 0] | 0 [0, 0] | 0 [0, 1] | |||
| Total phlebotomy losses, mL/kg | 32.3 [20.3–42.5] | 29.9 [20.4–42.5] | 24.9 [20.4–53.0] | .732 | .802 |
| 43.7 ± 45.3 | 42.5 ± 31.0 | 55.9 ± 53.9 | |||
| Pre study | 3.6 [1.6–7.2] | 5.8 [3.4–8.9] | 6.4 [3.7–9.0] | .137 | .267 |
| 5.6 ± 6.5 | 6.1 ± 4.4 | 7.9 ± 7.5 | |||
| During study | 32.3 [20.3–42.5] | 29.9 [20.4–42.5] | 24.9 [20.4–53.0] | .974 | .801 |
| 37.7 ± 43.1 | 35.5 ± 30.7 | 46.9 ± 49.5 | |||
| Post study | 0 [0–0] | 0.2 [0–1.3] | 0.3 [0–1.0] | .191 | .015 |
| 0.5 ± 1.1 | 0.9 ± 2.0 | 1.1 ± 2.1 |
Values represent mean ± SD and median [first quartile, third quartile]. Phlebotomy losses are represented by 95% confidence intervals. P1, Darbe plus Epo (combined) versus placebo; P2, Darbe versus Epo
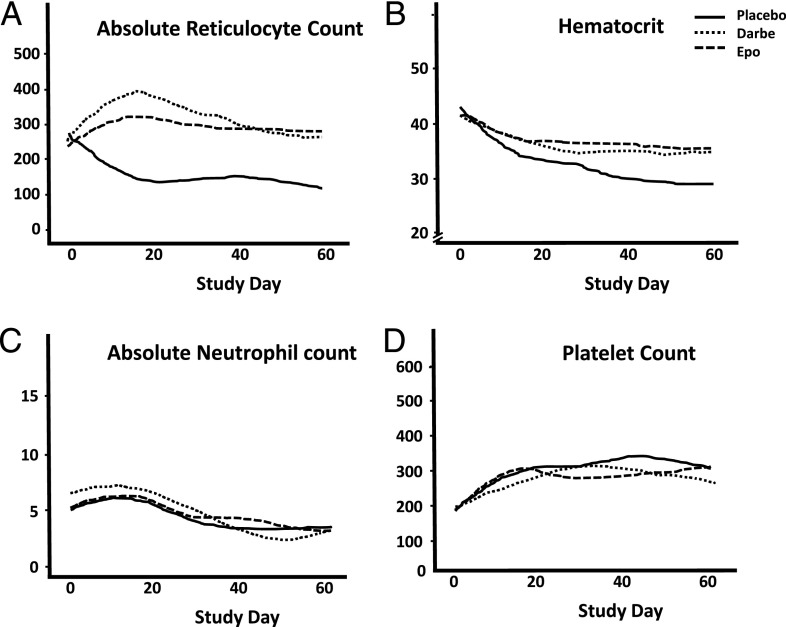
Changes in ARC are shown in Fig 1A. Infants treated with Darbe or Epo had significantly higher ARC than infants in the placebo group (P = .001). Similarly, hematocrits were significantly greater in the ESA groups compared with placebo (P = .001; Fig 1B). There were no differences in absolute neutrophil count (Fig 1C) or platelet counts (Fig 1D) among groups during the study.
FIGURE 1.
Hematologic indices during the study curves were generated for ARC (A), hematocrit (B), absolute neutrophil count (C), and platelet count (D) using nonparametric Lowess (locally weighted scatterplot smoothing) regression smoothers calculated for each treatment arm separately. Data were fit with linear mixed models where each subject was allowed a separate quadratic trajectory (random coefficient regression), with interaction allowed between time and treatment arm, and P values reported are for the fixed effects contrasts comparing groups. A, Changes in ARC. Infants treated with Darbe (dotted line) or Epo (hatched line) had significantly higher ARCs than infants in the placebo group (solid line; P = .001). Similarly, hematocrits were significantly greater in the ESA groups compared with placebo (P = .001; B). There were no differences in absolute neutrophil counts (C) or platelet counts (D) among groups during the study.
Ferritin concentrations were higher in the placebo group at days 14 and 42 of the study (Table 4). More infants in the ESA groups had their iron dose increased on day 14 and day 42 compared with the placebo group. More infants in the placebo group had their iron dose decreased on day 42 because of ferritin >400. The average number of parenteral iron doses per infant was similar, as was average age at which oral iron was started. No adverse events were attributed to administration of parenteral or enteral iron.
TABLE 4.
Iron Dosing and Ferritin Concentrations
| Darbe, n = 33 | Epo, n = 33 | Control, n = 33 | P = | |
|---|---|---|---|---|
| Ferritin d 14 | 121 [63, 184] | 104 [58, 197] | 204 [114, 348] | .003 |
| Ferritin d 42 | 50 [27, 77] | 61 [33, 99] | 127 [67, 216] | .002 |
| No. of intravenous iron doses | 1.7 ± 1.3 | 1.6 ± 1.5 | 1.9 ± 2.3 | .317 |
| Oral iron started, days of age | 15.5 ± 8.5 | 12.9 ± 8.4 | 14.5 ± 7.7 | .205 |
| Ferritin <50 d 14 | 7 | 5 | 2 | .024 |
| Ferritin <50 d 42 | 12 | 13 | 2 | <.001 |
| Ferritin >400 d 14 | 1 | 1 | 5 | .118 |
| Ferritin >400 d 42 | 3 | 0 | 5 | .045 |
Ferritin concentrations reported as median [first quartile, third quartile]; P values represent comparisons among groups.
Mortality and all preterm morbidities evaluated were similar among groups (Table 5). Specifically, the incidence of all stages of ROP was no different among groups. The incidence of ICH grade ≥3 was not statistically different among groups. Side effects (hypertension, thromboses, seizures, neutropenia) were minimal and similar among treatment groups (Table 5). There were no significant differences in mean blood pressures among groups.
TABLE 5.
Clinical Outcomes
| Darbe, n = 32 | Epo, n = 32 | Placebo, n = 30 | |
|---|---|---|---|
| CLD, n | 22 | 21 | 20 |
| Days on ventilation | 19 ± 52 | 13 ± 20 | 16 ± 20 |
| 3 [1, 18] | 4 [1, 15] | 7 [2, 26] | |
| No ROP, n | 22 | 22 | 21 |
| ROP ≤stage 2, n | 10 | 9 | 8 |
| ROP >stage 2-regressed, n | 0 | 1 | 2 |
| ROP >stage 2-required intervention, n | 2 | 1 | 2 |
| ICH ≥grade 3, n | 3 | 3 | 7 |
| NEC >stage 2 , n | 2 | 1 | 2 |
| Patent ductus arteriosus, n | 16 | 16 | 15 |
| Medically treated, n | 10 | 10 | 5 |
| Surgically treated, n | 3 | 2 | 7 |
| Positive culture, n | 6 | 5 | 5 |
| Hypertension, n | 2 | 0 | 1 |
| Seizures, n | 3 | 1 | 0 |
| Thromboses, n | 0 | 0 | 1 |
| Neutropenia, n | 0 | 0 | 0 |
| Mortality, n | 2 | 1 | 3 |
| Hospital stay, d | 76 ± 46 | 72 ± 26 | 74 ± 33 |
| 69 [51, 87] | 73 [55, 89] | 63 [52, 88] | |
| Weight at 36 wk, g | 2046 ± 346 | 2059 ± 417 | 2025 ± 507 |
| 2060 [1730, 2332] | 2126 [1779, 2258] | 1980 [1845, 2369] | |
| Head circumference at 36 wk, cm | 31.2 ± 2.7 | 31.1 ± 1.6 | 31.6 ± 2.1 |
| 30.7 [29.5, 32.5] | 31.0 [30.0, 31.9] | 31.5 [30.5, 32.1] |
Values represent mean ± SD and median [first quartile, third quartile]. All differences among groups in clinical outcomes were not significant (all P values >0.2).
None of the ESA recipients tested had evidence of antibody formation (D. Mytych, PhD, Amgen Scientific Director, personal communication, 2013). One infant in the placebo group had measurable anti-Epo antibody (2.5 μg/mL) and anti-Darbe antibody (8 μg/mL) concentrations. More detailed antibody isotype analysis detected anti-ESA immunoglobulin (Ig)M antibodies at day 56. No anti-ESA IgG was detected. This infant did not receive any blood products; nor did the infant receive Darbe, Epo, or other medications that could result in antibody formation. Two additional infants (1 placebo, 1 on Epo) had evidence of weak anti-Epo antibody formation that was not clinically relevant (<0.25 μg/mL).
Discussion
The number of transfusions preterm infants receive has gradually decreased in the past decade. Although PRBC transfusions are generally considered safe, there is a small but measurable risk of infection, transfusion reaction, or transfusion-associated morbidities, such as transfusion-related lung injury.21 In addition, with new evidence from recent studies that demonstrate potential side effects of red cell transfusions, such as extension of ICH and transfusion-associated NEC,11–13, 22 the need to study the safety and efficacy of ESAs, in combination with restrictive transfusion guidelines, urgently increases.
This is the first prospective, randomized, masked, multicenter study of early Darbe administered to preterm infants, and the first study of ESA administration to preterm infants that resulted in a significant decrease in both donor exposure and transfusion number. ESA-treated infants were exposed to approximately half the donors and received half the number of transfusions compared with the placebo group. In addition, although not the primary outcome of the study, 59% of the Darbe recipients and 52% of the Epo recipients did not receive any transfusions during their hospitalization. Although previous studies outside the United States have achieved similar levels of success,23 this is the first US study to report a >50% transfusion-free population of very low birth weight infants. Moreover, although the number of transfusions has decreased over the past 20 years, extremely low birth weight (ELBW) infants are still receiving 3 to 5 PRBC transfusions per hospitalization, and are being exposed to 2 to 3 donors.24,25
The success of this study compared with previous studies may be in part because of differences in study design. We compared early Darbe administration and the usual erythropoietic dose of Epo with placebo to determine if ESAs could decrease or prevent transfusions. Study medications were generally initiated before 60 hours of life, 12 to 24 hours earlier than previous studies.16 The earlier administration of ESAs might also benefit longer-term developmental outcomes. The absence of antibody formation was specifically tested in all available infant samples. Finally, we applied stringent transfusion guidelines at sites that were above 4800 feet elevation. Despite being at higher elevation, infants appeared to tolerate the restrictive guidelines, although long-term developmental outcomes require further study.
Instituting strict transfusion guidelines can have a significant impact on the number of transfusions infants receive. This is most beneficial to preterm infants in a practical sense, because it can be implemented without additional cost. A previous study reported $780 074 savings 1 year after implementation of transfusion guidelines.26 Moreover, the risks of transfusions continue to be evaluated. The 2006 Cochrane analysis of “early” Epo usage in the NICU included 23 studies involving 2074 preterm infants.27 Summary statistics indicated a modest reduction in early transfusions. Similarly, summary statistics of the effect of the “late” use of Epo28 indicated a significant but small reduction in transfusions. In both analyses the importance of 1 or 2 fewer transfusions was questioned.27,28 However, this benefit might have greater clinical significance in light of recent associations between early transfusions and severe ICH,12 late transfusions and the development of NEC,11,22 and potentially damaging concentrations of heavy metals, such as lead and mercury.29 It is possible that even 1 to 2 fewer transfusions at key periods during a NICU course could be beneficial.
Previous studies evaluating specific transfusion strategies reported conflicting results. Bell and colleagues6 reported an increase in serious ICH and PVL in those infants randomized to a restrictive transfusion strategy. Kirpalani and colleagues7 randomized 451 infants to low- or high-threshold hemoglobin strategies and reported no difference in neurologic morbidities in the low–hemoglobin threshold group; however, analyses at 2 years of age revealed increased mild cognitive impairment in the low–hemoglobin threshold group.8 Conversely, improved cognitive and MRI outcomes were reported in the restrictively transfused group of the Iowa study,9,10 underscoring the need for long-term evaluation and more comprehensive study.
Side effects were minimal and not different between ESA-treated and placebo groups. In addition, similar to adult studies (particularly Darbe studies), none of the ESA-treated infants tested developed antibodies. The presence of polyreactive IgM (which cross-reacts with multiple antigens) is not unexpected because of an immature immune response. Anti-ESA IgM reactivity to glycosylated therapeutic proteins has been previously demonstrated to ESAs in other patient populations.30 At present, there are no published reports of neonates receiving more than single doses of Darbe, and further study is required in a larger population of ELBW infants to identify side effects specific to neonates. This study was not powered to identify differences in the low incidence of any side effects, specifically ROP, a preterm morbidity that has been linked to early use of Epo.27 Similar to our previous randomized trials of Epo administration to preterm infants, we saw no difference in the incidence of any stage of ROP.16,31,32 Close ophthalmologic evaluation of all ESA-treated infants remains a priority in treatment studies. To determine a similar difference reported in the stud by Romagnoli et al33 (where ROP was a significant side effect), a total of 450 infants would be required. However, because that study33 began Epo in the second week of life, it is difficult to compare with our current study.
Recent animal studies have identified neuroprotective properties of both Darbe and Epo.15,33–41 In addition to stimulating erythropoiesis, ESAs are protective in the developing brain, making it possibly beneficial for very premature infants who are at risk for intraventricular hemorrhage, hypoxic-ischemic injury, and developmental delay.37 The neuroprotective mechanisms of ESAs include decreased neuronal apoptosis, decreased inflammation, increased neurogenesis, promotion of oligodendrocyte differentiation and maturation, and improved white matter survival.35–41 Recent clinical studies suggest a strong potential for neuroprotection.42–45 Our group previously reported an improved cognitive outcome in former ELBW infants with serum Epo concentrations ≥500 mU/mL.42 Brown and colleagues43 reported improved cognitive outcomes in preterm infants exposed to higher cumulative does of Epo, and European investigators reported improved developmental outcomes at 8 to 12 years in former Epo-treated preterm infants, especially those with significant brain injury during initial hospitalization.44 McAdams et al randomized ELBW infants to 3 doses of 500 to 2500 U/kg Epo and found improved neurodevelopmental outcomes compared with concurrent controls.45 Neurodevelopmental follow-up of study infants is ongoing.
We conclude that Darbe and Epo are effective in decreasing donors and transfusions in preterm infants without increasing morbidities. With increasing evidence of possible risks of transfusions, it was significant that more than 50% of the treated infants remained untransfused. Importantly, Darbe provides this effect with weekly dosing. Our study found no differences in preterm infant morbidities, and in particular, no differences in ROP between groups; however, conclusions regarding overall safety require larger randomized studies.
Acknowledgments
The authors are indebted to the parents for their willingness to allow their infants to participate in this study, and to the staff and nurses at the Universities of New Mexico and Colorado, and the LDS Hospital, Intermountain Medical Center, and McKay Dee Hospital NICUs for their support and contributions to this study. We also thank the General Clinical Research Center/Clinical Translational Science Center Research Coordinators (Carol Hartenberger, Sandra Brown, Christine Reed, Donna Rodden, Kathy Hale, and Lucy Fashaw) for their support and contributions to this study, to Hannah Peceny for management of the data collection system, and to members of the Data Safety Monitoring Board for their time and effort during the study. We thank Amgen for performing the antibody assay. Finally, the authors thank the Thrasher Research Fund for their support.
Glossary
- ARC
absolute reticulocyte count
- Darbe
darbepoetin alfa
- ELBW
extremely low birth weight
- Epo
erythropoietin
- ESA
erythropoiesis stimulating agents
- ICH
intracranial hemorrhage
- Ig
immunoglobulin
- NEC
necrotizing enterocolitis
- PRBC
packed red blood cells
- ROP
retinopathy of prematurity
Footnotes
Dr Ohls conceptualized and designed the study, performed analyses, and drafted the initial manuscript; Dr Christensen conceptualized and designed the study, was the principal investigator at the Ogden site, and reviewed and revised the manuscript; Dr Kamath-Rayne served as co–principal investigator and supervised data collection at the Colorado site, and critically reviewed and revised the manuscript; Dr Rosenberg was the principal investigator and supervised data collection at the Colorado site, and critically reviewed the manuscript; Dr Wiedmeier was the principal investigator and supervised data collection at the Salt Lake City site, and reviewed the manuscript; Ms Roohi and Ms Lacy coordinated the study and performed data collection at the New Mexico site, and critically reviewed and revised the manuscript; Ms Lambert coordinated the study and performed data collection at the Ogden site, and critically reviewed the manuscript; Ms Burnett coordinated the study and performed data collection at the Salt Lake City site, and critically reviewed the manuscript; Ms Pruckler coordinated the study and performed data collection at the Colorado site, and critically reviewed the manuscript; Dr Schrader performed the statistical analyses, and critically reviewed the manuscript; Dr Lowe performed statistical analyses, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT00334737; IND100138).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This project was supported in part by grants from the Thrasher Research Fund, the University of Colorado Clinical and Translational Sciences Institute (UL1 TR000154), and by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number 8UL1TR000041, The University of New Mexico Clinical and Translational Science Center. Funded by the National Institutes of Health.
References
- 1.Bishara N, Ohls RK. Current controversies in the management of the anemia of prematurity. Semin Perinatol. 2009;33(1):29–34 [DOI] [PubMed] [Google Scholar]
- 2.Maier RF, Obladen M, Müller-Hansen I, et al. European Multicenter Erythropoietin Beta Study Group . Early treatment with erythropoietin beta ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141(1):8–15 [DOI] [PubMed] [Google Scholar]
- 3.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer. 2001;84(suppl 1):3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warwood TL, Ohls RK, Wiedmeier SE, et al. Single-dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25(11):725–730 [DOI] [PubMed] [Google Scholar]
- 5.Warwood TL, Ohls RK, Lambert DK, et al. Intravenous administration of darbepoetin to NICU patients. J Perinatol. 2006;26(5):296–300 [DOI] [PubMed] [Google Scholar]
- 6.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115(6):1685–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149(3):301–307 [DOI] [PubMed] [Google Scholar]
- 8.Whyte RK, Kirpalani H, Asztalos EV, et al. PINTOS Study Group . Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123(1):207–213 [DOI] [PubMed] [Google Scholar]
- 9.McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011;17(4):347–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nopoulos PC, Conrad AL, Bell EF, et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011;165(5):443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen RD. Association between red blood cell transfusions and necrotizing enterocolitis. J Pediatr. 2011;158(3):349–350 [DOI] [PubMed] [Google Scholar]
- 12.Baer VL, Lambert DK, Henry E, Snow GL, Christensen RD. Red blood cell transfusion of preterm neonates with a Grade 1 intraventricular hemorrhage is associated with extension to a Grade 3 or 4 hemorrhage. Transfusion. 2011;51(9):1933–1939 [DOI] [PubMed] [Google Scholar]
- 13.Christensen RD, Lambert DK, Baer VL, et al. Postponing or eliminating red blood cell transfusions of very low birth weight neonates by obtaining all baseline laboratory blood tests from otherwise discarded fetal blood in the placenta. Transfusion. 2011;51(2):253–258 [DOI] [PubMed] [Google Scholar]
- 14.Roohi M, Peceny MC, Ohls RKA. Randomized, masked, dose response study of darbepoetin administered to preterm infants. Pediatr Res. 2007;61:14 [Google Scholar]
- 15.Belayev L, Khoutorova L, Zhao W, et al. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36(5):1071–1076 [DOI] [PubMed]
- 16.Ohls RK, Ehrenkranz RA, Wright LL, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics. 2001;108(4):934–942 [DOI] [PubMed] [Google Scholar]
- 17.Ehrenkranz RA, Walsh MC, Vohr BR, et al. National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360 [DOI] [PubMed] [Google Scholar]
- 18.The Committee for the Classification of Retinopathy of Prematurity . An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102(8):1130–1134 [DOI] [PubMed] [Google Scholar]
- 19.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534 [DOI] [PubMed] [Google Scholar]
- 20.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez R, Toy P. Transfusion related acute lung injury: a pediatric perspective. Pediatr Blood Cancer. 2005;45(3):248–255 [DOI] [PubMed] [Google Scholar]
- 22.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129(3):529–540 [DOI] [PubMed] [Google Scholar]
- 23.Haiden N, Schwindt J, Cardona F, et al. Effects of a combined therapy of erythropoietin, iron, folate, and vitamin B12 on the transfusion requirements of extremely low birth weight infants. Pediatrics. 2006;118(5):2004–2013 [DOI] [PubMed] [Google Scholar]
- 24.Carroll PD, Widness JA. Nonpharmacological, blood conservation techniques for preventing neonatal anemia—effective and promising strategies for reducing transfusion. Semin Perinatol. 2012;36(4):232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115(5):1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baer VL, Henry E, Lambert DK, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion. 2011;51(2):264–269 [DOI] [PubMed] [Google Scholar]
- 27.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;(3):CD004863. [DOI] [PubMed] [Google Scholar]
- 28.Aher S, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;(3):CD004868. [DOI] [PubMed] [Google Scholar]
- 29.Bearer CF, Linsalata N, Yomtovian R, Walsh M, Singer L. Blood transfusions: a hidden source of lead exposure. Lancet. 2003;362(9380):332. [DOI] [PubMed] [Google Scholar]
- 30.Barger TE, Wrona D, Goletz TJ, Mytych DT. A detailed examination of the antibody prevalence and characteristics of anti-ESA antibodies. Nephrol Dial Transplant. 2012;27(10):3892–3899 [DOI] [PubMed] [Google Scholar]
- 31.Ohls RK, Roohi M, Peceny HM, Schrader R, Bierer R. A randomized, masked study of weekly erythropoietin dosing in preterm infants. J Pediatr. 2012;160(5):790–795.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double-blind, placebo-controlled study. J Pediatr. 1997;131(5):661–665 [DOI] [PubMed] [Google Scholar]
- 33.Romagnoli C, Zecca E, Gallini F, Girlando P, Zuppa AA. Do recombinant human erythropoietin and iron supplementation increase the risk of retinopathy of prematurity? Eur J Pediatr. 2000;159(8):627–628 [DOI] [PubMed] [Google Scholar]
- 34.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008;26(1):103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412(6847):641–647 [DOI] [PubMed] [Google Scholar]
- 36.Kumral A, Baskin H, Gokmen N, et al. Selective inhibition of nitric oxide in hypoxic-ischemic brain model in newborn rats: is it an explanation for the protective role of erythropoietin? Biol Neonate. 2004;85(1):51–54 [DOI] [PubMed] [Google Scholar]
- 37.Chattopadhyay A, Choudhury TD, Bandyopadhyay D, Datta AG. Protective effect of erythropoietin on the oxidative damage of erythrocyte membrane by hydroxyl radical. Biochem Pharmacol. 2000;59(4):419–425 [DOI] [PubMed] [Google Scholar]
- 38.Akisu M, Tuzun S, Arslanoglu S, Yalaz M, Kultursay N. Effect of recombinant human erythropoietin administration on lipid peroxidation and antioxidant enzyme(s) activities in preterm infants. Acta Med Okayama. 2001;55(6):357–362 [DOI] [PubMed] [Google Scholar]
- 39.Genc S, Akhisaroglu M, Kuralay F, Genc K. Erythropoietin restores glutathione peroxidase activity in 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine-induced neurotoxicity in C57BL mice and stimulates murine astroglial glutathione peroxidase production in vitro. Neurosci Lett. 2002;321(1-2):73–76 [DOI] [PubMed] [Google Scholar]
- 40.Vairano M, Dello Russo C, Pozzoli G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16(4):584–592 [DOI] [PubMed] [Google Scholar]
- 41.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44(4):391–403 [DOI] [PubMed] [Google Scholar]
- 42.Beirer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e635 [DOI] [PubMed] [Google Scholar]
- 43.Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124(4). Available at: www.pediatrics.org/cgi/content/full/124/4/e681 [DOI] [PubMed] [Google Scholar]
- 44.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010;67(5):657–666 [DOI] [PubMed] [Google Scholar]
- 45.McAdams RM, McPherson RJ, Mayock DE, Juul SE. Outcomes of extremely low birth weight infants given early high-dose erythropoietin. J Perinatol. 2013;33(3):226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]