Abstract
Purpose
To systematically examine trends and applications of the disease risk score (DRS) as a confounder summary method.
Methods
We completed a systematic search of MEDLINE and Web of Science® to identify all English language articles that applied DRS methods. We tabulated the number of publications by year and type (empirical application, methodological contribution, or review paper) and summarized methods used in empirical applications overall and by publication year (<2000, ≥2000).
Results
Of 714 unique articles identified, 97 examined DRS methods and 86 were empirical applications. We observed a bimodal distribution in the number of publications over time, with a peak 1979-1980, and resurgence since 2000. The majority of applications with methodological detail derived DRS using logistic regression (47%), used DRS as a categorical variable in regression (93%), and applied DRS in a non-experimental cohort (47%) or case-control (42%) study. Few studies examined effect modification by outcome risk (23%).
Conclusion
Use of DRS methods has increased yet remains low. Comparative effectiveness research may benefit from more DRS applications, particularly to examine effect modification by outcome risk. Standardized terminology may facilitate identification, application, and comprehension of DRS methods. More research is needed to support the application of DRS methods, particularly in case-control studies.
Keywords: confounding factors (epidemiology), epidemiologic methods, pharmacoepidemiology, propensity score, review literature as topic
INTRODUCTION
Epidemiologic analyses often require investigators to control for many measured confounding variables. Restriction, stratification and matching allow for easily interpretable analysis, yet become complex as the number of variables for adjustment increase.1 Adjustment using multivariable regression techniques has thus become a standard method to control for confounding. In addition to conventional multivariable regression methods that include the exposure and potential confounding variables in a single outcome model, two methods of confounder summary score techniques have been proposed: the exposure propensity score (EPS), and the disease risk score (DRS).2-8 EPS reflect patients’ exposure probability conditional on measured confounders. This confounder summary score can then be used in place of the individual confounding variables in conventional adjustment methods, such as: matching, stratification, weighting, restriction, or as a covariate in the outcome model.3,4,9 Use of EPS has increased exponentially since its introduction in 1983.2,3 However, EPS is limited when exposure is rare and can be complicated when studying multiple exposures or multiple exposure levels.
The DRS is the prognostic analogue of the EPS, derived based on the predicted risk of disease outcome and was first proposed methodologically in 1976.5 Early simulation work published in 1979 concluded that the DRS method may overestimate the effect of confounders and thus bias results.10 A subsequent simulation published in 1989 concluded that overestimation of confounders may be rare, particularly when applying the DRS as a categorical variable.11 Recent evidence also identifies that in the setting of a large number of exposed individuals and outcomes, conventional multivariable regression, EPS and DRS methods yield similar results provided covariates are not highly correlated with exposure.3,6-8,12 Although the intention of EPS and DRS in summarizing confounders into a single summary score is similar, the logic behind the methods is distinct. EPS model the treatment selection process to balance treatment determinants, similar in concept to randomization in clinical trials. DRS do not share this feature of balancing baseline covariates across treatment groups. Rather, DRS seek to balance outcome determinants such that baseline outcome risk is similar between treatment groups. In contrast to EPS, DRS are not limited when exposure is rare or categorical, and they can provide a meaningful scale across which investigators can examine effect modification. Despite their advantages and even though they were initially proposed before EPS,5,13-15 DRS have received less attention in the epidemiologic literature.16 We sought to systematically examine trends the use and application of DRS as a confounder summary method.
METHODS
We conducted a systematic literature search to identify all English language articles that utilized DRS confounder summary score methods in studies of humans. We searched the MEDLINE database from 1965 to May 2011 with keyword terms: “disease$ risk$ score$”, “summary$ risk$ score$”, “multivariate$ risk$ score$”, “confounder$ score$”, and “Miettinen$ confounder$ score$”; it was important to include terms in quotations to avoid inclusion of papers that may have used comorbidity indices, such as the Charlson Index,17 or a risk scoring system, such as the Framingham risk score.18 We then used Web of Science® to perform a citation search to identify papers that referenced seminal DRS method papers,5,6,10,11 and an author search to identify articles published by investigators noted to have frequently used DRS (PG Arbogast and WA Ray). Two authors (MT, JJG) reviewed all abstracts to exclude articles that clearly did not meet eligibility criteria. Discrepancies were resolved by agreement. Full text articles were then reviewed to confirm eligibility. The number of eligible publications was plotted by calendar year and type, as: empirical application, methodological contribution or review paper. We then focused exclusively on the empirical applications and abstracted: author, journal, year of publications, terminology used to describe method, study design, sample size, exposure and outcome variable, number of outcome events, primary analytical methods to derive DRS (statistical method used, data type, number of covariates in model), and how DRS was applied. One author (MT) extracted all data and a second author (JJG) verified all extracted data. The area of study and methods of DRS derivation and application were tabulated overall, and stratified by calendar year of publication in print (before or after January 2000).
RESULTS
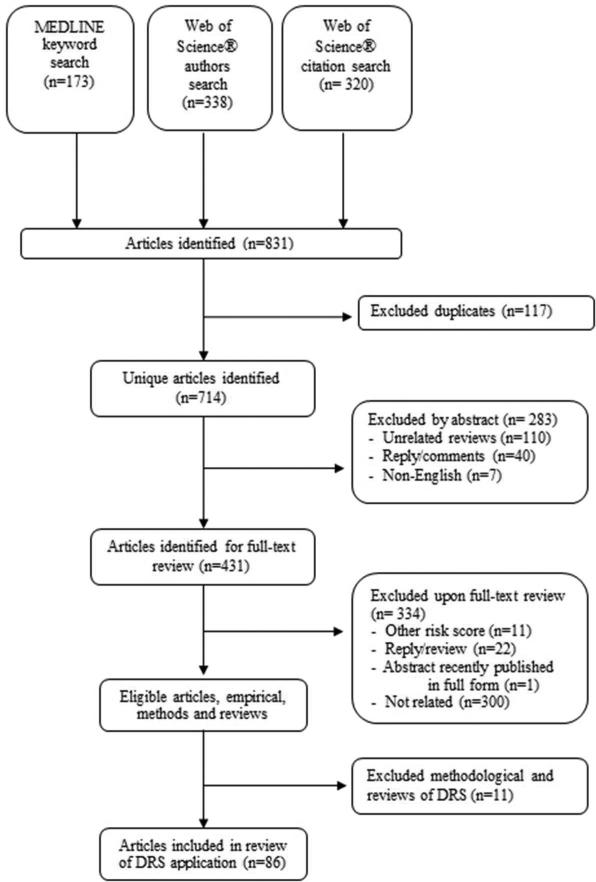
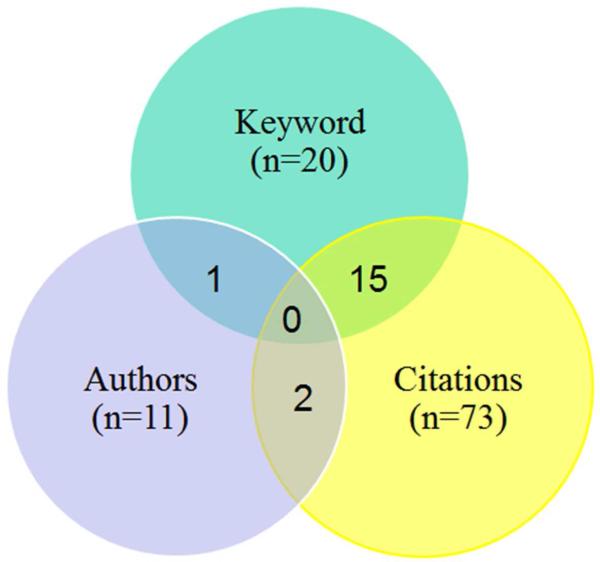
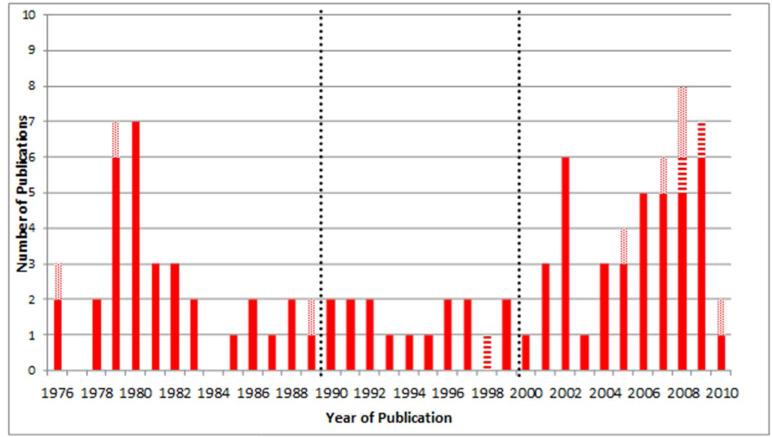
Of 714 unique articles identified, 97 studies were eligible: 8 methodological contributions,5-7,10-12,19,20 3 review papers,16,21,22 and 86 were empirical applications,23-108Figure 1. We retained one abstract that had not yet been published in full form,12 and excluded one abstract identified through the search that was recently published in a full-text article.8,20 The keyword search identified 173 articles (20 relevant empirical), the major paper citation search identified 338 articles (73 relevant empirical), and the author search identified 320 articles (11 relevant empirical) – each method yielded unique empirical papers, and no paper was identified by all three search methods, Figure 2. The empirical studies were published between March 1976 and May 2010 with a bimodal distribution; 32 (37%) articles were published prior to 1990, 15 (17%) in the 1990s, and 39 (45%) published since 2000 (Figure 3).
Figure 1.
Flow Diagram of Systematic Search Results
Figure 2.
Venn Diagram of Search Result Yield of Empirical Applications, by Search Strategy, N=86
Figure 3.
Number of Disease Risk Score Confounder Summary Score Publications, by Year of Publication, N=97. Empirical application (solid, n=86), methodological contribution (diagonal stripes, n=8) and review papers (horizontal stripe, n=3); 35 papers published before 1990, 16 papers published between 1990 and 1999, and 46 papers published since January 2000.
Table 1 summarizes the DRS derivation and application methods, with full details presented for each paper in the online Appendix. The most common terminology used to describe DRS methods included the words and/or combinations of: 1. summary, 2. confounder, 3. Miettinen, and 4. score; and many included disease specific terminology. Cohort (47%) and case-control (42%) studies were the most common study designs. Studies of cancer risk (27%) and drug effects (24%) were the most common applications. Application focus changed over time, with environmental and social exposures/outcomes (32%) and cancer risk (19%) the most common before 2000 and drug exposures (46%) and skin cancer risk (36%) dominating since 2000.
Table 1.
Characteristics of disease risk score confounder summary method applications, N=86
| Year of Publication | ||||||
|---|---|---|---|---|---|---|
| Total (n=86) | <2000 (n=47) |
≥2000 (n=39) |
||||
| No. | % | No. | % | No. | % | |
| Area of Study | ||||||
| Drug effects | 21 | 24.4 | 3 | 6.4 | 18 | 46.2 |
| Environmental and social exposure/disease | 18 | 20.9 | 15 | 31.9 | 3 | 7.7 |
| Hospital Care/Health Care | 10 | 11.6 | 8 | 17.0 | 2 | 5.1 |
| Cancer Risk | 23 | 26.7 | 9 | 19.1 | 14 | 35.9 |
| Skin Cancer Risk | 15 | 17.4 | 1 | 2.1 | 14 | 35.9 |
| Pregnancy outcomes | 8 | 9.3 | 7 | 14.9 | 1 | 2.6 |
| Surgical effectiveness | 4 | 4.7 | 3 | 6.4 | 1 | 2.6 |
| Ophthalmology | 2 | 2.3 | 2 | 4.3 | 0 | 0.0 |
| Study Design | ||||||
| Cohort | 40 | 46.5 | 19 | 40.4 | 21 | 53.8 |
| Case-Control | 36 | 41.9 | 23 | 48.9 | 13 | 33.3 |
| Cross-sectional | 5 | 5.8 | 3 | 6.4 | 2 | 5.1 |
| Clinical Trial | 5 | 5.8 | 2 | 4.3 | 3 | 7.7 |
| DRS Derivation (Regression Method) | ||||||
| Not Reported | 9 | 10.5 | 6 | 12.8 | 3 | 7.7 |
| Reported | 77 | 89.5 | 41 | 87.2 | 36 | 92.3 |
| Logistic | 36 | 46.8 | 15 | 36.6 | 21 | 58.3 |
| Discriminant | 13 | 16.9 | 13 | 31.7 | 0 | 0.0 |
| Linear | 8 | 10.4 | 8 | 19.5 | 0 | 0.0 |
| Poisson | 7 | 9.1 | 0 | 0.0 | 7 | 19.4 |
| Cox Proportional Hazards | 6 | 7.8 | 1 | 2.4 | 5 | 13.9 |
| Other | 7 | 9.1 | 4 | 9.8 | 3 | 8.3 |
| Primary DRS Application Method | ||||||
| Not Reported | 3 | 3.5 | 2 | 4.3 | 1 | 2.6 |
| Reported | 83 | 96.5 | 45 | 95.7 | 38 | 97.4 |
| Stratification | 50 | 60.2 | 40 | 88.9 | 10 | 26.3 |
| Included as covariate | 29 | 34.9 | 5 | 11.1 | 24 | 63.2 |
| Matching | 2 | 2.4 | 0 | 0.0 | 2 | 5.3 |
| Other | 2 | 2.4 | 0 | 0.0 | 2 | 5.3 |
| Primary DRS Application Variable | ||||||
| Not Reported | 13 | 15.1 | 10 | 21.3 | 3 | 7.7 |
| Reported | 73 | 84.9 | 37 | 78.7 | 36 | 92.3 |
| Continuous | 5 | 6.8 | 2 | 5.4 | 3 | 8.3 |
| Categorical* | 68 | 93.2 | 35 | 94.6 | 33 | 91.7 |
| 2 | 4 | 5.9 | 1 | 2.8 | 3 | 9.1 |
| 3 | 19 | 27.9 | 8 | 22.8 | 11 | 33.3 |
| 4 | 13 | 19.1 | 7 | 20.0 | 6 | 18.2 |
| 5 | 19 | 27.9 | 16 | 45.7 | 3 | 9.1 |
| 10 | 12 | 17.6 | 3 | 8.6 | 9 | 27.3 |
| 20 | 2 | 2.9 | 0 | 0.0 | 2 | 6.0 |
| Other | 5 | 7.3 | 4 | 11.4 | 1 | 3.0 |
Some applications examined several categories and thus proportions add to greater than 100%
Appendix.
Detailed Description of Studies Utilizing the Disease Risk Score Confounder Summary Method, N=86
| Author | Terminology | Study Design |
N (subjects unless stated) |
Exposure | Outcome(s) | No. Outcomes (cases in case-control) |
Measure Reported |
Primary DRS Derivation |
Primary DRS Application |
||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | No. Var | Method | Variable | ||||||||
| Applebaum 2007 (20) | Multivariate confounder score |
Case-control | 2,326 | Arsenic | BCC and SCC | BCC, n=880 SCC, n=666 |
Odds ratio | “Multivariate confounder method” (controls only) and Miettinen method |
6 | Covariate in multi-variable model |
Cat-4 |
| Applebaum 2009 (21) | Multivariate confounder score |
Case-control | 559 | Oral Contraceptives | SCC | 261 | Odds ratio | “Multivariate confounder method” (controls only) and Miettinen method |
6 | Covariate in multi- variable model |
Cat-4 |
| Axelson 1983 (22) | Miettinen c onfounder score technique |
Cohort | 84 pregnancies in 30 women | Work in tire building department of rubber factory |
Abnormal pregnancy | 16 | Rate ratio | Linear | 4 | Stratified (M-H) | Cat-5 |
| Boyce 1986 (23) | Confounder summarization procedure |
Cohort | 968 | Social and cultural factors |
Maternal and neonatal complications |
Maternal, n=445 Neonatal, n=136 |
Relative risk | Logistic | 7 | Stratified and linear trend |
Cat-5 |
| Bravata 2010 (24) | Risk adjustment score |
Cohort | 1,487 | Post-stroke or transient ischemic attach care processes (n=7) |
Combined (in-hospital mortality, discharge to hospice or skilled nursing facility) |
239 | Odds ratio | Logistic | 13 | Only covariate with exposures in multi-variable model |
Continuous |
| Chung 1982 (25) | Multivariate confounder score |
Cohort | 16,961 | Past induced abortion |
Spontaneous fetal loss in subsequent pregnancies |
NR | Relative risk | Logistic | 6 | Stratified (M-H) | Cat-5 |
| Cohen 1997 (26) | Multivariate risk score |
Clinical trial | 3,809 | Various risk factors for bleeds |
Bleeding | 421 | Odds ratio | Logistic | 10 | Stratified | Cat-4 |
| Dash 2006 (27) | Multivariate confounder score |
Cohort | 196 | Elective partial nephrectomy (PN) vs. radical nephrectomy (RN) |
Disease-free survival |
21 | Hazard ratio | Cox | 7 | Only covariate with exposures in multi-variable model |
Continuous |
| Daubs 1981 (28) | Multivariate risk score |
Case-control | N(1)=1,274; N(2)=1,002 | (1)Intraocular pressure; (2)Refractive error |
(1)Myopia (2)Primary open angle glaucoma |
(1)n=272 (2)n=672 |
Relative risk | Discriminant | 3,11 | Stratified (M-H) | Cat-4,5 |
| Elwood 1978 (29) | Risk score | Case-control | 6,391 | Geographical and ethnic influence |
Anencephalus | 1,391 | Risk ratio | Linear discriminant | 11 | Stratified | Cat-5 |
| Ensrud 2008 (30) | Summary fracture risk score |
Clinical trial | 10,101 | Raloxifene | Fractures: nonvertebral and clinical vertebral |
Nonvertebral, n=866 Clinical vertebral, n=161 |
Hazard ratio | Logistic (placebo group) |
14,3,2 | Stratified | Cat-3 |
| Fiebach 1990 (31) | Multivariate confounder score |
Cohort | 467 | Admission for chest pain to stepdown unit vs. coronary care unit |
Adverse outcomes ( death, life-threatening or other serious complications, or need for a major invasive procedure) |
175 | Relative risk | Logistic | >50 considered | Stratified | Cat-NR |
| Flodin 1986 (32) | Miettinen confounder score technique |
Case-control | 413 | Various e nvironmental and occupational exposures |
Acute myeloid leukemia |
59 | Rate ratio | Linear | 8 | Stratified (M-H) | Cat-3 |
| Flodin 1987 (33) | Miettinen confounder score technique |
Case-control | 562 | Various environmental and occupational exposures |
Multiple myeloma | 131 | Rate ratio | Linear | 10 | Stratified (M-H) | Cat-NR |
| Flodin 1988 (34) | Miettinen confounder technique |
Case-control | 550 | Various environmental and occupational exposures |
Multiple sclerosis | 83 | Rate ratio | Linear | 11 | Stratified (M-H) | Cat-NR |
| Flodin 1988(35) | Miettinen confounder score technique |
Case-control | 542 | Various environmental and occupational exposures |
Chronic lymphatic leukemia |
111 | Rate ratio | Linear | 8 | Stratified (M-H) | Cat-NR |
| Flodin 1990 (36) | Miettinen confounder score technique |
Case-control | 158 | Various environmental and occupational exposures |
Acute myeloid leukemia |
86 | Rate ratio | Linear | 14 | Stratified (M-H) | Cat-NR |
| Fung 2002 (37) | Multivariate risk score |
Cohort | 107,975 | Alcohol Intake | BCC | 6,088 | Relative risk | Logistic | NR | Stratified | Cat-3 |
| Fung 2002 (38) | Risk score | Cohort | 85,836 | Vitamins and carotenoids |
BCC | 5,392 | Relative risk | Logistic | NR | Stratified | Cat-3 |
| Giles-Corti 2002 (39) | Multivariate summarizeation score technique or determinant scores |
Cross-sectional | 1,773 | Individual, social, and physical variables |
Physical activity | 1,450 | Odds ratio | Logistic | 12 | Stratified | Cat-3,10 |
| Giles-Corti 2003 (40) | Multivariate summarizeation score technique or determinant scores |
Cross-sectional | 1,803 | Individual, social, and physical variables |
Walking | 310 | Odds ratio | Logistic | 12 | Stratified | Cat-3,10 |
| Gillum 1978 (41) | Multivariate confounder summarizing score |
Case-control | 1,708 | Sociocultural mobility | CHD, MI, Angina, and HTN |
CHD, n=88 MI, n=78 Angina, n=48 HTN, n=319 |
Incidence rate ratio |
Binary | 14,19 | Stratified (M-H) | Cat-5 |
| Graham 2005 (42) | CV risk score | Case-control | 39,639 | NSAIDS | Serious CHD | 8,143 | Odds ratio | Logistic (unexposed) |
32 | Covariate in multi- variable model |
Cat-10 |
| Grijalva 2007 (43) | Summary risk score |
Cohort | 14,932 | DMARDs | Non-persistence and adherence |
Non-persistence, n=8,835 |
Hazard ratio and model coefficient |
Persistence- Cox Adherence- Linear |
48 | Covariate in multi- variable model |
Cat-5 |
| Han 2004 (44) | Multivariate confounder score |
Nested case-control | 1,678 | Genetic polymorphisms of XRCCI |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300, SCC, n=286 melanoma, n=219 |
Odds ratio | Logistic | 6 | Covariate in multi-variable model |
Cat-3 |
| Han 2005 (45) | Multivariate confounder score |
Nested case-control | 1,679 | Genetic polymorphisms of XPD |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300, SCC, n=286 melanoma, n=219 |
Odds ratio | Logistic | 6 | Covariate in multi- variable model |
Cat-2 |
| Han 2006 (46) | Multivariate confounder score |
Nested case-control | 1,679 | Genetic polymorphisms of p53 Codon 72 |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300, SCC, n=286 melanoma, n=219 |
Odds ratio | Logistic | NR | Covariate in multi- variable model |
Cat-3 |
| Han 2006 (47) | Multivariate confounder score |
Nested case-control | 1,562 | Constitutional factors and sun exposure |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=283, SCC, n=275 melanoma, n=200 |
Odds ratio | Logistic | 5 | Covariate in multi- variable model |
Cat-3 |
| Han 2007 (48) | Multivariate confounder score |
Nested case-control | 1,678 | Genetic polymorphisms of V16A on MnSOD gene |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300, SCC, n=286 melanoma, n=219 |
Odds ratio | Logistic | 6 | Covariate in multi- variable model |
Cat-3 |
| Han 2007 (49) | Multivariate confounder score |
Nested case-control | 1,678 | Genetic polymorphisms (vitamin D and folate) |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300, SCC, n=286 melanoma, n=219 |
Odds ratio | Logistic | 6 | Covariate in multi- variable model |
Cat-3 |
| Hennekens 1976 (50) | Multivariate risk score |
Case-control | 1,298 | Coffee drinking | Death due to congestive heart failure |
649 | Risk ratio | Linear discriminate | 21 | Stratified | Cat-5 |
| Heyden 1980 (51) | Confounder summarization score |
Cohort | 1,165 | Sex | Diabetes-related coronary mortality |
124 | Rate ratio | NR | 6 | Only covariate with exposures in multi-variable model |
NR |
| Hill 2000 (52) | Confounder score | Cohort | 150 | Maternal smoking, drinking and familial susceptibility to alcohol dependence |
Psychiatric disorders | NR | Odds ratio | Logistic | 2 | Covariate in multi- variable model |
NR |
| Hirsch 2009 (53) | Estimated baseline risk | Clinical trial | 1,139 | In-hospital revascularization and early invasive vs. selective invasive treatment strategy |
Long-term mortality | 74 | Hazard ratio | Cox | 11 | Stratified | Cat-3 |
| Holman 1999 (54) | Comorbidity summarization score |
Cohort | 19,598 | Transurethral resection of p rostate vs. open prostatectomy |
Death | 4,845 | Rate ratio | Cox | 21 | Covariate in multi- variable model |
Fractional polynomial of continuous variable |
| Hooton 1981 (55) | Risk strata | Cohort | 169,518 | Various risk factors | Nosocomial infection | NR | Incidence rates | Risk tree model | 8,8,9,10 | Stratified | Cat-13,10, 9, 6 |
| Johnson 1992 (56) | Miettinen's multivariate confounder score |
Cross-sectional | 2,544 | Adolescent smoking, weight changes, and binge-purge behavior |
Secondary amenorrhea | 215 | Relative risk | NR | NR | NR | NR |
| Joseph 1996 (57) | Confounder score | Case-control | 4,061 | Major Tranquillizers | Death or near death from asthma |
131 | Relative risk | Logistic | NR | Covariate in multi- variable model |
Continuous |
| Journois 2005 (58) | Multivariate c onfounder score |
Cohort, historical comparator |
64 | Inhaled nitric oxide use in severe postoperative p ulmonary hypertension |
Early postoperative mortality |
27 | Odds ratio reported as risk ratio |
Logistic | NR | Matched | Continuous |
| Knowler 1980 (59) | Multivariate risk-indicator score |
Cohort | 163 | Systolic blood pressure |
Retinopathy | 54 | Rate difference and rate ratio |
Linear | 13 | Stratified | Cat-5 |
| Koopman 1991 (60) | Predictive risk score |
Cross-sectional | 3,408 households | Various risk factors |
Dengue infection | NR | Odds ratio | NR | NR | Covariate in multi- variable model |
NR |
| Levin 1980 (61) | Multivariate confounder score |
Case-control | 1,312 | Past induced abortions |
Pregnancy loss | 240 | Relative risk | Discriminant | 26 | Stratified | Cat-NR |
| Magnus 1979 (62) | Confounder summarizing score |
Case-control | 1,348 | Light physical activity |
Acute coronary events |
473 | Rate ratio | Linear discriminant | 14 | Stratified | Cat-10 |
| Matroos 1979 (63) | Summary score | Case-control | 1,390 | Cigarette or cigar smoking |
Acute coronary events | 499 | Rate ratio | Linear discriminant | 18 | Stratified | Cat-10 |
| Matthai 1994 (64) | Predictive model | Clinical trial | 2,166 | Low- vs. high-osmolality contrast agents in cardia angiography |
Adverse events | 78 | Odds ratio | Logistic | NR | Stratified | Cat-4 |
| Miller 2006 (65) | Multivariate confounder score |
Case-control | 2,364 | A23G single nucleotide polymorphism |
Skin Cancer (BCC, SCC) | BCC, n=886 SCC, n=682 |
Odds ratio | NR | 6 | Covariate in multi- variable model |
Cat-4 |
| Nan 2008 (66) | Multivariate confounder score |
Case-control | 1,679 | P53 codon 72 polymorphism and its interaction with melanocortin 1 receptor variants |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma | BCC, n=300 SCC, n=286 Melanoma, n=219 |
Odds ratio | Logistic | 6 | Stratified | Cat-3 |
| Nan 2009 (67) | Multivariate confounder score |
Case-control | 1,678 | MDM2 polymorphism and its interaction with the p53 Arg72Pro polymorphism |
Skin Cancer: 1.BCC, 2.SCC, 3.melanoma |
BCC, n=300 SCC, n=286 Melanoma, n=219 |
Odds ratio | Logistic | 6 | Covariate in multi- variable model |
Cat-2 |
| Nelemans 1993(68) | Sun-sensitivity summary score |
Case-control | 324 | Intermittent E xposure to Sunlight |
Melanoma | 141 | Odds ratio | Logistic | 6 | Stratified | Cat-2 |
| Olsen 1991 (69) | Multivariate confounder score |
Cohort | 1,752 | Social Network Strength | All-cause and cardiovascular mortality |
1,501 | Hazard ratio | NR | NR | Stratified (Cox) | Cat-5 |
| Orth-Gomer 1980 (70) | Multivariate confounder score |
Case-control | 150 | Psychological stress | Ischemic heart disease |
50 | Relative risk | Linear discriminate | NR | Stratified (M-H) | Cat-5 |
| Orth-Gomer 1980 (71) | Multivariate confounder score |
Case-control | 150 | Pattern-A behavior | Ischemic heart disease |
50 | Odds ratio | Linear discriminate | NR | Stratification | Cat-5 |
| Parker 2002 (72) | Risk group | Clinical trial | 6,797 | Enalapril | Combined (death or hospitalization for heart failure) |
2,275 | Relative risk | Logistic | NR | Stratified | Cat-3 |
| Pater 1979 (73) | Miettinen's Multivariate confounding score |
Cohort | 1,419 | Auxometry | 5-year breast cancer recurrence |
372 | Odds ratio | Linear | NR | Stratified | Cat-3 |
| Rajala 1980 (74) | Multivariate confounder summarizing score |
Cross-sectional | 212 | Tooth brushing | Dental caries | NR | Prevalence difference | Linear discriminant | 7 | Stratified | Cat-4,3 |
| Rantakallio 1992 (75) | Confounder score | Cohort | 5,966 | Maternal smoking in pregnancy |
Delinquency in offspring | 355 | Risk difference and risk ratio |
Logistic (unexposed) | NR | Stratified | Cat-5 |
| Rantakallio 1995 (76) | Confounder Score | Cohort | 20,097 | Maternal Build | Pregnancy outcome (preterm births, perinatal and childhood deaths, birth weight) |
NR | Odds Ratio | Logistic | 6 | Covariate in multi-variable model |
Cat-3 |
| Ray 2001 (77) | Summary CV risk score |
Cohort | 481,744 | Antipsychotics | Sudden cardiac death |
1,487 | Rate ratio | Poisson (unexposed) | NR | Stratified and covariate in multi-variable model |
Cat-4 |
| Ray 2002 (78) | Summary CV disease risk score |
Cohort | 656,875 | NSAIDS and Cox-2 | Serious coronary heart disease |
5,316 | Rate ratio | NR | NR | Covariate in multi- variable model |
NR |
| Ray 2004 (79) | Summary Score | Cohort | 1,246,943 PY | Erythromycin | Sudden cardiac deaths | 1,476 | Rate ratio | NR | NR | Covariate in multi- variable model |
Cat-10 |
| Ray 2004 (80) | Summary CV Risk Score |
Cohort | 481,744 | Cyclic anti- depressants |
Sudden cardiac deaths | 1,487 | Rate ratio | Poisson (unexposed) | NR | Covariate in multi- variable model |
Cat-10 |
| Ray 2002 (81) | Summary R isk Score |
Cohort | 69,314 | NSAIDS | Serious CHD | 6,362 | Rate ratio | Poisson (unexposed) | NR | Covariate in multi- variable model |
NR |
| Ray 2009 (82) | Summary CV risk score |
Cohort | 279,900 | Atypical antipsychotics |
Sudden cardiac deaths |
1,870 | Incidence rate-ratio |
Poisson (unexposed) | NR | Covariate in multi- variable model |
Cat-20 |
| Ray 2007 (83) | Baseline s ummary medical comorbidity score |
Cohort | 76,637 | NSAIDs w protective cotherapy vs. coxibs |
Peptic ulcer h ospitalization |
1,223 | Rate ratio | Poisson (former users with no gastroprotective therapy) |
NR | Covariate in multi-v ariable model |
Cat-10 |
| Ray 2009 (84) | CV Risk Score | Cohort | 48,566 | NSAIDs | CV risk | 3,600 | Rate ratio | Poisson (noncurrent users) |
NR | Covariate in multi- variable model |
Cat– 20 |
| Read 1983 (85) | Multivariate c onfounder score |
Cohort | 8,527 | Clinical setting | Antenatal diagnostic procedures |
3,786 | Proportion | Multivariate least square regression |
45 | Stratified | Cat-4 |
| Rosenberg 1982 (86) | Multivariate score | Case-control | 1,447 | Aspirin | Myocardial infarction |
551 | Odds ratio | Logistic (unexposed group) |
17 | Stratified | Cat-5 |
| Rothman 1980 (87) | Summary confounder score |
Case-control | 17,099 | Maternal age and birth rank |
Breast Cancer | 4,339 | Relative risk | NR | 11 | Stratified | Cat – 5 |
| Roumie 2008(88) | Vascular risk score | Cohort | 336,906 | NSAIDS | Stroke | 4,354 | Hazard ratio | Cox (non-users) | 22 | Covariate in multi- variable model |
Cat-10 |
| Roumie 2009 (89) | Summary risk score |
Cohort | 610, 001 | NSAIDS | CV events | 22,432 | Hazard ratio | Cox (non-users) | 24 | Covariate in multi- variable model |
Cat-10 |
| Salonen 1985 (90) | Multivariate confounder score |
Cohort | 102 | Health education program |
Smoking cessation after myocardial infarction |
25 | Rate ratio | Logistic | NR | Stratified | Cat-3 |
| Schachter 1982 (91) | Confounder summarization score |
Case-control | 883 | Chlamydia trachomatis |
Cervical neoplasia | 383 | Odds ratio | Logistic (whole population) |
NR | Stratified | Cat-4 |
| Scholer 1999 (92) | Risk score | Cohort | 18,768,162 infant years |
Socio-demographic factors |
Infant Injury deaths |
5,963 | Rates | Point system based on adjusted stratum calculated by Poisson |
5 | Stratified | Cat-5 |
| Shore 1979 (93) | Multivariate confounder score |
Case-control | 322 | Hair Dye | Breast cancer | 129 | Relative risk | Discriminate | 14 | Stratified | Cat–6 |
| Singer 1989 (94) | Multivariate baseline risk scores |
Cohort | 424 | Diabetes | Mortality after myocardial infarction |
94 | Relative risk | Logistic (unexposed ) |
3 | Stratified | Cat-3 |
| Siu 1979 (95) | Multivariate c onfounding score |
Cohort | 433 | Post-operative r adiotherapy |
Mortality | NR | Odds ratio | Discriminant | 11 | Stratified | Cat-3 |
| Solomon 2006 (96) | CV risk score | Cohort | 98,370 | NSAIDS | CV events | 4,850 | Relative risk | Cox (nonusers) | 23 | Stratified | Cat– 2 |
| Stason 1976 (97) | Multivariate confounder summarizing score |
Case-control | 2,885 | Alcohol consumption |
Nonfatal myocardial infarction |
399 | Rate ratio | Linear discriminant (whole cohort) |
20 | Stratified | Cat-5 |
| Strauss 1997 (98) | Multivariate c onfounder score |
Cohort | 4291 | Tube feeding | Mortality | 612 | Rate ratio | Logistic (unexposed) |
NR | Stratified | Cat-8 |
| Strauss 1996 (99) | Multivariate c onfounder score |
Cohort | 7,241 | Institutional placement vs. community living |
Mortality | 1,330 | Mortality rates | Logistic (unexposed) |
10 | Stratified | Cat-8 |
| Swan 1981 (100) | Multivariate score | Case-control | 285 | Oral contraceptives | Cervical carcinoma | 69 | Odds ratio | Linear discriminant | NR | Stratified | Cat-3 |
| van Rossum 2001 (101) | Multivariate risk score |
Cohort | 19,019 | Season | Death | 8,347 | Rate ratios | Logistic | NA | Stratified | Cat-3 |
| van Staa 2008 (102) | Disease risk score | Cohort | 1,172,341 | NSAID | Myocardial infarction | 31,019 | Rate ratios | Poisson (controls) |
21 | Matched | Cat-10 |
| van Staa 2001 (103) | Miettinen's multivariate confounder score |
Cohort | 244 235 | Oral corticosteroid | Fracture | NR | Incidence rates | Logistic (whole cohort) |
27 | Stratified | Cat-5 |
| Welsh 2008 (104) | Multivariate confounder score |
Case-control | 2464 | Genetic variation in the histidase gene |
Skin cancer (BCC, SCC) | SCC, n=702 BCC, n=914 |
Odds ratios | Logistic | 5 | Covariate in multi- variable model |
Cat- 3 |
| Wynder 1979 (105) | Miettinen confounder score method |
Case-control | 10,581 | Filter cigarette usage |
Lung and larynx cancer |
1,034 | Odds ratio | NR | NR | NR | NR |
BCC=Basal Cell Carcinoma, Cat=Categorical, CHD=Coronary Heart Disease, M-H=Mantel-Haenszel, MI=Myocardial Infarction, NR=Not Reported, PY=Person Years, SCC=Squamous Cell Carcinoma, Var=Variables
DRS methods were not clearly reported in up to 15 percent of empirical papers, Table 1. Of the empirical papers reporting derivation methods, logistic regression (47%), followed by discriminant analysis (17%) where the most common, with a shift away from discriminant analysis and no study using this method since 2000. The majority of papers did not specify the cohort used to derive DRS (70%). Of the 26 papers with methodological detail, 85% created DRS in an “unexposed” or subgroup. DRS were most commonly used as a categorical variable (93%), with a shift from stratification prior to 2000 (89% of applications published before January 2000) to use as a covariate in regression models (63% of applications) since January 2000. The most common number of groups were 3 (28%), 5 (28%), 4 (19%), and 10 (18%).
DISCUSSION
We examined use of DRS as a confounder summary score method over time and identified a bimodal distribution in DRS application with a peak 1979-1980 and resurgence since 2000. This bimodal distribution is not surprising given early simulation efforts. In 1976, Miettinen proposed the creation of a ‘multivariate confounder score’ in the unexposed group to be applied in the full cohort to examine exposure effects adjusted for confounding variables.5 However, early simulation work by Pike in 1979 concluded that the DRS method may overestimate the effect of confounders and thus bias results.10 A subsequent simulation by Cook and Goldman published in 1989 concluded that overestimation of confounders may be rare, particularly when applying the DRS as a categorical variable.11 These results likely rejuvenated interest and confidence in the methodology. The increase since 2000 may also partially relate to the recent increased demand and continued importance of comparative effectiveness research, particularly in the area of pharmacoepidemiology,9,109,110 with 46 percent of DRS application papers related to drug safety and effectiveness since 2000.
Our results show great variation in DRS application with differences in methods of score derivation, utilization, and naming. The most common method of DRS derivation was originally discriminant analysis and since 2000, has been logistic regression. This follows trends of analyses seen in the last 40 years of health science research with older studies utilizing discriminant analysis, and more recent publications utilizing logistic and other regression analyses. Miettinen's original derivation of the DRS used a discriminant function and discussed Cox, logistic, and linear models as possible alternatives.5
About half (42%) of empirical studies applied DRS in a case-control study, however only two methodological contributions have examined DRS using a case-control study design.10,12 Pike completed his simulation work that halted wide uptake of the DRS using a case-control study design,10 and a recent simulation published in abstract form concluded that the DRS may be appropriate in the case-control study when exposure is not highly correlated with its confounders.12 Use of the propensity score in a case-control has been shown to introduce artificial effect modification and reduce control of confounding.111 Further methodological work is needed to support DRS utilization in the case-control setting.
DRS were most commonly used as a categorical variable to control for confounding in the main outcome model. The number of categories varied, with 3 (28%) and 5 (28%) groups being the most common. Miettinen recommended that the initial analysis be completed with equal deciles and then adjacent strata combined to create five strata.5 In many cases, the most clinically relevant number of groups may be three with risk stratified into low, medium and high. However, few studies used DRS to communicate results by disease strata (23%). We believe this to be an underutilization of DRS benefits. The added advantage of graphical presentation may allow for easier communication of results and identify effect modification by baseline outcome risk. Stratifying results by disease risk strata may be particularly beneficial in drug effects studies to maximize the benefits and limit harms in patients. Oral bisphosphonates, as an example, are indicated to treat osteoporosis and reduce fracture risk among patients with low bone mineral density and/or major risk factors for fracture.113 Treating patients at low fracture risk may increase potential harms, with little benefit on fracture risk reduction. Prior evidence identifies little difference in fracture risk reduction between osteoporosis therapies among patients in low risk strata.7,114 Interestingly, few studies used matching on the DRS as the means to implement DRS, yet matching on EPS is common.3,4,9 In the presence of heterogeneous treatment effects, matching on the DRS or examining risk by DRS strata after adjusting for DRS in the regression, allows investigators to estimate exposure effects in well-defined populations and examine effect modification by disease (outcome) risk. Once treatment effect heterogeneity has been described, standardization methods such as matching on the DRS, stratifying the Cox proportional hazards model on DRS strata, or another method to adjust for the observed interaction in the regression model may be used if an overall treatment effect estimate is needed.
Our results show that, where reported, DRS were most commonly derived in a subgroup of the study population and then applied to the study population at large. These applications are similar to the recommendations made by Miettinen and Cook et al..5,11 These authors argued the importance of creating the score in the unexposed group so that exposure does not bias the underlying risk of outcome. However, more recent empirical and simulation work has identified that DRS creation in the full cohort may be important in settings where exposure is highly correlated with covariates.7,8 In other settings, such as in the context of new therapeutic agents, deriving DRS in an external historical cohort may be advantageous.115 Further research is needed to understand the relative advantages and disadvantages of different DRS approaches.
Our systematic review is subject to some limitations. First, although we completed a 3-step search, we recognize that due to the lack of standardized terminology to describe DRS methods, we may have missed some relevant applications. Indeed, upon discussion of our review with colleagues, five additional DRS applications were identified that were not found through our comprehensive search – three were early applications,13-15 and two described DRS as “propensity score” for the outcome.116,117 Although “propensity score” for the outcome is technically correct, this terminology makes the identification and interpretation of the DRS more challenging. Propensity Score became an official MeSH keyword heading in 2010, defined as the conditional probability of exposure to a treatment given observed covariates. As DRS applications increase, standardized terminology is recommended. Descriptions such as the “multivariate risk score” and “confounder score” may be confusing and vague, and therefore we encourage adoption of the recent terminology Disease Risk Score. Disease risk score is descriptive of the technique and unique versus propensity score and may thus minimize possible confusion between these two confounder summary score methods. Despite the limitations of our search strategy, it was interesting to note that all three search strategies (keyword, citation and author) captured different studies with only 18 of the 86 studies identified by two methods, and no paper identified by all three search methods. The citation search found the largest number of papers, 73 of the 86 papers. Despite potentially missing some applications, we feel our results and conclusions of the general trends would remain.
Second, given the lack of transparency or detail in how DRS was derived or applied, accurate description of some applications was difficult and supports our recommendation for improved transparency and a move toward standardized terminology in future applications. The great variation in the utilization of DRS highlights the need for further work. Additional simulation work is important to support the best means to utilize DRS, particularly in the case-control setting. Finally, although we summarized derivation methods, we did not consider the process of variable selection or its appropriateness. Considerations for variable selection in DRS are similar to those for EPS and a conventional multivariable regression strategy -- variables measured before the start of exposure are risk factors for the outcome of interest, i.e., are potential confounding variables.118-120
In summary, we identified an increase in DRS application, yet underutilization of DRS to examine potential effect modification by disease risk. Comparative safety and effectiveness research may benefit from DRS to help target interventions to those who benefit most. However, more work is needed to guide DRS applications, particularly in the case-control setting. A move towards better transparency in DRS derivation and utilization, and standardization of terminology will facilitate DRS application and interpretation. We recommend that future work consider utilizing the terminology Disease Risk Score when describing confounder summary scores derived based on the primary outcome model.
Key Points (5 max).
Disease Risk Scores (DRS) are confounder summary scores derived based on the probability of disease outcome and may be advantageous over other confounding adjustment techniques when exposure is rare, to study multiple exposures, and to study effect modification by outcome risk.
Use of DRS confounder summary methods has increased, yet remains low. We observed a bimodal distribution in the number of publications over time, with a peak 1979-1980, and resurgence since 2000. Close to half of empirical applications since 2000 have been associated with pharmacoepidemiology.
Great variation in DRS application exist with differences in methods of score derivation, utilization, and naming.
There is a general lack of transparency in methods used to derive and apply DRS methods, and few studies used DRS to its full potential by examining effect modification by outcome risk.
A move toward standardized terminology and providing methodological detail will facilitate DRS utilization and interpretation. We recommend that future work consider adopting the terminology Disease Risk Score when applying confounder summary scores derived based on the probability of disease outcome.
ACKNOLWEDGMENTS
We would like to thank Dr. Robert J. Glynn, Divisions of Preventive Medicine and Pharmacoepidemiology and Pharmacoeconomics, Harvard Medical School, Boston MA; and Dr. David N. Juurlink, Sunnybrook Research Institute, Toronto ON; for discussions and pointing out DRS applications not identified by our systematic search strategy.
Sponsors: This research was supported by an Ontario Ministry of Research and Innovation Early Researcher Award to Dr. Suzanne Cadarette. Dr. Cadarette is supported by a Canadian Institutes of Health Research (CIHR) New Investigator Award in Aging and Osteoporosis (MSH-95364). Dr. Mina Tadrous was supported by a CIHR Strategic Training Initiative in Health Research as part of the Drug Safety and Effectiveness Cross-disciplinary Training program in 2011, and is supported by a CIHR Fredrick Banting and Charles Best Canada Graduate Scholarship Doctoral Award (GSD-11342). Dr. Til Stürmer receives investigator-initiated research funding and support as Principal Investigator (RO1 AG023178) and Co-Investigator (RO1 AG018833) from the National Institute on Aging at the National Institutes of Health. He also receives research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality.
Footnotes
Conflict of Interest: None related to this work.
Prior Presentations: This work was presented at the Canadian Association for Population Therapeutics meeting, Montreal, May 2012; and the International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Barcelona Spain, August 2012.
REFRENCES
- 1.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Walters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2008. [Google Scholar]
- 2.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 3.Stürmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59:437–47. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miettinen OS. Stratification by a multivariate confounder score. Am J Epidemiol. 1976;104:609–20. doi: 10.1093/oxfordjournals.aje.a112339. [DOI] [PubMed] [Google Scholar]
- 6.Stürmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005;161:891–8. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cadarette SM, Gagne JJ, Solomon DH, Katz JN, Stürmer T. Confounder summary scores when comparing the effects of multiple drug exposures. Pharmacoepidemiol Drug Saf. 2010;19:2–9. doi: 10.1002/pds.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174:613–20. doi: 10.1093/aje/kwr143. [DOI] [PubMed] [Google Scholar]
- 9.Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–9. doi: 10.1111/j.1742-7843.2006.pto_293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pike MC, Anderson J, Day N. Some insights into Miettinen's multivariate confounder score approach to case-control study analysis. Epidemiol Community Health. 1979;33:104–6. doi: 10.1136/jech.33.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook EF, Goldman L. Performance of tests of significance based on stratification by a multivariate confounder score or by a propensity score. J Clin Epidemiol. 1989;42:317–24. doi: 10.1016/0895-4356(89)90036-x. [DOI] [PubMed] [Google Scholar]
- 12.Arbogast PG, Kaltenbach L, Ray WA. Performance of disease risk scores in case-control studies [abstract]. Pharmacoepidemiol Drug Saf. 2008;17:S106–7. [Google Scholar]
- 13.Cornfield J. The University Group Diabetes Program: a further statistical analysis of the mortality findings. JAMA. 1971;217:1676–1687. [PubMed] [Google Scholar]
- 14.Peters CC. A Method of Matching Groups for Experiment with No Loss of Population. J Educ Res. 1941;34:606–612. [Google Scholar]
- 15.Belson WA. A technique for studying the effects of a television broadcast. J R Stat Soc Ser C Appl Stat. 1956;5:195–202. [Google Scholar]
- 16.Arbogast PG, Ray WA. Use of disease risk scores in pharmacoepidemiologic studies. Stat Methods Med Res. 2009;18:67–80. doi: 10.1177/0962280208092347. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.D'Agostino RB, Sr., Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 19.Arbogast PG, Kaltenbach L, Ding H, Ray WA. Adjustment for multiple cardiovascular risk factors using a summary risk score. Epidemiology. 2008;19:30–7. doi: 10.1097/EDE.0b013e31815be000. [DOI] [PubMed] [Google Scholar]
- 20.Arbogast PG, Kaltenbach L, Ray WA. Performance of summary risk scores, propensity scores, and multivariable regression in the presence of multiple confounders [abstract]. Pharmacoepidemiol Drug Saf. 2007;16:S1–2. [Google Scholar]
- 21.Strauss D. On Miettinen's multivariate confounder score. J Clin Epidemiol. 1998;51:233–6. doi: 10.1016/s0895-4356(97)00283-7. [DOI] [PubMed] [Google Scholar]
- 22.Cummings P. Propensity scores. Arch Pediatr Adolesc Med. 2008;162:734–7. doi: 10.1001/archpedi.162.8.734. [DOI] [PubMed] [Google Scholar]
- 23.Applebaum KM, Karagas MR, Hunter DJ, Catalano PJ, Byler SH, Morris S, Nelson HH. Polymorphisms in nucleotide excision repair genes, arsenic exposure, and non-melanoma skin cancer in New Hampshire. Environ Health Perspect. 2007;115:1231–1236. doi: 10.1289/ehp.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Applebaum KM, Nelson HH, Zens MS, Stukel TA, Spencer SK, Karagas MR. Oral contraceptives: A risk factor for squamous cell carcinoma? J Invest Dermatol. 2009;129:2760–2765. doi: 10.1038/jid.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Axelson O, Edling C, Andersson L. Pregnancy outcome among women in a Swedish rubber plant. Scand J Work Environ Health. 1983;9(Suppl 2):79–83. [PubMed] [Google Scholar]
- 26.Boyce WT, Schaefer C, Harrison HR, Haffner WHJ, Lewis M, Wright AL. Social and cultural factors in pregnancy complications among Navajo women. Am J Epidemiol. 1986;124:242–253. doi: 10.1093/oxfordjournals.aje.a114382. [DOI] [PubMed] [Google Scholar]
- 27.Bravata DM, Wells CK, Lo AC, Nadeau SE, Melillo J, Chodkowski D, Struve F, Williams LS, Peixoto AJ, Gorman M, Goel P, Acompora G, McClain V, Ranjbar N, Tabereaux PB, Boice JL, Jacewicz M, Concato J. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170:804–810. doi: 10.1001/archinternmed.2010.92. [DOI] [PubMed] [Google Scholar]
- 28.Chung CS, Smith RG, Steinhoff PG, Mi MP. Induced abortion and spontaneous fetal loss in subsequent pregnancies. Am J Public Health. 1982;72:548–554. doi: 10.2105/ajph.72.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen AT, Wagner MB, Mohamed MS. Risk factors for bleeding in major abdominal surgery using heparin thromboprophylaxis. Am J Surg. 1997;174:1–5. doi: 10.1016/S0002-9610(97)00050-0. [DOI] [PubMed] [Google Scholar]
- 30.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int. 2006;97:939–945. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 31.Daubs JG, Crick RP. Effect of refractive error on the risk of ocular hypertension and open angle glaucoma. Trans Ophthalmol Soc U K. 1981;101:121–126. [PubMed] [Google Scholar]
- 32.Elwood JM, Mousseau G. Geographical, secular and ethnic influences in anencephalus. J Chronic Dis. 1978;31:483–491. doi: 10.1016/0021-9681(78)90012-7. [DOI] [PubMed] [Google Scholar]
- 33.Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw KT, Zhao Q, Agnusdei D, Cauley JA, Ensrud KE, Stock JL, Barrett-Connor E, Grady D, Mosca L, Khaw K-T, Zhao Q, Agnusdei D, Cauley JA. Effects of raloxifene on fracture risk in postmenopausal women: the Raloxifene Use for the Heart Trial. J Bone Miner Res. 2008;23:112–20. doi: 10.1359/jbmr.070904. [DOI] [PubMed] [Google Scholar]
- 34.Fiebach NH, Cook EF, Lee TH, Brand DA, Rouan GW, Weisberg M, Goldman L. Outcomes in patients with myocardial infarction who are initially admitted to stepdown units: data from the Multicenter Chest Pain Study. Am J Med. 1990;89:15–20. doi: 10.1016/0002-9343(90)90091-q. [DOI] [PubMed] [Google Scholar]
- 35.Flodin U, Fredriksson M, Axelson O, Persson B, Hardell L. Background radiation, electrical work and some other exposures associated with acute myeoid-leukemia in a case-referent study. Arch Environ Health. 1986;41:77–84. doi: 10.1080/00039896.1986.9937413. [DOI] [PubMed] [Google Scholar]
- 36.Flodin U, Fredriksson M, Persson B. Multiple myeloma and engine exhausts, fresh wood, and creosote: a case-referent study. Am J Ind Med. 1987;12:519–29. doi: 10.1002/ajim.4700120506. [DOI] [PubMed] [Google Scholar]
- 37.Flodin U, Fredriksson M, Persson B, Axelson O. Chronic lymphatic leukaemia and engine exhausts, fresh wood, and DDT: a case-referent study. Br J Ind Med. 1988;45:33–8. doi: 10.1136/oem.45.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Flodin U, Fredriksson M, Persson B, Axelson O. Acute myeloid-leukemia and background radiation in an expanded case-referent study. Arch Environ Health. 1990;45:364–366. doi: 10.1080/00039896.1990.10118756. [DOI] [PubMed] [Google Scholar]
- 39.Flodin U, Soderfeldt B, Noorlindbrage H, Fredriksson M, Axelson O. Multiple sclerosis, solvents and pets: a case-referent study. Arch Neurol. 1988;45:620–623. doi: 10.1001/archneur.1988.00520300038015. [DOI] [PubMed] [Google Scholar]
- 40.Fung TT, Hunter DJ, Spiegelman D, Colditz GA, Rimm EB, Willett WC. Intake of alcohol and alcoholic beverages and the risk of basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2002;11:1119–1122. [PubMed] [Google Scholar]
- 41.Fung TT, Hunter DJ, Spiegelman D, Colditz GA, Speizer FE, Willett WC. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221–230. doi: 10.1023/a:1015036317596. [DOI] [PubMed] [Google Scholar]
- 42.Giles-Corti B, Donovan RJ. The relative influence of individual, social and physical environment determinants of physical activity. Soc Sci Med. 2002;54:1793–1812. doi: 10.1016/s0277-9536(01)00150-2. [DOI] [PubMed] [Google Scholar]
- 43.Giles-Corti B, Donovan RJ. Relative influences of individual, social environmental, and physical environmental correlates of walking. Am J Public Health. 2003;93:1583–1589. doi: 10.2105/ajph.93.9.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gillum RF, Paffenbarger RS. Chronic disease in former college students: socioculural mobility as a precursor of coronary heart disease and hypertension. Am J Epidemiol. 1978;108:289–298. doi: 10.1093/oxfordjournals.aje.a112622. [DOI] [PubMed] [Google Scholar]
- 45.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclooxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-ontrol study. Lancet. 2005;365:475–81. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 46.Grijalva CG, Chung CP, Arbogast PG, Stein CM, Mitchel EF, Griffin MR. Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care. 2007;45:S66–S76. doi: 10.1097/MLR.0b013e318041384c. [DOI] [PubMed] [Google Scholar]
- 47.Hamynen H, Vartiainen E, Sahi T, Pallonen U, Salonen JT. Social, personality and environmental determinants of smoking in young Finnish men. Scand J Public Health. 1987;15:219–224. doi: 10.1177/140349488701500403. [DOI] [PubMed] [Google Scholar]
- 48.Han J, Hankinson SE, Colditz GA, Hunter DJ. Genetic variation in XRCC1, sun exposure, and risk of skin cancer. British Journal of Cancer. 2004;91:1604–1609. doi: 10.1038/sj.bjc.6602174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han JL, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case-control study within the Nurses’ Health Study. Int J Epidemiol. 2006;35:1514–1521. doi: 10.1093/ije/dyl197. [DOI] [PubMed] [Google Scholar]
- 50.Han JL, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28:390–397. doi: 10.1093/carcin/bgl156. [DOI] [PubMed] [Google Scholar]
- 51.Han JL, Colditz GA, Hunter DJ. Manganese superoxide dismutase polymorphism and risk of skin cancer (United States). Cancer Causes Control. 2007;18:79–89. doi: 10.1007/s10552-006-0079-6. [DOI] [PubMed] [Google Scholar]
- 52.Han JL, Colditz GA, Liu JS, Hunter DJ. Genetic variation in XPD, sun exposure, and risk of skin cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1539–1544. doi: 10.1158/1055-9965.EPI-04-0846. [DOI] [PubMed] [Google Scholar]
- 53.Han JL, Cox DG, Colditz GA, Hunter DJ. The p53 codon 72 polymorphism, sunburns, and risk of skin cancer in US Caucasian women. Mol Carcinog. 2006;45:694–700. doi: 10.1002/mc.20190. [DOI] [PubMed] [Google Scholar]
- 54.Hennekens CH, Drolette ME, Jesse MJ, Davies JE, Hutchison GB. Coffee drinking and death due to coronary heart disease. N Engl J Med. 1976;294:633–6. doi: 10.1056/NEJM197603182941203. [DOI] [PubMed] [Google Scholar]
- 55.Heyden S, Heiss G, Bartel AG, Hames CG. Sex differences in coronary mortality among diabetics in Evans County, Georgia. J Chronic Dis. 1980;33:265–273. doi: 10.1016/0021-9681(80)90021-1. [DOI] [PubMed] [Google Scholar]
- 56.Hill SY, Lowers L, Locke-Wellman J, Shen SA. Maternal smoking and drinking during pregnancy and the risk for child and adolescent psychiatric disorders. J Stud Alcohol. 2000;61:661–8. doi: 10.15288/jsa.2000.61.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hirsch A, Windhausen F, Tijssen JGP, Ophuis A, van der Giessen WJ, van der Zee PM, Cornel JH, Verheugt FWA, de Winter RJ. Diverging associations of an intended early invasive strategy compared with actual revascularization, and outcome in patients with non-ST-segment elevation acute coronary syndrome: the problem of treatment selection bias. Eur Heart J. 2009;30:645–654. doi: 10.1093/eurheartj/ehn438. [DOI] [PubMed] [Google Scholar]
- 58.Holman CDJ, Wisniewski ZS, Semmens JB, Rouse IL, Bass AJ. Mortality and prostate cancer risk in 19 598 men after surgery for benign prostatic hyperplasia. BJU Int. 1999;84:37–42. doi: 10.1046/j.1464-410x.1999.00123.x. [DOI] [PubMed] [Google Scholar]
- 59.Hooton TM, Haley RW, Culver DH, White JW, Morgan WM, Carroll RJ. The joint associations of multiple risk factors with the occurence of nosocomial infection. Am J Med. 1981;70:960–970. doi: 10.1016/0002-9343(81)90562-3. [DOI] [PubMed] [Google Scholar]
- 60.Johnson J, Whitaker AH. Adolescent smoking, weight changes and binge-purge behaviour: associations with secondary amenorrhea. Am J Public Health. 1992;82:47–54. doi: 10.2105/ajph.82.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Joseph KS, Blais L, Ernst P, Suissa S. Increased morbidity and mortality related to asthma among asthmatic patients who use major tranquillisers. BMJ. 1996;312:79–82. doi: 10.1136/bmj.312.7023.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Journois D, Baufreton C, Mauriat P, Pouard P, Vouh, x00E, Safran D, Journois D, Baufreton C, Mauriat P, Pouard P, Pascal, Safran D. Effects of inhaled nitric oxide administration on early postoperative mortality in patients operated for correction of atrioventricular canal defects. Chest. 2005;128:3537–44. doi: 10.1378/chest.128.5.3537. [DOI] [PubMed] [Google Scholar]
- 63.Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood-pressure: 6-year follow-up study in Pima Indians. N Engl J Med. 1980;302:645–650. doi: 10.1056/NEJM198003203021201. [DOI] [PubMed] [Google Scholar]
- 64.Koopman JS, Prevots DR, Marin MAV, Dantes HG, Aquino MLZ, Longini IM, Amor JS. Determinants and predictors of dengue infection in Mexico. Am J Epidemiol. 1991;133:1168–1178. doi: 10.1093/oxfordjournals.aje.a115829. [DOI] [PubMed] [Google Scholar]
- 65.Levin AA, Schoenbaum SC, Monson RR, Stubblefield PG, Ryan KJ. Association of induced-abortion with subsequent pregancy loss. JAMA. 1980;243:2495–2499. [PubMed] [Google Scholar]
- 66.Magnus K, Matroos A, Strackee J. Walking, cycling or gardening with or without seasonal interruptions in relation to acute coronary events. Am J Epidemiol. 1979;110:724–733. doi: 10.1093/oxfordjournals.aje.a112853. [DOI] [PubMed] [Google Scholar]
- 67.Matroos A, Magnus K, Strackee J. Fatal and nonfatal coronary attacks in relation to smoking in some Dutch communities. Am J Epidemiol. 1979;109:145–151. doi: 10.1093/oxfordjournals.aje.a112669. [DOI] [PubMed] [Google Scholar]
- 68.Matthai WH, Kussmaul WG, Krol J, Goin JE, Schwartz JS, Hirshfeld JW. A comparison of low-osmolality with high-osmalality contrast agents in cardia angiography identification of criteria for selective use. Circulation. 1994;89:291–301. doi: 10.1161/01.cir.89.1.291. [DOI] [PubMed] [Google Scholar]
- 69.Miller KL, Karagas MR, Kraft P, Hunter DJ, Catalano PJ, Byler SH, Nelson HH. XPA, haplotypes, and risk of basal and squamous cell carcinoma. Carcinogenesis. 2006;27:1670–1675. doi: 10.1093/carcin/bgi376. [DOI] [PubMed] [Google Scholar]
- 70.Nan H, Qureshi AA, Hunter DJ, Han J. Interaction between p53 codon 72 polymorphism and melanocortin 1 receptor variants on suntan response and cutaneous melanoma risk. Br J Dermatol. 2008;159:314–321. doi: 10.1111/j.1365-2133.2008.08624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nan HM, Qureshi AA, Hunter DJ, Han JL. A functional SNP in the MDM2 promoter, pigmentary phenotypes, and risk of skin cancer. Cancer Causes Control. 2009;20:171–179. doi: 10.1007/s10552-008-9231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nelemans PJ, Groenendal H, Kiemeney L, Rampen FHJ, Ruiter DJ, Verbeek ALM. Effect of intermittent exposure to sunlight on melanoma risk among indoor workers and sun-sensitive individuals. Environ Health Perspect. 1993;101:252–255. doi: 10.1289/ehp.93101252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olsen RB, Olsen J, Gunnersvensson F, Waldstrom B. Social networks and longevity - a 14 year follow-up study among elderly in Denmark. Soc Sci Med. 1991;33:1189–1195. doi: 10.1016/0277-9536(91)90235-5. [DOI] [PubMed] [Google Scholar]
- 74.Orth G, x00E, r K, Ahlbom A. Impact of psychological stress on ischemic heart disease when controlling for conventional risk indicators. J Human Stress. 1980;6:7–15. doi: 10.1080/0097840X.1980.9935013. [DOI] [PubMed] [Google Scholar]
- 75.Orthgomer K, Ahlbom A, Theorell T. Impact of pattern-A behavior on ischemic heart disease when controlling for conventional risk indicators. J Human Stress. 1980;6:6–13. doi: 10.1080/0097840X.1980.9936093. [DOI] [PubMed] [Google Scholar]
- 76.Parker AB, Yusuf S, Naylor CD. The relevance of subgroup-specific treatment effects: The Studies Of Left Ventricular Dysfunction (SOLVD) revisited. Am Heart J. 2002;144:941–947. doi: 10.1067/mhj.2002.126446. [DOI] [PubMed] [Google Scholar]
- 77.Pater JL, Loeb M, Siu TO. Multivariate analysis of the contribution of auxometry to prognosis in breast cancer. J Chronic Dis. 1979;32:375–384. doi: 10.1016/0021-9681(79)90079-1. [DOI] [PubMed] [Google Scholar]
- 78.Rajala M, Selkainaho K, Paunio I. Relationship between reported toothbrushing and dental caries in adutls. Community Dent Oral Epidemiol. 1980;8:128–131. doi: 10.1111/j.1600-0528.1980.tb01272.x. [DOI] [PubMed] [Google Scholar]
- 79.Rantakallio P, Laara E, Koiranen M, Sarpola A. Maternal build and pregnancy outcome. J Clin Epidemiol. 1995;48:199–207. doi: 10.1016/0895-4356(94)00130-i. [DOI] [PubMed] [Google Scholar]
- 80.Rantakallio P, x00E, Isohanni M, Moilanen I, E Maternal smoking during pregnancy and delinquency of the offspring: an association without causation? Int J Epidemiol. 1992;21:1106–13. doi: 10.1093/ije/21.6.1106. [DOI] [PubMed] [Google Scholar]
- 81.Ray WA, Chung CP, Stein CM, Smalley WE, Hall K, Arbogast PG, Griffin MR. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterology. 2007;133:790–798. doi: 10.1053/j.gastro.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 82.Ray WA, Meredith S, Thapa PB, Hall K, Murray KT. Cyclic antidepressants and the risk of sudden cardiac death. Clin Pharmacol Ther. 2004;75:234–241. doi: 10.1016/j.clpt.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 83.Ray WA, Meredith S, Thapa PB, Meador KG, Hall K, Murray KT. Antipsychotics and the risk of sudden cardiac death. Arch Gen Psychiatry. 2001;58:1161–1167. doi: 10.1001/archpsyc.58.12.1161. [DOI] [PubMed] [Google Scholar]
- 84.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein CM. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–1096. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 85.Ray WA, Stein CM, Daugherty JR, Hall K, Arbogast PG, Griffin MR. COX-2 selective non-steroidal anti-inflammatory drugs and risk of serious coronary heart disease. Lancet. 2002;360:1071–1073. doi: 10.1016/S0140-6736(02)11131-7. [DOI] [PubMed] [Google Scholar]
- 86.Ray WA, Stein CM, Hall K, Daugherty JR, Griffin MR. Non-steroidal anti-inflamatory drugs and risk of serious coronary heart disease: an observational cohort study. Lancet. 2002;359:118–123. doi: 10.1016/S0140-6736(02)07370-1. [DOI] [PubMed] [Google Scholar]
- 87.Ray WA, Varas-Lorenzo C, Chung CP, Castellsague J, Murray KT, Stein CM, Daugherty JR, Arbogast PG, Garcia-Rodriguez LA. Cardiovascular risks of nonsteroidal antiinflammatory drugs in patients after hospitalization for serious coronary heart disease. Circ Cardiovasc Qual Outcomes. 2009;2:155–163. doi: 10.1161/CIRCOUTCOMES.108.805689. [DOI] [PubMed] [Google Scholar]
- 88.Read JL, Stern RS, Thibodeau LA, Geer DE, Jr., Klapholz H. Variation in antenatal testing over time and between clinic settings. JAMA. 1983;249:1605–9. [PubMed] [Google Scholar]
- 89.Rosenberg L, Slone D, Shapiro S, Kaufman DW, Miettinen OS, Stolley PD. Aspirin and myocardial infarctgion in young women. Am J Public Health. 1982;72:389–391. doi: 10.2105/ajph.72.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rothman KJ, MacMahon B, Lin TM, Lowe CR, Mirra AP, Ravnihar B, Salber EJ, Trichopoulos D, Yuasa S. Maternal age and birth rank of women with breast cancer. J Natl Cancer Inst. 1980;65:719–22. doi: 10.1093/jnci/65.4.719. [DOI] [PubMed] [Google Scholar]
- 91.Roumie CL, Choma NN, Kaltenbach L, Mitchel EF, Arbogast PG, Griffin MR. Non-aspirin NSAIDs, cyclooxygenase-2 inhibitors and risk for cardiovascular events-stroke, acute myocardial infarction, and death from coronary heart disease. Pharmacoepidemiol Drug Saf. 2009;18:1053–1063. doi: 10.1002/pds.1820. [DOI] [PubMed] [Google Scholar]
- 92.Roumie CL, Mitchel EF, Kaltenbach L, Arbogast PG, Gideon P, Griffin MR. Nonaspirin NSAIDs, cyclooxygenase 2 inhibitors, and the risk for stroke. Stroke. 2008;39:2037–2045. doi: 10.1161/STROKEAHA.107.508549. [DOI] [PubMed] [Google Scholar]
- 93.Salonen JT, Hamynen H, Heinonen OP. Impact of a health education program and other factors on stopping smoking after heart attack. Scand J Public Health. 1985;13:103–108. doi: 10.1177/140349488501300306. [DOI] [PubMed] [Google Scholar]
- 94.Schachter J, Hill EC, King EB, Heilbron DC, Ray RM, Margolis AJ, Greenwood SA. Chlamydia trachomatis and cervical neoplasia. JAMA. 1982;248:2134–2138. [PubMed] [Google Scholar]
- 95.Scholer SJ, Hickson GB, Ray WA. Sociodemographic factors identify US infants at high risk of injury mortality. Pediatrics. 1999;103:1183–1188. doi: 10.1542/peds.103.6.1183. [DOI] [PubMed] [Google Scholar]
- 96.Shore RE, Pasternack BS, Thiessen EU, Sadow M, Forbes R, Albert RE. Case-control study of hair dye use and breast cancer. J Natl Cancer Inst. 1979;62:277–283. [PubMed] [Google Scholar]
- 97.Singer DE, Moulton AW, Nathan DM. Diabetic myocardial infarction: interaction of diabetes with other preinfarction risk factors. Diabetes. 1989;38:350–357. doi: 10.2337/diab.38.3.350. [DOI] [PubMed] [Google Scholar]
- 98.Siu TO, Loeb M, Pater JL. Variable effects of postoperative radiotherapy on disease-free survival in subgroups of patients with node-negative breast carcinoma. J Chronic Dis. 1980;33:471–484. doi: 10.1016/0021-9681(80)90072-7. [DOI] [PubMed] [Google Scholar]
- 99.Solomon DH, Avorn J, Sturmer T, Glynn RJ, Mogun H, Schneeweiss S. Cardiovascular outcomes in new users of coxibs and nonsteroidal antiinflammatory drugs: high-risk subgroups and time course of risk. Arthritis Rheum. 2006;54:1378–89. doi: 10.1002/art.21887. [DOI] [PubMed] [Google Scholar]
- 100.Stason WB, Neff RK, Miettinen OS, Jick H. Alcohol consumption and nonfatal myocardial infarction. Am J Epidemiol. 1976;104:603–608. doi: 10.1093/oxfordjournals.aje.a112338. [DOI] [PubMed] [Google Scholar]
- 101.Strauss D, Eyman RK, Grossman HJ. Predictors of mortality in children with severe mental retardation: The effect of placement. Am J Public Health. 1996;86:1422–1429. doi: 10.2105/ajph.86.10.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strauss D, Kastner T, Ashwal S, White J. Tubefeeding and mortality in children with severe disabilities and mental retardation. Pediatrics. 1997;99:358–362. doi: 10.1542/peds.99.3.358. [DOI] [PubMed] [Google Scholar]
- 103.Swan SH, Brown WL. Oral-contraceptive use, sexual activity and cervical carcinoma. Am J Obstet Gynecol. 1981;139:52–57. doi: 10.1016/0002-9378(81)90411-7. [DOI] [PubMed] [Google Scholar]
- 104.van Rossum CT, Shipley MJ, Hemingway H, Grobbee DE, Mackenbach JP, Marmot MG. Seasonal variation in cause-specific mortality: are there high-risk groups? 25-year follow-up of civil servants from the first Whitehall study. Int J Epidemiol. 2001;30:1109–16. doi: 10.1093/ije/30.5.1109. [DOI] [PubMed] [Google Scholar]
- 105.van Staa TP, Abenhaim L, Cooper C, Zhang B, Leufkens HGM. Public health impact of adverse bone effects of oral corticosteroids. Br J Clin Pharmacol. 2001;51:601–607. doi: 10.1046/j.0306-5251.2001.1385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Staa TP, Rietbrock S, Setakis E, Leufkens HG, Leufkens HGM. Does the varied use of NSAIDs explain the differences in the risk of myocardial infarction? J Intern Med. 2008;264:481–92. doi: 10.1111/j.1365-2796.2008.01991.x. [DOI] [PubMed] [Google Scholar]
- 107.Welsh MM, Karagas MR, Applebaum KM, Spencer SK, Perry AE, Nelson HH. A role for ultraviolet radiation immunosuppression in non-melanoma skin cancer as evidenced by gene-environment interactions. Carcinogenesis. 2008;29:1950–1954. doi: 10.1093/carcin/bgn160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wynder EL, Stellman SD. Impact of long-term filter cigarette usage on lung and larynx cancer risk: case-control study. J Natl Cancer Inst. 1979;62:471–477. doi: 10.1093/jnci/62.3.471. [DOI] [PubMed] [Google Scholar]
- 109.National Pharmaceutical Council [April 5, 2012];A brief history of comparative effectiveness research and evidence-based medicine. 2012 http://www.npcnow.org/Public/Issues/i_cer/cer_toolkit/A_Brief_History_of_Comparative_Effectiveness_Research_And_Evidence-Based_Medicine.aspx.
- 110.Schneeweiss S. Developments in post-marketing comparative effectiveness research. Clin Pharmacol Ther. 2007;82:143–56. doi: 10.1038/sj.clpt.6100249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mansson R, Joffe MM, Sun W, Hennessy S. On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol. 2007;166:332–9. doi: 10.1093/aje/kwm069. [DOI] [PubMed] [Google Scholar]
- 112.Messer LC, Oakes JM, Mason S. Effects of socioeconomic and racial residential segregation on preterm birth: a cautionary tale of structural confounding. Am J Epidemiol. 2010;171:664–673. doi: 10.1093/aje/kwp435. [DOI] [PubMed] [Google Scholar]
- 113.MacLean C, Newberry S, Maglione M, McMahon M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A, McNamara M, Desai SB, Zhou A, Chen S, Carter J, Tringale C, Valentine D, Johnsen B, Grossman J. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213. doi: 10.7326/0003-4819-148-3-200802050-00198. [DOI] [PubMed] [Google Scholar]
- 114.Cadarette SM, Katz JN, Brookhart MA, Stürmer T, Stedman MR, Solomon DH. Relative effectiveness of osteoporosis drugs for preventing nonvertebral fracture. Ann Intern Med. 2008;148:637–46. doi: 10.7326/0003-4819-148-9-200805060-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glynn RJ, Gagne JJ, Schneeweiss S. Role of disease risk scores in comparative effectiveness research with emerging therapies. Pharmacoepidemiol Drug Saf. 2012 May;21(Suppl 2):138–147. doi: 10.1002/pds.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Juurlink DN, Mamdani MM, Kopp A, Redelmeier DA. The risk of suicide with selective serotonin reuptake inhibitors in the elderly. Am J Psychiatry. 2006;163:813–21. doi: 10.1176/ajp.2006.163.5.813. [DOI] [PubMed] [Google Scholar]
- 117.Park-Wyllie LY, Mamdani MM, Li P, Gill SS, Laupacis A, Juurlink DN. Cholinesterase inhibitors and hospitalization for bradycardia: a population-based study. PLoS Med. 2009;6:e1000157. doi: 10.1371/journal.pmed.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myers JA, Rassen JA, Gagne JJ, et al. Effects of adjusting for instrumental variables on bias and precision of effect estimates. Am J Epidemiol. 2011;174:1213–1222. doi: 10.1093/aje/kwr364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arbogast PG, Ray WA. Performance of disease risk scores, propensity scores, and traditional multivariable outcome regression in the presence of multiple confounders. Am J Epidemiol. 2011;174:613–620. doi: 10.1093/aje/kwr143. [DOI] [PubMed] [Google Scholar]