Abstract
Neonatal exposure to endocrine disrupting compounds (EDCs) can impair reproductive physiology, but the specific mechanisms by which this occurs remain largely unknown. Growing evidence suggests that kisspeptin (KISS) neurons play a significant role in the regulation of pubertal onset and ovulation, therefore disruption of KISS signaling could be a mechanism by which EDCs impair reproductive maturation and function. We have previously demonstrated that neonatal exposure to phytoestrogens decreases KISS fiber density in the anterior hypothalamus of female rats, an effect which was associated with early persistent estrus and the impaired activation gonadotropin releasing hormone (GnRH) neurons. The goals of the present study were to (1) determine if an ERα selective agonist (PPT) or bisphenol-A (BPA) could produce similar effects on hypothalamic KISS content in female rats and (2) to determine if male KISS fiber density was also vulnerable to disruption by EDCs. We first examined the effects of neonatal exposure to PPT, a low (50 μg/kg bw) BPA dose, and a high (50 mg/kg bw) BPA dose on KISS immunoreactivity (-ir) in the anterior ventral periventricular (AVPV) and arcuate (ARC) nuclei of adult female rats, using estradiol benzoate (EB) and a sesame oil vehicle as controls. AVPV KISS-ir, following ovariectomy (OVX) and hormone priming, was significantly lower in the EB and PPT groups but not the BPA groups. ARC KISS-ir levels were significantly diminished in the EB and high dose BPA groups, and there was a nonsignificant trend for lower KISS-ir in the PPT group. We next examined effects of neonatal exposure to a low (50μg/kg bw) dose of BPA and the phytoestrogens genistein (GEN) and equol (EQ) on KISS-ir in the AVPV and ARC of adult male rats, using OVX females as an additional control group. None of the compounds affected KISS-ir in the male hypothalamus. Our results suggest that the organization of hypothalamic KISS fibers may be vulnerable to disruption by EDC exposure and that females might be more sensitive than males.
Keywords: bisphenol, phytoestrogens, soy, genistein, equol, gonadotropins, development, disruption, estrogen, estrogen receptor, GnRH, KISS
1. Introduction
Endocrine disrupting compounds (EDCs) have previously been shown in multiple studies to impair reproduction in a diverse range of species and are suspected of impacting human fecundity (Freni-Titulaer et al., 1986; Guillette and Gunderson, 2001; Howdeshell et al., 1999; Schoental, 1983). The specific mechanisms by which this occurs presumably require steroid hormone receptors, especially estrogen receptors (ERs), but remain relatively unclear. There is rapidly emerging evidence demonstrating that the KiSS-1 gene, which codes for a family of proteins called kisspeptins (KISS; previously called metastins), is vital for the initiation of puberty and the release of preovulatory gonadotropin releasing hormone (GnRH) in many species, including humans (Kauffman et al., 2007a; Navarro et al., 2004; Smith et al., 2006a; Smith et al., 2006b) KISS neurons co-express both ER subtypes (ERα and ERβ) and are exquisitely sensitive to estrogens throughout life (Clarkson and Herbison, 2006; Kauffman et al., 2007b; Smith et al., 2006b). We therefore hypothesized that disruption of KISS signaling on GnRH neurons by EDCs could be a mechanism underlying both the advancement puberty and the impairment of reproduction. We further hypothesized that ERα plays a critical mechanistic role in this process.
Within the hypothalamic-pituitary-gonadal (HPG) axis, reproductive maturation and function is coordinated by the release of GnRH (Elkind-Hirsch et al., 1981; Gorski et al., 1975). Although GnRH neurons express ERβ throughout development (Herbison and Pape, 2001; Hrabovszky et al., 2000; Hrabovszky et al., 2001) and thus may respond to EDCs directly, it is generally accepted that hormonal and other environmental signals are largely conveyed to GnRH neurons from other estrogen-responsive neurons within the hypothalamus. In rodents, the two most significant regions appear to be the anterior ventral periventricular (AVPV) and arcuate (ARC) nuclei (Gu and Simerly, 1997; Polston et al., 2004; Polston and Simerly, 2006; Shughrue et al., 1997; Simerly et al., 1990). KISS neurons reside in both of these regions and the two populations are thought to have distinctly different functional roles. AVPV KISS neurons are more numerous in females than males and are thought to be essential for steroid positive feedback and the initiation of the preovulatory GnRH surge (Clarkson et al., 2008; Gottsch et al., 2004; Irwig et al., 2004; Kauffman et al., 2007a; Roa et al., 2006; Smith et al., 2006b). This sexual dimorphism of KISS distribution and function is organized during the neonatal period by estrogens aromatized from testicular androgens (Bateman and Patisaul, 2008; Clarkson and Herbison, 2006; Gonzalez-Martinez et al., 2008; Kauffman et al., 2007b; Simerly, 2002). In contrast, KiSS mRNA expression in the ARC is not thought to be sexually dimorphic and appears to be important for the regulation of steroid negative feedback (Kauffman et al., 2007b). In other species, including primates, the two functionally distinct populations are also present but both are thought to reside in the ARC. The receptor for KISS is the G protein-coupled receptor GPR54 and is constitutively expressed in GnRH neurons (Irwig et al., 2004; Kotani et al., 2001; Lee et al., 1999; Muir et al., 2001). Activation of this receptor was shown to be required for the initiation of pubertal onset with the discovery that humans or mice lacking functional GPR54 receptors are hypogonadal and fail to enter puberty (de Roux et al., 2003; Seminara et al., 2003).
We have previously demonstrated that neonatal exposure to estradiol benzoate (EB), the soy-derived phytoestrogen genistein (GEN) or the phytoestrogen metabolite equol (EQ) results in a significantly lower density of KISS immunolabeled fibers in the anterior AVPV of female rats (Bateman and Patisaul, 2008). This reduction in AVPV KISS immunoreactivity (KISS-ir) was accompanied by disrupted estrous cycles and an impaired ability to activate GnRH neurons (as measured by the colocalization of GnRH and Fos immunolabeling) in response to hormone priming. Therefore, significantly reduced KISS-ir in the female AVPV is likely indicative of an impaired ability to generate the surge of GnRH needed to initiate ovulation. It remains unclear which ER subtype mediates the disruption of KISS fiber density in the AVPV or if other EDCs can modify KISS content in the AVPV. It is also unknown whether or not EDC exposure can alter KISS-ir in the male AVPV or ARC. Therefore, the present study had two goals: (1) to determine if neonatal exposure to the ERα selective agonist propyl-pyrazole-triol (PPT) or the plastics component bisphenol-A (BPA) could affect KISS fiber density in the AVPV and ARC of the female rat and (2) to determine if BPA, GEN or EQ could disrupt KISS fiber density in the AVPV or ARC of the male rat. We hypothesized that PPT would suppress hypothalamic KISS fiber density in the females to a similar degree as EB, and that BPA would also significantly impact KISS-ir in the AVPV, as we have seen with GEN. We further hypothesized that hypothalamic KISS fiber density in males would be demasculinized by neonatal exposure to the endocrine disrupting compounds BPA, GEN and EQ.
BPA entered commercial production in the 1950s and is now used primarily to make polycarbonate plastic and epoxy resins including dental sealants. Exposure to BPA during the pre- or postnatal period has previously been shown in numerous studies to impact female reproductive physiology including the timing of pubertal onset and the induction of early, persistent estrus in rodents (Rubin et al., 2001; vom Saal and Hughes, 2005; Welshons et al., 2006). Masculinization of KISS system could be a mechanism underlying these physiological effects. At the time of this writing, the no observed adverse effect level (NOAEL) for BPA published by the United States Environmental Protection Agency (EPA) was 50 mg/kg bw per day. The lowest observed adverse effects level (LOAEL) established by the United States Food and Drug Administration (FDA) for BPA was 50 μg/kg bw. For the present experiments, we used the EPA NOAEL as our “high dose” and the FDA LOAEL as our “low dose.”
Similarly, perinatal exposure to GEN has previously been shown to impair reproductive behavior and function in males (Wisniewski et al., 2003), advance pubertal onset in females, and ultimately result in persistent estrus in mature females (Bateman and Patisaul, 2008; Jefferson et al., 2005; Jefferson et al., 2006; Lewis et al., 2003; Patisaul et al., 2007). In contrast, less is known about how the isoflavone metabolite EQ might affect reproductive physiology in either sex. EQ is a metabolite of the soy isoflavone daidzein (Setchell et al., 2003) and is generated entirely by intestinal microflora. Not all humans are capable of biotransforming daidzein to EQ (Setchell et al., 2002). For example, infants cannot generate EQ, but EQ produced by the mother, as well as GEN and other phytoestrogens, readily cross the placenta (Todaka et al., 2005). We previously reported that neonatal exposure to 10 mg/kg bw EQ can impair the capacity to generate a GnRH surge following hormone stimulation in female rats, and results in a premature acyclic state. Both effects were also observed with an equivalent dose of GEN (Bateman and Patisaul, 2008). Neonatal exposure to GEN, but not EQ, significantly decreased KISS fiber density in the female rat AVPV. The potential for GEN and EQ to alter hypothalamic KISS fiber density in males is unknown, and therefore was an important goal of this study.
2. Materials and Methods
2.1 Animals and neonatal treatment
Many of the male rats used in the present study were siblings of the females used in our prior, related study (Bateman and Patisaul, 2008). The remainder were obtained from cross-fostered litters born to timed pregnant Long Evans rats (n = 10; Charles River, NC). Females were obtained from a different set of cross fostered litters born to timed pregnant Long Evans rats (n = 10, Charles River, NC). All dams were individually housed in a humidity and temperature controlled room with a 12h light cycle (lights on from 7:00 to 19:00) at 23°C and 50% average relative humidity at the Biological Resource Facility at North Carolina State University (NCSU). Because standard lab chows are soy-based and thus contain significant amounts of phytoestrogens (Boettger-Tong et al., 1998; Brown and Setchell, 2001; Thigpen et al., 1999), all of the animals were fed a semi-purified, phytoestrogen-free diet ad libitum for the duration of the experiment (AIN-93G, Test Diet, Richmond, IN).
Beginning on the day of birth, the females (n = 8 - 11 per group) were subcutaneously (sc) injected for four days with vehicle (0.05 ml, Sigma, St. Louis), estradiol benzoate (EB, 25 μg, Sigma), 50 μg/kg bw bisphenol-A (Low Dose BPA, Sigma), 50 mg/kg bw bisphenol-A (High Dose BPA), or the ERα agonist propyl-pyrazole-triol (PPT; 1 mg/kg bw, Tocris Biosciences, Ellisville, MS). PPT, is a selective agonist for ERα, with a 400-fold preference for ERα and minimal binding to ERβ (Stauffer et al., 2000; Sun et al., 2002). Although sc injection does not mimic a “natural” exposure and therefore may not considered ideal, this route of administration was chosen because it is not known if PPT can survive oral administration. This delivery method may also be advantageous for BPA because a recent report has demonstrated that injection produces equivalent or lower plasma BPA levels in neonatal rodents than oral administration, therefore making injection an appropriate route of administration when considering potential effects in humans (Taylor et al., 2008). All compounds were dissolved in ethanol, and then sesame oil at a ratio of 10% EtOH and 90% oil as we have done previously (Bateman and Patisaul, 2008; Patisaul et al., 2006). The vehicle was also prepared with this ratio. We have found this vehicle to cause less skin irritation than the alternative vehicle, DMSO.
In a separate experiment, the males were sc injected with sesame oil (0.05 ml, Sigma, St. Louis), bisphenol-A (BPA, 50 μg/kg/bw, Sigma), racemic equol (EQ; 10 mg/kg bw, generously supplied by Mike Adams of Wake Forest University) or genistein (GEN; 10 mg/kg bw, Indofine Chemical Company, Hillsborough, NJ). All compounds were prepared in the same vehicle described above. This dose of GEN is similar to the total amount of soy phytoestrogens consumed daily by children fed soy infant formula (Setchell et al., 1997) and the dose of EQ was chosen to match. Although two doses of BPA were used for the female experiment, only the low dose was used here because it was considered to be the most environmentally relevant. The animals (n = 6 – 11 per group) received injections daily from the day of birth (PND 0) through PND 3 (4 injections total). Tissue collected from age-matched females (obtained from different litters) that had been ovariectomized (OVX; n = 7) was used as an additional control. This was done because female rats have more KISS neurons in the AVPV than males. The sex difference is augmented by estrogen in females, thus by using OVX females as a control group, we hoped to determine if any demasculinizing effect on AVPV KISS fiber density observed in the males following EDC exposure could significantly minimize this baseline sex difference.
All pups were weaned into same-sex littermate pairs on PND 22, ear tagged, and maintained on a reverse light schedule (lights off from 10:00 to 22:00) for the remainder of the experiment. Because neonatal administration of the EDCs impaired the estrous cycle, females were ovariectomized (OVX; at ovariectomy all of the control females were cycling normally, all of the EB and PPT females were in persistent estrus, 67% of the High Dose BPA females were no longer cycling, and 14% of the Low Dose BPA females also showed signs of persistent estrus) under isoflurane anesthesia (completed over two weeks beginning on postnatal day 148). Four weeks later they were injected sc with 10 μg EB dissolved in sesame oil at 0900 h, followed 48 hours later by a sc injection of 500 μg of progesterone dissolved in the same vehicle to stimulate GnRH secretion. Males were left gonadally intact.
Animal care, maintenance and surgery were conducted in accordance with the applicable portions of the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for the Care and use of Laboratory Animals.” All experimental procedures involving animals were approved by the NCSU Institutional Animal Care and Use Committee.
2.2 Tissue collection, and immunohistochemistry
All animals were sacrificed by transcardial perfusion with 4% paraformaldehyde. Females were sacrificed 5-7 hours after the progesterone injection as was done in the prior, related, experiment (Bateman and Patisaul, 2008). Brains were removed, post-fixed in 20% sucrose paraformaldehyde, cryoprotected overnight in potassium phosphate buffer containing 20% sucrose, rapidly frozen in powdered dry ice, and stored at -80°C. Brains were then sliced into 35 μm coronal sections, and divided into four series of free-floating alternating sections, one of which was used for the present study. Tissue was stored in antifreeze solution (20% glycerol, 30% ethylene glycol in KPBS) at -20°C until immunohistochemical processing.
The male AVPV tissue was processed first. A set of anatomically matched sections from untreated, age matched, OVX females obtained previously was used as an additional control group for the male experiment. For each animal, sections comprising the organum vasculosum of the lamina terminalis (OVLT) through the caudal border of the AVPV were immunolabeled for KISS using immunohistochemistry (IHC) methods described in detail elsewhere (Patisaul et al., 2008). Kisspeptin was detected with a primary antibody directed against kisspeptin-10, raised in rabbit and generously gifted by Alain Caraty of Institut National de la Recherche Agronomique/Centre National de la Recherche Scientifique, Université de Tours, at 1:6,000. After incubation in the primary antibody, sections for the male AVPV experiment were placed in a biotinylated anti-rabbit immunoglobiulin G (IgG) secondary antibody (1:200, Vector Labs, Burlingame, CA) for 90 minutes at room temperature then amplified using an avidin-biotin complex (ABC Elite, Vector Labs) and developed in nickel enhanced-3,3′-diaminobenzidine (NiDAB) chromagen (Vector Labs). Sections were mounted onto slides (Superfrost Plus, Fisher, Pitsburgh, PA) and cover-slipped with DPX mountant (VWR International, INC., Poole, England). With these methods, KISS-ir was easily visualized but technically difficult to quantify. Therefore, after primary incubation, the remaining tissue (male ARC, female AVPV and ARC) was placed in Alexa-Fluor goat anti-rabbit 488 secondary antibodies, rinsed, mounted onto slides (Superfrost Plus, Fisher, Pittsburgh, PA), and coverslipped using a standard glycerol mountant. The remaining tissue was used for other experiments.
2.4 Quantification of KISS fiber density in the AVPV and ARC
In the AVPV, KISS immunoreactivity (-ir) has previously been shown to be localized to extended lengths of fibers throughout and rostral to the AVPV as well as within cell bodies located in the medial and caudal AVPV (Bateman and Patisaul, 2008; Clarkson and Herbison, 2006). We aimed to quantify the density of AVPV KISS fibers projecting rostrally from the AVPV to GnRH neurons within the OVLT, therefore one anterior AVPV section was selected for each animal using a brain atlas (Paxinos and Watson, 1998; Paxinos and Watson, 2004). In the ARC, KISS-ir has been shown to be localized to fibers and cell bodies throughout the ARC so one midlevel section of the ARC was selected for imaging and quantification. Thus, the selected regions are representative of the AVPV and ARC but do not include all KISS fibers present in either. NiDAB labeled KISS-ir in the AVPV of the males was visualized on a Leica 5000DM fitted with a 40X objective lens. Fibers were thresholded and quantified using the MCID Elite Image Analysis (Interfocus Imaging Ltd., Cambridge, England) software package. In this analysis the proportion of immunolabeling (total grain area) within a region of interest (AVPV) was quantified for each section. Therefore the data represent the proportional area occupied by labeled fibers, not the specific number of fibers.
Immunofluorescent KISS-ir was visualized in the remaining tissue (female AVPV, male and female ARC) on a Leica TCS SPE confocal microscope fitted with a 63X oil corrective objective lens. For each scan, a set of serial image planes (z-step distance = 1 μm) was collected through the entire thickness of each section. Image stacks were analyzed using the Image J software package (National Institutes of Health (NIH), Bethesda, MD) as previously described (Bateman and Patisaul, 2008; Patisaul et al., 2008). To control for variations in tissue thickness that would result in unequal numbers of image planes, substacks of consecutive image planes that excluded the rostral and caudal edges of the tissue sections were created for each set of scans. Substacks consisted of 21 sequential image planes for the AVPV and 20 sequential image planes for the ARC. Only data from sections with consistent staining throughout the entire thickness were included in the analysis. Individual images contained within each substack were first binarized and depixelated to minimize the inclusion of background fluorescence. Fibers were then skeletonized to a thickness of one pixel to compensate for differences in individual fiber thickness and brightness. The number of resulting bright pixels in each image plane was then quantified using the Image J Voxel Counter plug-in (NIH). The voxel counts were averaged within the substack to obtain a single measure for each section that was then used as a quantitative representation of average KISS fiber density within the volume sampled (Bateman and Patisaul, 2008; Patisaul et al., 2008).
Because the tissue from the male and female experiments was immunolabled at different times, and KISS fiber density in the male AVPV was assessed using different analytical methods, it should be emphasized that the results from the two experiments are not directly comparable. Each experiment was designed, conducted, and statistically analyzed separately.
2.5 Statistical Analysis
For the analysis of the male results, the age matched OVX females were considered to be a treatment group, resulting in five treatment conditions: OIL, BPA (50 μg/kg), GEN, EQ, and OVX. For the analysis of the female results, no male tissue was included, resulting in five treatment conditions: OIL, EB, PPT, low dose BPA, and high dose BPA. For each experiment, the density of voxels containing KISS fibers was compared by one-way analysis of variance (ANOVA) followed by Fisher’s Least Significant Difference (LSD) post hoc tests when appropriate. In all cases, the significance level was set at p < 0.05 (SYSTAT).
3. Results
3.1 In the females, EB and PPT, but neither dose of BPA, reduced KISS-ir
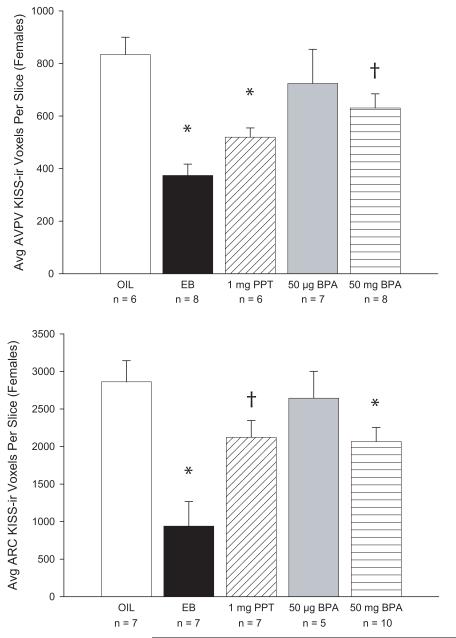
There was a significant effect of neonatal treatment on KISS-ir in the female AVPV (F(4,30) = 5.741, p < 0.001; Fig 1). Both EB and the ERα agonist PPT significantly decreased KISS fiber density in the AVPV compared to the vehicle treated control females (p < 0.01). Neither the high nor the low dose of BPA produced a significant effect, although the effect of the high dose approached statistical significance (p = 0.066). Similarly, neonatal treatment also significantly affected KISS-ir in the ARC (F(4,31) = 7.398, p < 0.001). ARC KISS-ir was significantly lower in the EB (p < 0.001) and high dose BPA groups (p < 0.03) but not the low dose BPA group (p = 0.61) compared to the vehicle treated controls. There was a trend for lower KISS-ir levels in the PPT group but this effect did not quite reach statistical significance (p < 0.06).
FIG. 1.
(A) AVPV KISS-ir was significantly lower in the females neonatally treated with EB or the ERα agonist PPT compared to the oil treated controls (OIL). KISS-ir was lower in the high dose BPA group compared to the OIL females, but this effect did not reach statistical significance. (B) ARC KISS-ir was significantly lower in the EB group compared the OIL group. The high dose of BPA also resulted in significantly reduced KISS-ir in the ARC. Although ARC KISS-ir levels were also lower, compared to the OIL group, in the PPT females, this effect did not reach statistical significance. (†p < 0.07; *p < 0.03)
3.2 Male AVPV and ARC KISS-ir was not affected by neonatal exposure to EDCs
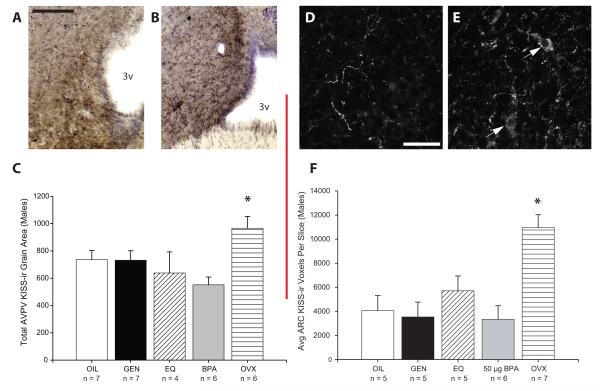
There was a significant effect of treatment group on AVPV KISS fiber density (F(4,23) = 4.1; p < 0.01; Fig 2) which was largely attributable to significantly higher levels in the OVX females compared to the males, regardless of treatment (p < 0.03). There was no significant difference in KISS-ir between any of the male groups. It should be noted that, because it turned out to be very difficult to reliably quantify KISS-ir fiber density using NiDAB as a chromagen, the number of EQ treated animals deemed acceptable for analysis was undesirably small (n = 4). In the ARC, KISS-ir was labeled using fluorescent secondary antibodies and visualized using confocal microscopy to achieve better spatial resolution.
FIG. 2.
Representative NiDAB KISS-ir labeling depicting the extended lengths of KISS-ir fibers present in the rostral AVPV of an (A) oil treated control male (OIL) and an (B) ovariectomized (OVX) female. Please note that the images presented here were photographed at a lower magnification than those used for the quantification and analysis of the KISS-ir fibers. AVPV KISS-ir was more abundant in the OVX females compared to the males, regardless of treatment (C). Representative confocal images (4 merged optical planes) depicting KISS-ir in the ARC of a representative OIL male (D) and OVX female (E). Labeling was readily observed within extended lengths of fibers as well as within neuronal cell bodies (white arrows) and as punctuate structures in close apposition to those cell bodies. KISS-ir cell bodies were visible in the ARC of all animals sampled, two of which are indicated (white arrows) in the OVX female image (D). ARC KISS-ir was significantly higher in the OVX females compared to the OIL males, and not significantly affected by treatment in the males. (*p < 0.03; black scale bar = 100 μm; white scale bar = 20 μm; 3v = 3rd ventricle)
Exposure effects in the ARC were similar to those observed in the AVPV. There was also a significant effect of treatment group on KISS content in the ARC (F(4,23) = 8.6; p < 0.001) which, again, mostly resulted from significantly higher levels in the OVX females compared to the males (p < 0.004; Fig 2). KISS-ir did not statistically differ among the male groups regardless of treatment.
4. Discussion
In the female rats, hypothalamic KISS-ir was significantly reduced by neonatal administration of EB or the ERα agonist PPT. These observations demonstrate that ERα plays a significant mechanistic role in the masculinization of hypothalamic KISS content by estrogens. We have previously reported a significant effect of PPT on KISS fiber density in the female ARC (Bateman and Patisaul, 2008) and the present results are consistent with that prior result, although they do not reach statistical significance in this instance. This masculinization is functionally significant as we have previously established that females neonatally treated with EB or PPT are incapable of maintaining a regular estrous cycle, and fail to activate GnRH neurons in response to hormone priming (Bateman and Patisaul, 2008). In contrast, we have also shown that neonatal administration of the ERβ agonist DPN at the same dose (1 mg/kg bw) does not produce a similar degree of impairment to either estrous cycle function or GnRH activation. It remains to be determined if higher or lower doses of ERβ agonists can produce effects that are as profound as those observed with the ERα selective agonist PPT. Collectively, the results from the present study, and our prior study (Bateman and Patisaul, 2008), support the hypothesis that ERα plays a more instrumental role in the masculinization of signaling pathways between KISS and GnRH neurons than ERβ.
Our findings are consistent with a paper that was published as this manuscript was in the final stages of revision (Navarro et al., 2008). In that study, Navarro and colleagues found that a single injection of EB on the day of birth resulted in significantly lower KiSS-1 mRNA levels (compared to control animals) in whole hypothalamic fragments obtained on PND 30. The precise location of the reduced mRNA levels was not determined, but our data indicate that it likely occurred in both the AVPV and the ARC, and further demonstrate that the effect persists beyond pubertal onset and into adulthood. Interestingly, Navarro and colleagues also found that reduced hypothalamic KiSS-1 mRNA levels were accompanied by a persistent decrease in basal LH levels, suggesting that steroid negative feedback was impaired. Within the present study, the observed reduction in ARC KISS-ir in the EB treated animals is consistent with that hypothesis. Finally, intracerebral administration of kisspeptin-10 provoked an LH surge in the EB treated animals of the Navarro study (Navarro et al., 2008) indicating that GnRH neurons remain capable of responding to kisspeptin. In our prior paper (Bateman and Patisaul, 2008), we showed that GnRH activation following hormone priming is nearly absent in adult females neonatally treated with EB or PPT. Collectively, these observations suggest that in animals neonatally exposed to EB or an ERα agonist, disrupted GnRH secretion does not result from the inability of GnRH neurons to release GnRH in response to kisspeptin. Instead, it is likely that the functional organization of hormone sensitive afferents to GnRH neurons has been disrupted such that, in animals neonatally exposed to EB or PPT, AVPV KISS-ir neurons do not respond to hormone priming as they should and therefore do not potentiate the GnRH surge. An alternative hypothesis is that the lower density of KISS-ir fibers in the AVPV means that the amount of kisspeptin generated in response to hormone priming is insufficient to result in a GnRH surge. Future work is needed to explore these possibilities. In general, our data support the hypothesis that neonatal exposure to estrogenic compounds disrupts the organization of the GnRH system and shows that ERα, rather than ERβ, likely plays a critical role in this process.
The demonstration that ERα agonism during the neonatal critical period can effectively masculinize hypothalamic KISS content (both in the present and our prior, related study) implies that ERα is expressed in the hypothalamus during this developmental window. Although it has previously been shown that nearly all KISS neurons in the adult AVPV and ARC of both sexes contain ERα (Smith et al., 2005) (while ERβ colocalizes with far fewer), whether or not ERα is present in KISS neurons during the neonatal period remains to be definitively established. A pair of studies conducted by the same research group (Orikasa et al., 2002; Orikasa and Sakuma, 2003) have identified both ERβ-ir and ERβ mRNA in the AVPV of male and female rats on PND 7 and beyond (day of birth defined as PND 1 in these studies) but did not look earlier. In contrast, a study conducted by a different research group observed no ERβ-ir within the AVPV in the first 2 weeks of life (Perez et al., 2003). Thus the neonatal distribution of ERα and ERβ in the rat AVPV remains undefined. It is possible that ERα-expressing neurons with afferents to KISS neurons, rather than KISS neurons themselves, are mitigating the decrease in KISS-ir. Regardless of how or where PPT is stimulating ERα activity, our data show that the net result is masculinized KISS-ir in the AVPV and ARC of the female hypothalamus and suggest that agonism of ERα by EDCs during the period in which the hypothalamus is undergoing steroid directed sexual differentiation (neonatal period in rodents, gestation in primates) could permanently impair GnRH secretion in the adult female.
Neither the low nor the high dose of BPA significantly affected KISS-ir in the female AVPV but the high dose reduced KISS-ir in the ARC. These results indicate that the sex specific organization of hypothalamic KISS-ir is vulnerable to disruption by estrogenic EDCs in females. The observation is consistent with the recent report (Navarro et al., 2008) that neonatal exposure to significantly higher doses of BPA (100 or 500 μg per rat) resulted in significantly decreased hypothalamic KiSS-1 mRNA levels at PND 30. That study did not isolate the specific location within the hypothalamus where this occurs, but our data indicate that the effect is most likely strongest within the ARC.
Intriguingly, the effect of BPA on female rat KISS fiber density differs from what we have previously observed with the phytoestrogens GEN and EQ, both of which significantly reduced KISS-ir in the AVPV but had no appreciable effect in the ARC (Bateman and Patisaul, 2008). Although it is possible that the effect of BPA on KISS-ir in the AVPV would have reached statistical significant if the group size was larger, in our prior study there was no indication that the phytoestrogens might have a meaningful impact on KISS-ir in the ARC. The differential effects of BPA and the phytoestrogens on hypothalamic KISS fiber density may be related to their ability to bind and activate ERs in vivo. GEN and EQ have substantially higher relative binding affinities for both ERα and ERβ in vitro than BPA (Kuiper et al., 1998; Mueller et al., 2003; Nikov et al., 2000) suggesting that they may be more potent agonists of ERs than BPA and other synthetic EDCs in vivo. It is also possible that the phytoestrogens are altering AVPV KISS-ir through ER-independent mechanisms. For example, it is well established that GEN is a tyrosine kinase inhibitor (Dixon and Ferreira, 2002; Gaudette and Holub, 1990; Huang et al., 1999) and EQ can bind and sequester the potent androgen dihydrotestosterone (Lund et al., 2004).
Because the AVPV and ARC populations of KISS neurons are thought to have distinct functional roles, it is possible that the physiological consequences of disrupting the organization of each may also differ. The AVPV population is thought to be critical for the regulation of steroid positive feedback and the initiation of the preovulatory surge of GnRH in females, while the ARC population is thought to be important for steroid negative feedback in both sexes (Clarkson et al., 2008; Gottsch et al., 2004; Irwig et al., 2004; Kauffman et al., 2007a; Kauffman et al., 2007b; Roa et al., 2006; Smith et al., 2006b). This would suggest that EDCs which affect the organization and function of the AVPV population of KISS neurons could ultimately impair the GnRH surge, and therefore ovulation. This hypothesis is supported by our prior observation that females neonatally treated with GEN or EQ show significantly lower levels of GnRH activation following hormone priming than vehicle treated control females. Because BPA significantly affected the ARC population of KISS neurons, but not the AVPV population, it is possible that GnRH neuronal activation following hormone priming is relatively normal while the ability to regulate steroid negative feedback is not. We are currently exploring this possibility. In general, our data support the hypothesis that disrupted signaling between KISS and GnRH neurons could be an underlying mechanism by which EDCs impair female reproductive physiology.
Neither BPA nor the phytoestrogens EQ or GEN significantly affected KISS-ir in either the AVPV or the ARC of the males, suggesting that, at the doses tested, none were able to block or augment the masculinizing role of endogenous estrogen on KISS-ir in these regions. These data imply that the KISS system is potentially less vulnerable to disruption by EDCs in males than females. However, because the data from the male tissue (a set which included OVX female tissue) was obtained using different analytical methods than the female tissue discussed above it should be emphasized that the results from the two experiments are not directly comparable. AVPV KISS-ir levels were notably lower, but not significantly so, in the BPA treated males than the control males, a result which is consistent with what was recently reported by Navarro and colleagues using significantly higher doses of BPA (Navarro et al., 2008). These data suggest that BPA tended to hypermasculinize, rather than feminize, KISS-ir in this region. This contrasts with our previous work which demonstrated that higher levels of GEN and BPA can feminize the total volume of the male AVPV and the number of tyrosine hydroxylase (TH) immunolabeled cells within it (Patisaul et al., 2006; Patisaul et al., 2007). However, in those prior studies, we ultimately determined that these males did not show feminized patterns of GnRH activation, as measured by the co-localization of GnRH and Fos immunoreactivity, when stimulated by the sequential administration of EB and progesterone. Therefore, despite the feminization of volume and TH-ir, we had previously concluded that the AVPV was not functionally feminized. Collectively our data suggest that significantly higher AVPV volume is likely not an accurate or reliable biomarker of feminized AVPV function in males. Thus caution must be taken when using nuclear volume as a biomarker of endocrine disruption in the brain. In addition, the functional consequence of feminized TH within the male, if any, remains to be determined.
Finally, it should be noted that a sex difference in KISS-ir was observed in both the AVPV and the ARC between the OVX females and control (gonadally intact) males of the second experiment (Fig 2). This result is at least partially explained by the gonadal status of the animals. It has previously been established that in the rodent AVPV, KISS-ir (and KiSS-1 mRNA expression) is significantly higher in females compared to males, and greatly enhanced by hormone replacement (Irwig et al., 2004; Navarro et al., 2004; Smith et al., 2005; Smith et al., 2006b). Our observation that AVPV KISS fiber density is significantly higher in OVX females compared to gonadally intact males is consistent with these data. We ultimately elected to use OVX females as a control group in the male experiment because we were primarily interested in determining if neonatal EDC exposure in males could result in AVPV KISS-ir levels that were comparable to baseline levels in females. This turned out not to be the case. The effect of steroid hormones on KISS-ir is very different in the rat ARC. In the ARC, KISS-ir (and KiSS-1 mRNA expression) in both sexes decreases as steroid hormone levels increase (Irwig et al., 2004; Navarro et al., 2004; Smith et al., 2005; Smith et al., 2006b). Therefore it must be emphasized that the difference in KISS-ir between the control males and the OVX females (Fig 2) would have been lower if we had used gonadally intact or hormone replaced females instead of the OVX females. Interestingly, reduced KISS-ir in the female ARC following the neonatal administration of EB or PPT (observed in the first experiment, Fig 1) suggests that an underlying sex difference in KISS-ir content exists in the ARC, and that this difference is organized through an ERα dependent mechanism in the neonatal period. It has previously been reported that KiSS-1 mRNA expression levels in the ARC do not differ between the sexes (Kauffman et al., 2007b) but our results are not consistent with that conclusion.
The results of the present study collectively considered with our prior, related study (Bateman and Patisaul, 2008), demonstrate that disruption of the sex specific organization of KISS-ir in the female AVPV may be a mechanism by which some EDCs impact female reproductive physiology. The degree to which this can occur is likely dependent on how effectively the EDC in question can agonize ERα. BPA, at either the high or the low dose, failed to significantly alter AVPV KISS-ir in females suggesting that any reproductive defects observed following neonatal exposure to BPA likely originate via a different mechanism, possibly within the pituitary or the ovary. These results emphasize that although a variety of EDCs may produce similar deficits in female reproductive function and behavior, the mechanisms that underlie these effects may differ and warrant further exploration.
Acknowledgements
We thank Mindy Hill and Rebecca Pareja for assisting with the tissue slicing, Alain Caraty for generously gifting the kisspeptin antibody, and Mike Adams for gifting the equol. We are also grateful for the animal care staff at NCSU, particularly B.J. Welker and Linda Hester. This research was supported by NIEHS grant R01ES016001 to H.B.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008 doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger-Tong H, Murthy L, Chiappetta C, Kirkland JL, Goodwin B, Adlercreutz H, Stancel GM, Mäkelä S. A case of a laboratory animal feed with high estrogenic activity and its impact on in vivo responses to exogenously administered estrogens. Environmental Health Perspectives. 1998;106:369–73. doi: 10.1289/ehp.98106369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–47. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28:8691–7. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205–11. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- Elkind-Hirsch K, King JC, Gerall AA, Arimura AA. The luteinizing hormone-releasing hormone (LHRH) system in normal and estrogenized neonatal rats. Brain Res Bull. 1981;7:645–54. doi: 10.1016/0361-9230(81)90112-x. [DOI] [PubMed] [Google Scholar]
- Freni-Titulaer LW, Cordero JF, Haddock L, Lebron G, Martinez R, Mills JL. Premature thelarche in Puerto Rico. A search for environmental factors. Am J Dis Child. 1986;140:1263–7. doi: 10.1001/archpedi.1986.02140260065028. [DOI] [PubMed] [Google Scholar]
- Gaudette DC, Holub BJ. Effect of genistein, a tyrosine kinase inhibitor, on U46619-induced phosphoinositide phosphorylation in human platelets. Biochemistry and Biophysiology Res Comm. 1990;170:238–42. doi: 10.1016/0006-291x(90)91265-t. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez D, De Mees C, Douhard Q, Szpirer C, Bakker J. Absence of GnRH1 and Kiss1 activation in alpha-fetoprotein knockout mice: prenatal estrogens defeminize the potential to show preovulatory LH surges. Endocrinology. 2008 doi: 10.1210/en.2007-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA, Mennin SP, Kubo K. The neural and hormonal bases of the reproductive cycle of the rat. Adv Exp Med Biol. 1975;54:115–53. doi: 10.1007/978-1-4684-8715-2_6. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J Comp Neurol. 1997;384:142–64. [PubMed] [Google Scholar]
- Guillette LJ, Jr., Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–64. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Pape JR. New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol. 2001;22:292–308. doi: 10.1006/frne.2001.0219. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–9. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–4. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Fang MJ, Dillon GH. The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Brain Res Mol Brain Res. 1999;67:177–83. doi: 10.1016/s0169-328x(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Padilla-Banks E, Newbold RR. Studies of the effects of neonatal exposure to genistein on the developing female reproductive system. J AOAC Int. 2006;89:1189–96. [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007a;30:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007b;148:1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Safe SH, Van Der Saag PT, Van Der Berg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, Coulombe N, Tan CP, Tang-Nguyen AT, George SR, O’Dowd BF. Discovery of a receptor related to the galanin receptors. FEBS Lett. 1999;446:103–7. doi: 10.1016/s0014-5793(99)00009-5. [DOI] [PubMed] [Google Scholar]
- Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- Mueller SO, Kling M, Arifin Firzani P, Mecky A, Duranti E, Shields-Botella J, Delansorne R, Broschard T, Kramer PJ. Activation of estrogen receptor alpha and ERbeta by 4-methylbenzylidene-camphor in human and rat cells: comparison with phyto- and xenoestrogens. Toxicol Lett. 2003;142:89–101. doi: 10.1016/s0378-4274(03)00016-x. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–75. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–86. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Sanchez-Garrido MA, Castellano JM, Roa J, Garcia-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M. Persistent Impairment of Hypothalamic KiSS-1 System Following Exposures to Estrogenic Compounds at Critical Periods of Brain Sex Differentiation. Endocrinology. 2008 doi: 10.1210/en.2008-0580. [DOI] [PubMed] [Google Scholar]
- Nikov GN, Hopkins NE, Boue S, Alworth WL. Interactions of dietary estrogens with human estrogen receptors and the effect on estrogen receptor-estrogen response element complex formation. Environmental Health Perspectives. 2000;108:867–72. doi: 10.1289/ehp.00108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C, Kondo Y, Hayashi S, McEwen BS, Sakuma Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: implication in luteinizing hormone surge. Proc Natl Acad Sci U S A. 2002;99:3306–11. doi: 10.1073/pnas.052707299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orikasa C, Sakuma Y. Possible involvement of preoptic estrogen receptor beta positive cells in luteinizing hormone surge in the rat. Domest Anim Endocrinol. 2003;25:83–92. doi: 10.1016/s0739-7240(03)00047-x. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–8. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Sex differences in serotonergic but not gamma-aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:397–408. doi: 10.1210/en.2007-0666. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates, vol. Academic Press; San Diego: 1998. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates : [the new coronal set], vol. Elsevier Academic; London: 2004. [Google Scholar]
- Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res Dev Brain Res. 2003;145:117–39. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Polston EK, Simerly RB. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J Comp Neurol. 2006;495:122–32. doi: 10.1002/cne.20874. [DOI] [PubMed] [Google Scholar]
- Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–78. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Murray MK, Damassa DA, King JC, Soto AM. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect. 2001;109:675–80. doi: 10.1289/ehp.01109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoental R. Precocious sexual development in Puerto Rico and oestrogenic mycotoxins (zearalenone) Lancet. 1983;1:537. doi: 10.1016/s0140-6736(83)92229-8. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003;133:1027–35. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- Shughrue PL, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor α and β mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388:507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006a;131:623–30. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006b;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–47. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143:941–7. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Welshons WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol. 2008;25:169–76. doi: 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, Goelz MF, Haseman JK, Newbold RR, Forsythe DB. Phytoestrogen content of purified open- and closed-formula laboratory animal diets. Laboratory Animal Sciences. 1999;49:530–36. [PubMed] [Google Scholar]
- Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T, Mori C. Fetal exposure to phytoestrogens--the difference in phytoestrogen status between mother and fetus. Environ Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–33. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol. 2003;169:1582–6. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]