Abstract
Objective
Development of the secondary palate in mammals is a complex process that can be easily perturbed leading to the common and distressing birth defect, cleft palate (CP). Animal models are particularly useful tools for dissecting underlying genetic components of CP. We describe a new CP model resulting from a transgene insertion mutation.
Results
Transgene insertion mutagenesis disrupts the genomic organization and expression of the Ap2β1 gene located on chromosome 11. This gene encodes the β2-adaptin subunit of the heterotetrameric adaptor protein 2 (AP-2) complex involved in clathrin-dependent endocytosis. Homozygous CP mutant mice express no Ap2β1 mRNA or β2-adaptin protein, and die during the perinatal period. Heterozygous mice are phenotypically normal despite expressing diminished β2-adaptin mRNA and protein compared to wildtype. Remarkably, the paralogous β1-adaptin subunit of the AP-1 complex partially substitutes for the missing β2-adaptin in embryonic fibroblasts from homozygous mutant mice, resulting in assembly of reduced levels of an AP-2 complex bearing β1-adaptin. This variant AP-2 complex is therefore apparently capable of maintaining viability of the homozygous mutant embryos until birth, but insufficient to support palatogenesis.
Conclusion
Non-syndromic CP in an animal model is associated with disruption of the Ap2β1 gene.
Keywords: cleft palate, mouse, transgene insertion mutagenesis, β2-adaptin, clathrin coated pits
During morphogenesis of the secondary palate (palatogenesis) bilateral extensions of the maxillary processes (palatal shelves) reorient from a vertical position to a horizontal position over the tongue. Palatal fusion occurs when there is transformation of the medial edge epithelia (MEE) along with remodeling of the extracellular matrix (ECM). Perturbation of this complex cascade of events can lead to cleft palate (CP)(Ferguson, 1988). Non-syndromic CP (i.e. no other recognizable defects or disabilities) represents a common clinical outcome of genetically heterogeneous etiologies. Growing number of genes encoding transcription factors, signaling molecules, growth factors, growth factor receptors, and extracellular matrix proteins have been implicated in the pathogenesis of syndromic and non-syndromic orofacial clefts (Schutte and Murray, 1999; Chong, et al., 2002; Lidral, et al., 2008; Marazita, et al., 2009; Shi, et al., 2009). Transgene insertion mutagenesis is a common occurrence during the generation of transgenic mice (Meisler, 1992) and has led to the identification a number of developmentally important genes (Shawlot, et al., 1989; Bishop, et al., 2000; Overbeek, et al., 2001; Cunningham, et al., 2002).
Adaptor protein (AP) complexes are composed of subunits (adaptins) that are involved in the formation of intracellular transport vesicles and in the selection of cargo for incorporation into vesicles (Boehm and Bonifacino, 2001). Several heterotetrameric adaptor complexes (AP-1, AP-2, AP-3, and AP-4) are associated with vesicles. Adaptor complexes contain adaptin proteins (α, γ, δ, or ɛ and β1, β2, or β4, respectively) that are linked to a medium chain (µ1, µ2, µ3, or µ4) and one small chain (ο1, ο2, ο3, or ο4). The AP-2 adaptor complex (α, β2, µ2, and ο2) is key to successful clathrin dependent endocytosis from the plasma membrane whereas the AP-1 adaptor complex (γ, β1, µ1, and ο1) is associated with the trans-Golgi network and endosomes (Traub, 1997; Traub, 2003; Robinson, 2004). The Ap2β1 gene located on mouse chromosome 11 encodes the AP-2 complex subunit beta-1 protein (AP2B1, UniProtKB/Swiss-Prot Q9DBG3). AP2B1 has been widely studied under several alternative names including adaptor-related protein complex 2 beta-1 subunit; β2-adaptin; β-adaptin; plasma membrane adaptor HA2/AP2 adaptin beta subunit; clathrin assembly protein complex 2 beta large chain; and AP105B. The Ap1β1 gene encodes the AP-1 complex subunit beta-1 (AP1B1) (UniProtKB/Swiss-Prot O35643). Common alternative names for AP1B1 include: adaptor-related protein complex 1 subunit beta-1; adaptor protein complex AP-1 subunit beta-1; β-prime adaptin 1; β1-adaptin; Golgi adaptor HA1/AP1 adaptin beta subunit; clathrin assembly protein complex 1 beta large chain; and AP105A. To minimize confusion the common alternate names for the products of the Ap2β1 and Ap1β1 genes β2-adaptin and β1-adaptin, respectively are used through this present study.
Herein we report the use of transgene insertion mutagenesis to show that disruption of the Ap2β1 gene encoding the β2-adaptin subunit of the AP-2 complex causes perinatal mortality and non-syndromic CP.
METHODS
Generation of transgenic mice
Transgenic mice were generated by microinjection of a tyrosinase minigene (TYBS) into single-cell mouse embryos (Fig. 1A) (Yokoyama, et al., 1990; Overbeek, et al., 1991). Albino FVB/NJ female mice mated to FVB/NJ males were used as embryo donors. ICR females bred to vasectomized BDF1 males were used as embryo recipients and surrogate mothers. All the animal experiments were performed with the approval of the Indiana University School of Dentistry and Baylor College of Medicine Animal Care and Use Committees.
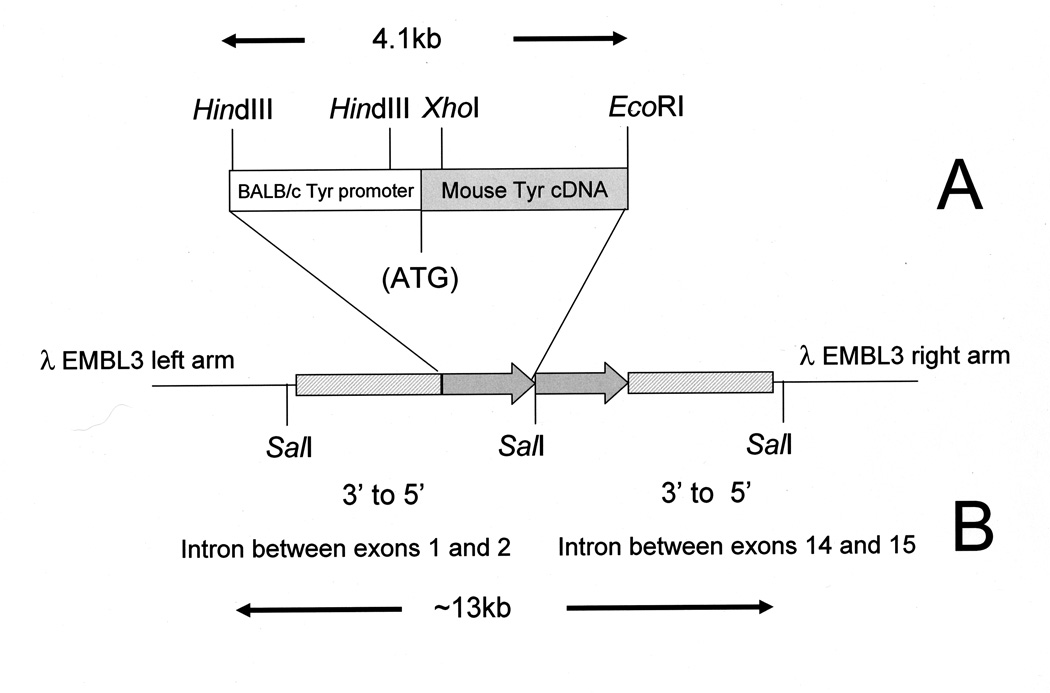
Fig. 1.
Schematic representation of the TYBS transgene and genomic clone. The 4.1 kb TYBS minigene (Panel A) is present as two copies (shaded arrows) in the ~13 kb λ EMBL3 genomic clone (Panel B). Genomic DNA flanking the transgene complex corresponds to intronic sequences between exons 14 and 15 and exons 1 and 2 of the Ap2β1 gene.
Genotyping of transgenic mice
The identification of transgenic mice was initially made by the presence of coat color and pigmented eyes. The BALB/c promoter driving the tyrosinase cDNA in the tyrosinase minigene provides rescue of the albino phenotype of FVB/NJ mice (Yokoyama, et al., 1990; Overbeek, et al., 1991). The presence of the transgene in genomic DNA was also confirmed by standard PCR using GeneAmp® PCR reagents (Applied Biosystems, Foster City, CA)and TYBS specific primers (TYBS 636F ctgaaatatggagggacattgatt and TYBS1034R tcaaactcagacaaaattccacatt) to amplify a 400-bp portion of the TYBS transgene. These primers do not amplify the endogenous Tyr gene located on chromosome 7. Tg/+ and Tg/Tg embryos were distinguished using TaqMan® Gene Expression Assays. Total cellular RNA was prepared from whole embryos using the RNAqueous - 4PCR kit (Ambion, Austin, TX) and reverse transcribed to cDNA, High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) followed by TaqMan® Gene Expression Assays using TaqMan® Universal PCR Master mix (Applied Biosystems), Ap2b1 (Mm00551136_m1), and Pgk (Mm00435617_m1) as an endogenous control.
Genomic library construction and screening
Genomic DNA was prepared from a pool of 3 homozygous OVE427 CP embryos using the Pure™ Tissue DNA Isolation Systems (DNA Technologies, Inc., Gaithersburg, MD). Following partial digestion with Sau3AI the DNA was size fractionated using sucrose gradients into two size ranges (9.0–13.0 kb and 13.0–23.0 kb) and then ligated to BamHI digested EMBL3 vectors, packaged using Gigapack III packaging extract, and plated on the XL1-Blue MRA (P2) strain (Stratagene , La Jolla, CA). Libraries contained ~5 to 8×106 clones and unamplified titers of ~1×1010 pfu/ml. Aliquots of the unamplified 9.0–13.0 kb and 13.0–23.0 kb libraries were screened using PCR (primers described above). The PCR positive OVE427 library containing 13.0 to 23.0 kb, inserts was plated and the plaque lysates screened by PCR. PCR positive plates were then subjected to a minimum of three rounds of plaque purification using non-isotopic DNA-DNA hybridization with a DIG-labeled TYBS minigene fragment as a probe (DIG-High Prime DNA labeling) and chemiluminescent detection (Roche Diagnostics Corporation, Indianapolis, IN). Two clones were chosen for subsequent amplification and subcloning in pBluescript (Stratagene).
Northern analyses
Total cellular RNA was prepared from OVE427 Tg/Tg, Tg/+, and wildtype (+/+) whole 18d.p.c. embryos or adult OVE427 +/+ tissues using Tri-Reagent (Molecular Research Center, Cincinnati, OH). Twenty to 35 micrograms of RNA was separated using a denaturing formaldehyde-1.5% agarose gel and transferred to a MagnaGraph nylon membrane (M.S.I., Westboro, MA). Filters were pre-hybridized/hybridized using DIG Easy Hyb (Roche Diagnostics Corporation, Indianapolis, IN) and a DIG-labeled cocktail of Ap2β1 C-terminal and N-terminal probes subcloned from a full length Ap2β1 cDNA probe (Open Biosystems, Huntsville, AL), washed at high stringency (0.5X SSC / 0.1% w/v SDS at 62°C) then used for chemiluminescent detection on Fuji Super RX medical x-ray film (Fujifilm Medical Systems, Stamford, CT). The membranes were subsequently stripped and reprobed with a DIG-labeled mouse β-actin probe.
Mouse embryonic fibroblasts (MEFs)
Skin from the dorsum of E17-E18 embryos generated by crossing wildtype (+/+) with OVE427 heterozygotes (Tg/+) and by crossing Tg/+ by Tg/+ was finely minced and placed in DMEM (Gibco, Grand Island, NY) containing 4.5g/L D-glucose, L-glutamine, sodium pyruvate, 10% FCS (Sigma-Aldrich, St. Louis, MO), and Pen/Strep (Sigma-Aldrich). Cells were cultured at 37°C / 5% CO2 and passaged by lifting with 0.5g/L trypsin / 0.2g/L EDTA (Sigma-Aldrich) when cells reached 80% confluence.
Antibodies
Mouse anti-β2 adaptin, mouse anti-α (A isoform)(Robinson, 1989), and mouse anti-γ (γ1 isoform) monoclonal antibodies (BD Transduction Laboratories, San Jose, CA) were used for immunoblotting and immunoprecipitations in conjunction with HRP-conjugated anti-mouse or anti-rabbit IgG (GE Healthcare/Amersham Bioscience, Piscataway, NJ). For immunofluorescence mouse anti- α (Affinity BioReagents, Golden, CO); rabbit anti-epsin, a gift from Dr. Linton Traub (University of Pittsburgh School of Medicine, Pittsburgh, PA); rabbit and mouse antibodies anti- β1+ β2 were provided by Dr. James Keen (Kimmel Cancer Institute, Philadelphia, PA) and Dr. Tomas Kirchhausen (Harvard Medical School, Boston, MA), respectively were used in conjunction with goat anti-mouse or goat anti-rabbit IgG conjugated to Alexa Fluor 488 or 555 (Molecular Probes, Eugene, OR).
Immunofluorescence microscopy
Wild-type (+/+) and homozygous mutant (Tg/Tg) mouse embryonic fibroblasts (MEFs) were grown on coverslips and fixed in methanol/acetone (1:1, v/v) for 10 min at −20 C. Incubation with primary antibodies diluted in PBS, 0.1% w/v saponin and 0.1% w/v BSA, was carried out for 1h at room temperature. Unbound antibodies were removed by rinsing with PBS for 5 min, and cells were subsequently incubated with secondary antibody (Alexa555 or Alexa488-conjugated goat anti-rabbit or anti-mouse Ig) diluted in PBS, 0.1% w/v saponin and 0.1% BSA, for 30–60 min at room temperature. After a final rinse with PBS, coverslips were mounted onto glass slides with Fluoromount G (Southern Biotechnology Associates, Birmingham, AL). Fluorescence images were acquired on an LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY).
Immunoblotting and immunoprecipitation
Fibroblasts were washed with ice-cold PBS, extracted in ice-cold lysis buffer (25 mM Tris–HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 0.5% v/v Triton X-100) supplemented with a protease inhibitor cocktail, Sigma P-8340 (Sigma-Aldrich Co., St. Louis, MO), centrifuged at 16,000xg for 10 min, and supernatants were collected. For immunoprecipitation, lysates were incubated with 5µl of anti-α (Affinity BioReagents) and protein G-Sepharose (Amersham Bioscience, Piscataway, NJ) at 4°C overnight. Immunoprecipitates were then collected, washed 4 times with PBS, and eluted by incubation with Laemmli sample buffer (2% w/v SDS, 10% v/v glycerol, 5% v/v 2-mercaptoethanol, 0.002% v/v bromophenol blue and 0.0625M Tris HCl, pH 6.8) for 10 min at room temperature. Samples were analyzed by SDS–PAGE (4–20% gradient gels) under reducing conditions and transferred onto nitrocellulose. The membranes were then blocked with 1X PBS, 0.05% v/v Tween-20, 10% w/v non-fat milk and incubated with the appropriate antibodies. Enhanced chemiluminescence reagent (Amersham Biosciences) was used for protein detection.
RESULTS
Cleft palate mutant strain developed by insertional mutagenesis
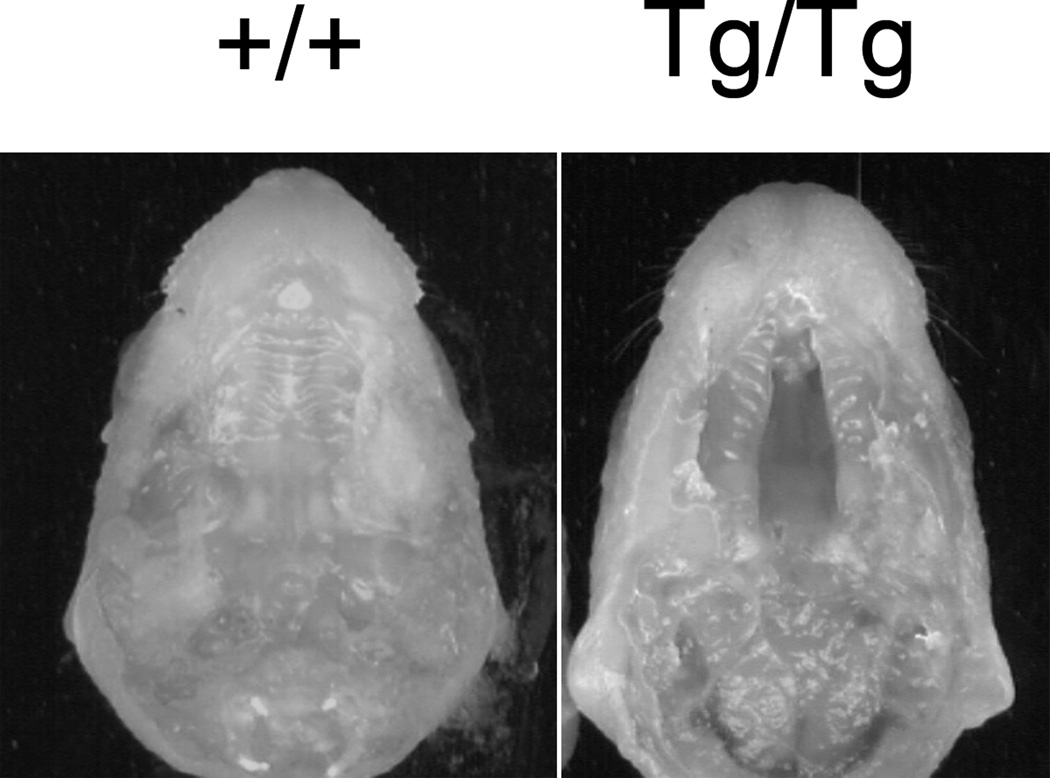
The transgenic line OVE427 was found to have perinatal lethality when bred to homozygosity. Complete clefting of the secondary palate was observed in 25% of late gestation embryos produced from intercrossing heterozygous OVE427 Tg/+ mice (Fig. 2). No craniofacial dysmorphology, or anomalies involving the limbs or developing skeleton were noted. Coronal cross-sections of the secondary palate of Tg/Tg embryos at E17 and E18 showed evidence of palatal shelf elevation and the apparent failure of the shelves to fuse. Additional histological examination through serial cross-sections of entire embryos did not reveal differences in major internal organs (heart, brain, lungs, kidneys, and gastrointestinal tract) between wildtype and homozygous littermates other than CP (data not shown).
Fig. 2.
OVE427 wildtype and homozygous embryos. OVE427 Tg/+ male and female mice were mated and 18 d.p.c. embryos collected following cesarean section. CP is present in the OVE427 Tg/Tg embryo.
Identification of the Ap2β1 gene as the CP locus
Following SalI digestion of a single OVE427 lambda clone, four DNA fragments of approximately 20kb, 9kb, 8kb and 5kb were identified. Southern transfer and hybridization using the TYBS minigene fragment revealed hybridization signals over the 5kb and 8kb bands and not the 9kb and 20kb bands (data not shown). The 9kb and 20kb bands corresponded to the expected sizes of the lambda EMBL3 vector arms. DNA sequencing inward from the EMBL3 vector arms in the undigested lambda clone and of the 5kb and 8kb SalI bands subcloned into pBluescript was performed. The SalI fragments were subjected to secondary digestion using XbaI and those resultant fragments were subcloned and subjected to DNA sequencing. Sequences were analyzed by BLAST® (Basic Local Alignment Search Tool) (http://blast.ncbi.nlm.nih.gov/Blast.cgi) using the megaBLAST program (compares highly related nucleotide sequences) against all mouse genome assemblies (http://www.ncbi.nlm.nih.gov/genome/seq/BlastGen/databases.shtml). From the left arm a 2.3kb genomic DNA fragment at 99% identity mapped to chromosome 11 and corresponded to intronic sequence between exons 1 and 2 of the Ap2β1 (adaptor protein complex 2 β1 subunit) gene (Fig. 1B). From the right arm a 4.2kb genomic DNA fragment at 99% identity mapped to chromosome 11 and corresponded to intronic sequence between exons 14 and 15 of the Ap2β1 gene. These intronic sequences were inverted in orientation in the clone and normally reside ~50kb apart in the Ap2β1 gene. Sequencing within and between the TYBS transgene in this clone confirmed its presence and indicated that 2 copies of the TYBS transgene integrated in the genome.
Absence of Ap2β1 transcript in homozygous mutant mice
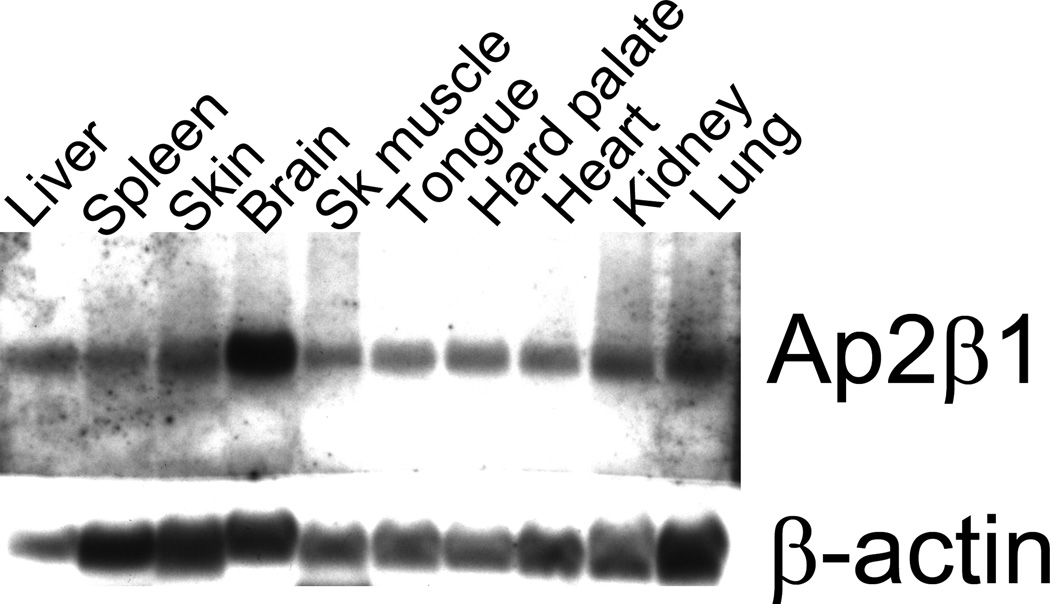
We next investigated Ap2β1 gene expression by Northern blot. A ~5.8kb Ap2β1 transcript was detected in wildtype (+/+) and heterozygous (Tg/+) embryos but not in RNA prepared from homozygous mutant (Tg/Tg) embryos (Fig. 3). The Ap2β1 transcript was reduced in intensity in the heterozygote when compared to wildtype. A multi-tissue Northern blot showed Ap2β1 to be widely expressed in wildtype mice, including palatal tissue and with greatest expression in the brain (Fig. 4).
Fig. 3.
Northern blot analysis of mouse Ap2βb1 and β-actin transcripts in total RNA from OVE427 Tg/Tg, Tg/+, and +/+ embryos. Twenty five micrograms of total RNA was blotted in each lane. The 5.8kb Ap2β1 transcript is present in heterozygous (Tg/+) and wildtype (+/+) lanes. After hybridization with a cocktail of Ap2β1 C-terminal and N-terminal probes the filter was stripped and hybridized with a murine β-actin probe.
Fig. 4.
Multiple tissue Northern blot analysis of mouse Ap2β1 and β-actin transcripts in total RNA. Thirty-five micrograms of total RNA was blotted in each lane. The blot was hybridized with a cocktail of Ap2β1 C-terminal and N-terminal probes, stripped, and re-hybridized with a 600-bp murine β-actin probe. Tissues surveyed were the liver, spleen, skin, brain, skeletal muscle, tongue, hard palate, heart, kidney and lung.
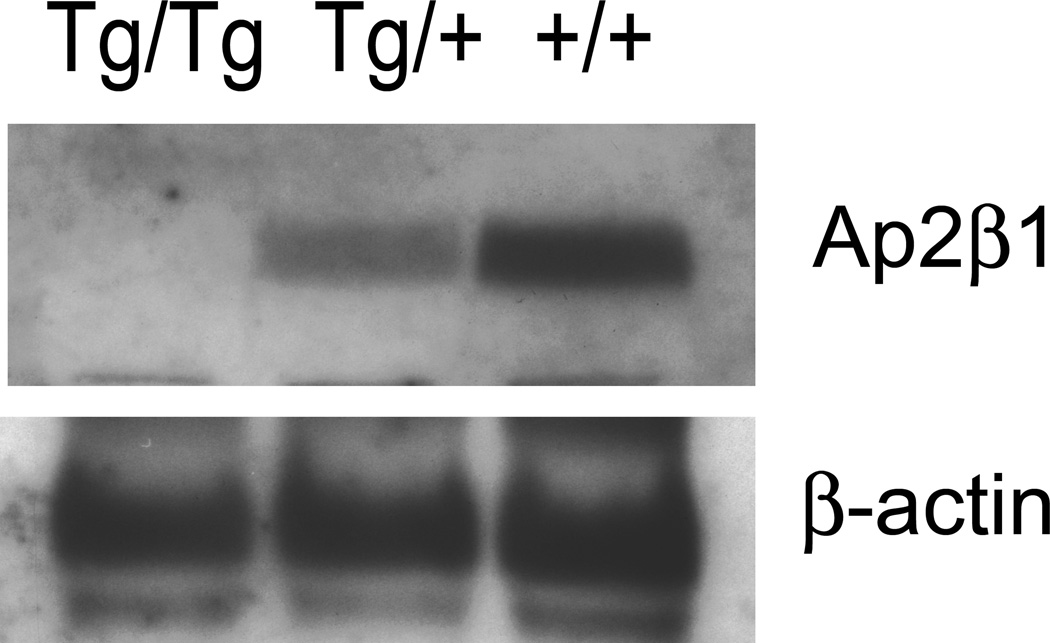
Absence of β2-adaptin protein in cells and tissues from homozygous mutant mice
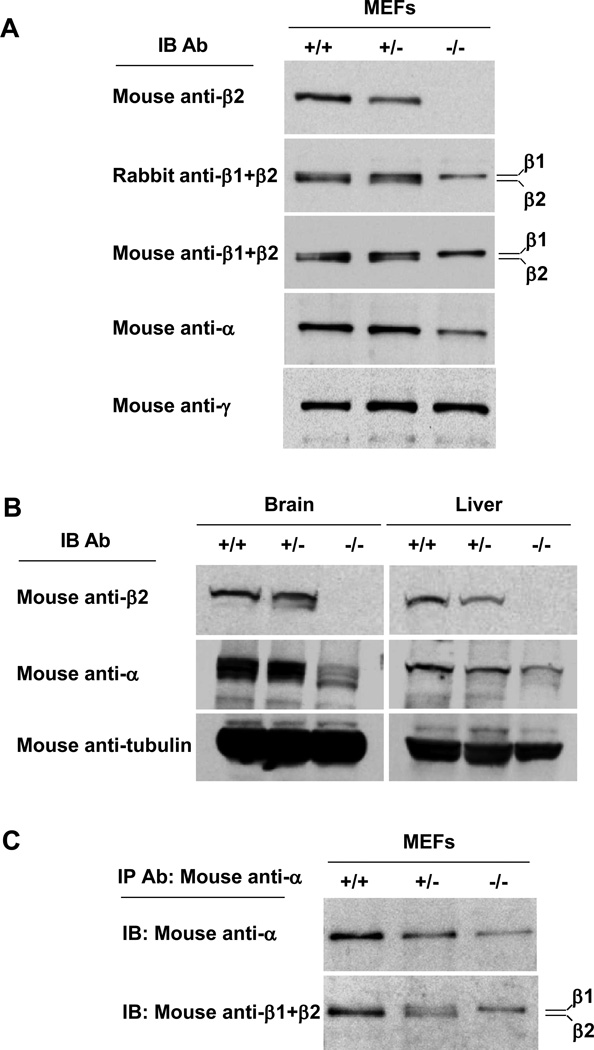
The Ap2β1 gene encodes a protein named β2-adaptin that, together with α-adaptin, µ2 and σ2, assemble into the heterotetrameric adaptor protein-2 (AP-2) complex involved in clathrin dependent endocytosis (Boehm and Bonifacino, 2002; Kirchhausen, 2002; Robinson, 2004). Consistent with the Northern analysis, immunoblot analysis with an antibody specific to β2-adaptin revealed the absence of this protein in mouse embryonic fibroblasts (MEFs) (Fig. 5A) and tissues (i.e., brain and liver) (Fig. 5B) from homozygous mutant (Tg/Tg) animals. MEFs and tissues from heterozygous (Tg/+) mice exhibited reduced levels (~50%) of β2-adaptin relative to those from wildtype mice. Immunoblot analysis of wildtype MEFs with two different antibodies that recognize both β2-adaptin and the homologous β1-adaptin subunit of the AP-1 complex showed a doublet corresponding to the faster migrating β2 and the more slowly migrating β1 (Fig. 5A). MEFs from heterozygous mice exhibited reduced levels of β2 and unchanged levels of β1, whereas those from homozygous mutant mice showed only β1 expression (Fig. 5A). Levels of α-adaptin were reduced in homozygous mutant MEFs (Fig. 5A) and tissues (Fig. 5B), suggesting that the absence of β2-adaptin partially destabilizes α-adaptin. In contrast, the levels of the γ-adaptin subunit of AP-1 were unchanged (Fig. 5A). These analyses thus showed that homozygous mutant mice did not synthesize any β2-adaptin and that this deficiency coincided with reduced the levels of α-adaptin.
Fig. 5.
Characterization of the AP-2 complex in Ap2β1 mutant mice. Embryonic fibroblasts (A) or brain and liver tissues (B) were collected from wild-type (+/+), heterozygous (Tg/+), and homozygous mutant (Tg/Tg) mice. After lysis, equal protein loadings of homogenates were analyzed by SDS–PAGE and immunoblotting with the indicated antibodies. (C) Lysates from (+/+), (Tg/+), and (Tg/Tg) mouse embryonic fibroblasts were immunoprecipitated with mouse monoclonal anti-α as described in Materials and Methods. Immunoprecipitates were resolved by SDS-PAGE and immunoblotting with antibodies against α (upper panel) or β1+β2 (lower panel).
Partial substitution of β1-adaptin for β2-adaptin in homozygous mutant mice
To examine the assembly status of α-adaptin in MEFs from wildtype, heterozygous and homozygous mutant mice, we performed immunoprecipitation of α-adaptin from cell lysates followed by immunoblotting with an antibody that recognizes both β1 and β2 (Fig. 5C). We found that in wildtype MEFs α co-precipitated almost exclusively with β2, as expected for two subunits of the AP-2 complex (Fig. 5C). Strikingly, α co-precipitated with equal amounts of β2 and β1 in heterozygous cells, and only with β1 in homozygous mutant cells (Fig. 5C). This indicates that β1 can substitute for β2 in the AP-2 complex. The amount of β1 co-precipitated with α in homozygous mutant cells was higher than that in wildtype cells (in which there is β2) (Fig. 5C). This means that under normal conditions α prefers to assemble with β2 over β1, but accepts β1 when there is no β2.
Normal distribution of the variant AP-2 complex containing β1-adaptin
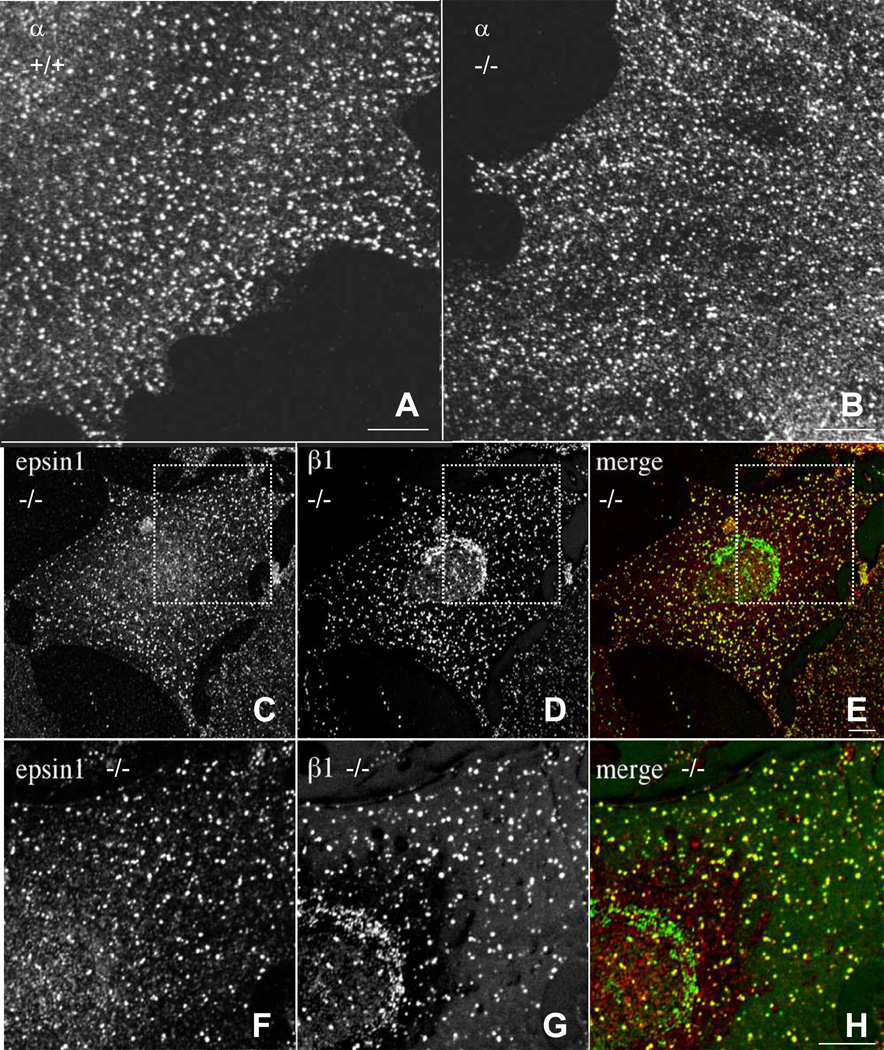
We next examined by immunofluorescence microscopy the distribution of the variant AP-2 complex containing β1-adaptin in wildtype (+/+) and homozygous mutant (Tg/Tg) MEFs. We observed that α-adaptin localized to punctate foci at the plasma membrane corresponding to clathrin-coated pits in both wildtype and homozygous mutant MEFs (Fig. 6, A and B). Interestingly, whereas in wildtype cells β1-adaptin localizes to the trans-Golgi network (TGN) (Robinson, 2004 and data not shown), in homozygous mutant MEFs β1-adaptin was found on both the TGN and plasma membrane puncta (Fig. 6, D and G). These puncta, but not the TGN structure, contained another plasma membrane clathrin-associated adaptor, epsin 1 (Fig. 6, C and F), identifying them as clathrin-coated pits and vesicles. From these experiments we concluded that incorporation into the AP-2 complex draws β1 to plasma membrane clathrin-coated pits and that the substitution of β1 for β2 in the AP-2 complex has no detectable effect on the localization of this complex.
Fig. 6.
β1 subunit is partially incorporated into AP-2 complexes in Ap2β1 mutant mice. (A,B) Wild-type (+/+) and homozygous (Tg/Tg) mouse embryonic fibroblasts were grown on coverslips, fixed in methanol:acetone (1:1), and stained with mouse monoclonal antibodies against α followed by Alexa488-conjugated goat anti mouse IgG. Cells were examined by confocal fluorescence microscopy. Scale bar, 10 µm. (C-E) (Tg/Tg) fibroblasts were fixed and double-stained with antibodies against epsin (red) (C) and β1 (green) (D). Bound antibodies were revealed by Alexa-488 conjugated antibody to mouse IgG and Alexa-555-conjugated antibody to rabbit IgG. All images were obtained by confocal microscopy. Merging images in the red and green channels generated the third panel (E and H) on each row; yellow indicates overlapping localization. Panels F, G, and H are two-fold magnification of the regions shown in panels C, D, and E. Scale bar = 10 µm.
DISCUSSION
We have identified a new mouse transgenic insertional mutant that results in autosomal recessive non-syndromic cleft palate. The transgene integration site is located within the Ap2β1 gene on chromosome 11 disrupting the genomic organization. Homozygous (Tg/Tg) embryos lack expression of the Ap2β1 gene as well as the β2-adaptin protein. Heterozygous (Tg/+) embryos express roughly half the level of wildtype Ap2β1 transcripts and are completely normal. The Ap2β1 gene is widely expressed in adult mice including the hard palate and is most abundant in brain.
The mechanism by which the loss of β2-adaptin function leads to CP is not entirely clear. β2-adaptin is a subunit of the heterotetrameric AP-2 complex involved in clathrin-dependent endocytosis of receptors from the plasma membrane (Kirchhausen, 2002; Robinson, 2004). It is thus likely that CP in β2-adaptin-mutant mice results from defective endocytosis of one or more receptors or other cell surface proteins involved in palatogenesis. TGF-β superfamily members regulate a wide range of biological processes by binding to two transmembrane serine/threonine kinase receptors (Feng and Derynck, 2005). The TGF-beta/Smad signaling pathway is known to play a critical role during the process of epithelial-mesenchymal transformation of medial edge epithelial cells (MEE) in palatogenesis (Cui and Shuler, 2000; Tudela, et al., 2002; Cui, et al., 2003; Cui, et al., 2005; Nakajima, et al., 2007). Disruption of TGF-beta signaling can lead to cleft palate (Proetzel, et al., 1995; Sanford, et al., 1997). Ligand binding leads to both signal transduction and receptor downregulation. TGF-β receptor downregulation has been shown to occur through concentration within clathrin-coated pits and internalization into clathrin-coated vesicles for eventual delivery to endosomes (Anders, et al., 1997; Anders, et al., 1998; Ehrlich, et al., 2001; Yao, et al., 2002). Clathrin-mediated endocytosis of TGF-β receptors depends on the interaction of their cytosolic tails with the β2-adaptin subunit of AP-2 (Yao, et al., 2002). This interaction is in contrast to that of other endocytic receptors, which bind to the µ2 subunit (Ohno, et al., 1995; Boll, et al., 1996; Ohno, et al., 1996) or a combination of the α and σ2 subunits of AP-2 (Chaudhuri, et al., 2007; Doray, et al., 2007; Mitchell, et al., 2008). It is likely that the absence of β2-adaptin impairs internalization of TGF-β receptors from the cell surface, resulting in either sustained signaling from the plasma membrane or decreased signaling from the endosomes (Hayes, et al., 2002; Itoh, et al., 2002; Di Guglielmo, et al., 2003; Runyan, et al., 2005). Therefore, the CP phenotype of the β2-adaptin-deficient mice might result in part from perturbation of TGF-β signaling.
Perturbation of GABAergic signaling also leads to cleft palate (Homanics, et al., 1997; Hagiwara, et al., 2003). Interestingly, GABA(A) receptors cycle between the synaptic membrane and intracellular sites, and their AP-2-dependent recruitment into clathrin-coated pits represents an important mechanism in the postsynaptic modulation of inhibitory synaptic transmission (Kittler, et al., 2000; Herring, et al., 2003; Kittler, et al., 2005). Therefore, defective GABA(A) receptor internalization and signaling due to the presence of the variant, β1-adaptin-containing AP-2 complex could also contribute to the cleft palate phenotype of the β2-adaptin-deficient mice.
In addition to the substitution of β1-adaptin for β2-adaptin, the lower levels of the variant AP-2 complex (~50%, as inferred from the levels of α-adaptin) in the β2-deficient mice could also be a contributing factor leading to cleft palate. Such substitution of β1-adaptin for β2-adaptin has been observed (Keyel, et al., 2008). Interestingly, heterozygous µ2-deficient mice also have reduced levels of the β2-adaptin AP-2 complex (~70% of α-adaptin levels seen in wildtype) but display no phenotypic abnormalities (Mitsunari, et al., 2005).
Homozygous β2-adaptin-deficient embryos survive development and die perinatally due to CP. This phenotype contrasts sharply with that of homozygous µ2-deficient embryos, which die before day 3.5 p.c. (Mitsunari, et al., 2005). As shown here the reason for the different outcomes likely lies in the unique ability of β1-adaptin to substitute for β2-adaptin in the AP-2 complex. Indeed, mouse β1- and β2-adaptins are 84% identical in amino acid sequence, a level that to a large extent allows them to behave as interchangeable subunit isoforms (Ahle, et al., 1988; Kirchhausen, et al., 1989; Ponnambalam, et al., 1990; Guilbaud, et al., 1997; Boehm and Bonifacino, 2001). This notion is supported by previous work showing that both β1- and β2-adaptins promiscuously interact with other subunits from AP-1 and AP-2 in co-precipitation and yeast two-hybrid analyses (Page and Robinson, 1995). An extreme case is D. melanogaster, in which a single β1/2-adaptin is a component of both AP-1 and AP-2 (Camidge and Pearse, 1994). The situation is different for the other three subunits of AP-1 and AP-2, which exhibit 29–45% amino acid sequence identity and are not interchangeable (Page and Robinson, 1995). Thus, the inability of µ1 to be incorporated into the AP-2 complex likely accounts for the early embryonic lethality of µ2-deficient (Mitsunari, et al., 2005). The converse is also the case, as µ2 does not assemble into the AP-1 complex in µ1A-deficient mice, resulting in mid-gestation embryonic lethality (Meyer, et al., 2000). Thus, the survival of β2-adaptin-deficient embryos until birth likely results from the rescue of most AP-2 functions by substitution with β1-adaptin.
Acknowledgements
We thank Ms. Deidra Faust for her technical assistance with the timed matings and embryo collections. This work was supported by Public Health Service grant DE-015180 from the NIDCR (ETE) and by the Intramural Program of NICHD, NIH (JSB).
Footnotes
The authors state that they have no conflicts of interest.
Contributor Information
Wei Li, Postdoctoral Fellow Department of Oral Facial Development Indiana University School of Dentistry.
Rosa Puertollano, Postdoctoral Fellow Cell Biology and Metabolism Program Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Institutes of Health (NIH).
Juan S. Bonifacino, Senior Investigator Cell Biology and Metabolism Program Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Institutes of Health (NIH)
Paul A. Overbeek, Professor Department of Molecular and Cellular Biology Baylor College of Medicine
Eric T. Everett, Associate Professor Department of Oral Facial Development Indiana University School of Dentistry.
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. Embo J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Arline SL, Dore JJ, Leof EB. Distinct endocytic responses of heteromeric and homomeric transforming growth factor beta receptors. Mol Biol Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Dore JJ, Jr, Arline SL, Garamszegi N, Leof EB. Differential requirement for type I and type II transforming growth factor beta receptor kinase activity in ligand-mediated receptor endocytosis. J Biol Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, Behringer RR, Overbeek PA. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Genetic analyses of adaptin function from yeast to mammals. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. Embo J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Camidge DR, Pearse BM. Cloning of Drosophila beta-adaptin and its localization on expression in mammalian cells. J Cell Sci. 1994;107(Pt 3):709–718. [PubMed] [Google Scholar]
- Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol. 2007;81:3877–3890. doi: 10.1128/JVI.02725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong SS, Cheah FSH, Jabs EW. Genes implicated in lip and palate development. In: Wyszynski DF, editor. Cleft Lip and Palate From Origin To Treatment. New York: Oxford University Press; 2002. pp. 25–39. [Google Scholar]
- Cui XM, Shuler CF. The TGF-beta type III receptor is localized to the medial edge epithelium during palatal fusion. Int J Dev Biol. 2000;44:397–402. [PubMed] [Google Scholar]
- Cui XM, Chai Y, Chen J, Yamamoto T, Ito Y, Bringas P, Shuler CF. TGF-beta3-dependent SMAD2 phosphorylation and inhibition of MEE proliferation during palatal fusion. Dev Dyn. 2003;227:387–394. doi: 10.1002/dvdy.10326. [DOI] [PubMed] [Google Scholar]
- Cui XM, Shiomi N, Chen J, Saito T, Yamamoto T, Ito Y, Bringas P, Chai Y, Shuler CF. Overexpression of Smad2 in Tgf-beta3-null mutant mice rescues cleft palate. Dev Biol. 2005;278:193–202. doi: 10.1016/j.ydbio.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Cunningham D, Xiao Q, Chatterjee A, Sulik K, Juriloff D, Elder F, Harrison W, Schuster G, Overbeek PA, Herman GE. exma: an X-linked insertional mutation that disrupts forebrain and eye development. Mamm Genome. 2002;13:179–185. doi: 10.1007/s00335-001-2121-z. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18:1887–1896. doi: 10.1091/mbc.E07-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Shmuely A, Henis YI. A single internalization signal from the di-leucine family is critical for constitutive endocytosis of the type II TGF-beta receptor. J Cell Sci. 2001;114:1777–1786. doi: 10.1242/jcs.114.9.1777. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Ferguson MW. Palate development. Development. 1988;103(Suppl):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Guilbaud C, Peyrard M, Fransson I, Clifton SW, Roe BA, Carter NP, Dumanski JP. Characterization of the mouse beta-prime adaptin gene; cDNA sequence, genomic structure, and chromosomal localization. Mamm Genome. 1997;8:651–656. doi: 10.1007/s003359900531. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Katarova Z, Siracusa LD, Brilliant MH. Nonneuronal expression of the GABA(A) beta3 subunit gene is required for normal palate development in mice. Dev Biol. 2003;254:93–101. doi: 10.1016/s0012-1606(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring D, Huang R, Singh M, Robinson LC, Dillon GH, Leidenheimer NJ. Constitutive GABAA receptor endocytosis is dynamin-mediated and dependent on a dileucine AP2 adaptin-binding motif within the beta 2 subunit of the receptor. J Biol Chem. 2003;278:24046–24052. doi: 10.1074/jbc.M301420200. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CE, Korpi ER, Makela R, Brilliant MH, Hagiwara N, Ferguson C, Snyder K, Olsen RW. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci U S A. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Divecha N, Brocks L, Oomen L, Janssen H, Calafat J, Itoh S, Dijke Pt P. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes Cells. 2002;7:321–331. doi: 10.1046/j.1365-2443.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- Keyel PA, Thieman JR, Roth R, Erkan E, Everett ET, Watkins SC, Heuser JE, Traub LM. The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis. Mol Biol Cell. 2008;19:5309–5326. doi: 10.1091/mbc.E08-07-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T, Nathanson KL, Matsui W, Vaisberg A, Chow EP, Burne C, Keen JH, Davis AE. Structural and functional division into two domains of the large (100- to 115-kDa) chains of the clathrin-associated protein complex AP-2. Proc Natl Acad Sci U S A. 1989;86:2612–2616. doi: 10.1073/pnas.86.8.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. Clathrin adaptors really adapt. Cell. 2002;109:413–416. doi: 10.1016/s0092-8674(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Delmas P, Jovanovic JN, Brown DA, Smart TG, Moss SJ. Constitutive endocytosis of GABAA receptors by an association with the adaptin AP2 complex modulates inhibitory synaptic currents in hippocampal neurons. J Neurosci. 2000;20:7972–7977. doi: 10.1523/JNEUROSCI.20-21-07972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci U S A. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidral AC, Moreno LM, Bullard SA. Genetic Factors and Orofacial Clefting. Semin Orthod. 2008;14:103–114. doi: 10.1053/j.sodo.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazita ML, Lidral AC, Murray JC, Field LL, Maher BS, Goldstein McHenry T, Cooper ME, Govil M, Daack-Hirsch S, Riley B, Jugessur A, Felix T, Morene L, Mansilla MA, Vieira AR, Doheny K, Pugh E, Valencia-Ramirez C, Arcos-Burgos M. Genome scan, fine-mapping, and candidate gene analysis of non-syndromic cleft lip with or without cleft palate reveals phenotype-specific differences in linkage and association results. Hum Hered. 2009;68:151–170. doi: 10.1159/000224636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisler MH. Insertional mutation of 'classical' and novel genes in transgenic mice. Trends Genet. 1992;8:341–344. doi: 10.1016/0168-9525(92)90278-c. [DOI] [PubMed] [Google Scholar]
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. Embo J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RS, Chaudhuri R, Lindwasser OW, Tanaka KA, Lau D, Murillo R, Bonifacino JS, Guatelli JC. Competition model for upregulation of the major histocompatibility complex class II-associated invariant chain by human immunodeficiency virus type 1 Nef. J Virol. 2008;82:7758–7767. doi: 10.1128/JVI.02668-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunari T, Nakatsu F, Shioda N, Love PE, Grinberg A, Bonifacino JS, Ohno H. Clathrin adaptor AP-2 is essential for early embryonal development. Mol Cell Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima A, Ito Y, Asano M, Maeno M, Iwata K, Mitsui N, Shimizu N, Cui XM, Shuler CF. Functional role of transforming growth factor-beta type III receptor during palatal fusion. Dev Dyn. 2007;236:791–801. doi: 10.1002/dvdy.21090. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Overbeek PA, Aguilar-Cordova E, Hanten G, Schaffner DL, Patel P, Lebovitz RM, Lieberman MW. Coinjection strategy for visual identification of transgenic mice. Transgenic Res. 1991;1:31–37. doi: 10.1007/BF02512994. [DOI] [PubMed] [Google Scholar]
- Overbeek PA, Gorlov IP, Sutherland RW, Houston JB, Harrison WR, Boettger-Tong HL, Bishop CE, Agoulnik AI. A transgenic insertion causing cryptorchidism in mice. Genesis. 2001;30:26–35. doi: 10.1002/gene.1029. [DOI] [PubMed] [Google Scholar]
- Page LJ, Robinson MS. Targeting signals and subunit interactions in coated vesicle adaptor complexes. J Cell Biol. 1995;131:619–630. doi: 10.1083/jcb.131.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnambalam S, Robinson MS, Jackson AP, Peiperl L, Parham P. Conservation and diversity in families of coated vesicle adaptins. J Biol Chem. 1990;265:4814–4820. [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Cloning of cDNAs encoding two related 100-kD coated vesicle proteins (alpha-adaptins) J Cell Biol. 1989;108:833–842. doi: 10.1083/jcb.108.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Runyan CE, Schnaper HW, Poncelet AC. The role of internalization in transforming growth factor beta1-induced Smad2 association with Smad anchor for receptor activation (SARA) and Smad2-dependent signaling in human mesangial cells. J Biol Chem. 2005;280:8300–8308. doi: 10.1074/jbc.M407939200. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGF beta2 knockout mice have multiple developmental defects that are non-overlapping with other TGF beta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte BC, Murray JC. The many faces and factors of orofacial clefts. Hum Mol Genet. 1999;8:1853–1859. doi: 10.1093/hmg/8.10.1853. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Siciliano MJ, Stallings RL, Overbeek PA. Insertional inactivation of the downless gene in a family of transgenic mice. Mol Biol Med. 1989;6:299–307. [PubMed] [Google Scholar]
- Shi M, Mostowska A, Jugessur A, Johnson MK, Mansilla MA, Christensen K, Lie RT, Wilcox AJ, Murray JC. Identification of microdeletions in candidate genes for cleft lip and/or palate. Birth Defects Res A Clin Mol Teratol. 2009;85:42–51. doi: 10.1002/bdra.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM. Clathrin-associated adaptor proteins - putting it all together. Trends Cell Biol. 1997;7:43–46. doi: 10.1016/S0962-8924(96)20042-X. [DOI] [PubMed] [Google Scholar]
- Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudela C, Formoso MA, Martinez T, Perez R, Aparicio M, Maestro C, Del Rio A, Martinez E, Ferguson M, Martinez-Alvarez C. TGF-beta3 is required for the adhesion and intercalation of medial edge epithelial cells during palate fusion. Int J Dev Biol. 2002;46:333–336. [PubMed] [Google Scholar]
- Yao D, Ehrlich M, Henis YI, Leof EB. Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol Biol Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Silversides DW, Waymire KG, Kwon BS, Takeuchi T, Overbeek PA. Conserved cysteine to serine mutation in tyrosinase is responsible for the classical albino mutation in laboratory mice. Nucleic Acids Res. 1990;18:7293–7298. doi: 10.1093/nar/18.24.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]