Abstract
The paper described a novel technique for semen collection in large psittacines (patent pending), a procedure which was not routinely possible before. For the first time, a large set of semen samples is now available for analysis as well as for artificial insemination. Semen samples of more than 100 psittacine taxa were collected and analysed; data demonstrate large differences in the spermatological parameters between families, indicating an ecological relationship with breeding behaviour (polygamous versus monogamous birds). Using semen samples for artificial insemination resulted in the production of offspring in various families, such as Macaws and Cockatoos, for the first time ever. The present technique represents a breakthrough in species conservation programs and will enable future research into the ecology and environmental factors influencing endangered species.
Many psittacine species are threatened by the destruction of their natural habitat or poaching. Therefore, captive breeding programs are important to preserve their genetic material and to allow the later reintroduction of birds back to the wild. In some highly endangered species [e.g. Spix's Macaw (Cyanopsitta spixii)1; St. Vincent Amazon (Amazona guildingii)2], only a few individuals have survived in captivity. For captive breeding, birds are often forced-paired, resulting frequently in infertile eggs, which is similar to the case of male infertility. Artificial insemination would overcome such problems, which would also allow the inclusion of surplus males or females in the breeding population3. Artificial insemination can also be used to increase the representation of genetically valuable specimens within a population. Semen collection and the subsequent evaluation of sperm can also provide valuable information about male fertility.
In birds of prey4, turkeys5, cranes6 or pigeons7, semen collection and subsequent artificial insemination is frequently used. In contrast, semen collection in larger psittacine species has only been reported anecdotally8,9,10, and has never been successful in a larger set of different species or individuals. Consequently, successful artificial insemination has only been reported for a few small psittacine species or single cases, but never for larger endangered parrots9,11,12,13. In this report we describe a novel technique of semen collection which was successful in 109 different psittacine species. The collected semen was employed for quality evaluation and artificial insemination which was successful in several species. The results of this study represent a breakthrough in species conservation projects of large psittacines and allow further research on spermatological parameters and their link to ecological niches of the species.
Results
Semen collection
Applying the novel method in 243 different males (see Table 1), 230 out of 344 attempts (66.9%) at semen collection were successful. In 72 cases, the males were classified as Group 1 (see Methods), of which 67 were successful ( = 93.1%). Of 272 attempts from Group 2 (see Methods), 163 attempts ( = 59.9%) were successful.
Table 1. List of parrots in which semen collection was attempted. Group 1 represents paired and sexually active males in which successful semen collection was expected, as they have previously produced fertile clutches. Group 2 represents single housed or sexually inactive males or birds which had previously produced infertile clutches, so successful semen collection was not expected.
| Species | Attempts of semen collection | Successful semen collection | ||
|---|---|---|---|---|
| Total | Total | Group 1 | Group 2 | |
| Cacatua leadbeateri | 1 | 1 | 0 | 1 |
| Cacatua haematuropygia | 11 | 11 | 1 | 10 |
| Cacatua sulphurea sulphurea | 1 | 1 | 0 | 1 |
| Cacatua sulphurea citrinocristata | 1 | 1 | 0 | 1 |
| Cacatua sulphurea abbotti | 1 | 1 | 0 | 1 |
| Cacatua alba | 1 | 0 | 0 | 1 |
| Cacatua galerita galerita | 4 | 4 | 0 | 4 |
| Cacatua pastinator | 1 | 1 | 0 | 1 |
| Cacatua tenuirostris | 2 | 1 | 0 | 2 |
| Cacatua ducorpsii | 1 | 1 | 0 | 1 |
| Cacatua sanguinea sanguinea | 1 | 1 | 0 | 1 |
| Cacatua goffini | 2 | 1 | 0 | 2 |
| Cacatua ducorpsii | 1 | 1 | 1 | 0 |
| Cacatua opthalmica | 1 | 1 | 0 | 1 |
| Cacatua moluccensis | 1 | 1 | 1 | 0 |
| Calyptorhynchus magnificus banksii | 2 | 2 | 0 | 2 |
| Calyptorhynchus funereus funereus | 1 | 1 | 0 | 1 |
| Calyptorhynchus funereus latirostris | 5 | 5 | 4 | 1 |
| Callocephalon fimbriatum | 4 | 4 | 0 | 4 |
| Eolophus roseicapilla | 1 | 1 | 0 | 1 |
| Nymphicus hollandicus | 1 | 1 | 1 | 0 |
| Probosciger aterimus aterimus | 2 | 2 | 0 | 2 |
| Probosciger aterimus goliath | 1 | 1 | 0 | 1 |
| Cockatoos total: | 47 | 44 | Success rate: 93.6% | |
| Anodorhynchus hyacinthinus | 3 | 3 | 3 | 0 |
| Anodorhynchus leari | 2 | 1 | 1 | 1 |
| Ara ambiguus | 3 | 2 | 1 | 2 |
| Ara chloropterus | 7 | 5 | 4 | 3 |
| Ara glaucogularis | 9 | 4 | 3 | 6 |
| Ara militaris militaris | 1 | 1 | 0 | 1 |
| Ara nobilis cumanensis | 2 | 1 | 0 | 2 |
| Ara severus | 1 | 0 | 0 | 1 |
| Dipsittaca nobilis nobilis | 1 | 0 | 0 | 1 |
| Primolius auricollis | 1 | 0 | 0 | 1 |
| Ara ararauna | 2 | 0 | 0 | 2 |
| Ara macao | 1 | 0 | 0 | 1 |
| Ara rubrogenys | 1 | 0 | 0 | 1 |
| Cyanopsitta spixii | 4 | 3 | 0 | 4 |
| Primolius couloni | 6 | 4 | 2 | 4 |
| Macaws total: | 44 | 24 | Success rate: 54.5% | |
| Amazona guildingii | 3 | 3 | 1 | 2 |
| Amazona pretrei | 32 | 24 | 2 | 30 |
| Amazona vinacea | 3 | 2 | 1 | 2 |
| Amazona xantholora | 6 | 4 | 0 | 6 |
| Amazona rhodocorytha | 2 | 1 | 0 | 2 |
| Amazona brasiliensis | 1 | 1 | 1 | 0 |
| Amazona albifrons nana | 1 | 1 | 0 | 1 |
| Amazona tucumana | 4 | 4 | 1 | 3 |
| Amazona amazonica | 1 | 1 | 0 | 1 |
| Amazona finschii | 2 | 2 | 0 | 2 |
| Amazona viridigenalis | 3 | 3 | 2 | 1 |
| Amazona barbadensis | 1 | 1 | 0 | 1 |
| Amazona ochrocephala oratrix | 1 | 0 | 0 | 1 |
| Amazona ochrocephala xatholaema | 1 | 0 | 0 | 1 |
| Amazona auropaliata | 1 | 0 | 0 | 1 |
| Amazona mercenaria | 1 | 0 | 0 | 1 |
| Amazona leucocephala | 1 | 0 | 0 | 1 |
| Amazona festiva festiva | 1 | 0 | 0 | 1 |
| Amazona dufresniana | 3 | 1 | 0 | 3 |
| Amazona farinosa guatemalae | 1 | 1 | 0 | 1 |
| Amazona farinosa virenticeps | 1 | 1 | 1 | 0 |
| Amazona festiva bodini | 8 | 3 | 3 | 5 |
| Amazona autumnalis lilacina | 7 | 5 | 1 | 6 |
| Amazona autumnalis autumnalis | 9 | 7 | 1 | 8 |
| Amazona ventralis | 1 | 1 | 1 | 0 |
| Alipiopsitta xanthops | 2 | 2 | 0 | 2 |
| Amazons total: | 97 | 68 | Success rate: 68.7% | |
| Eos histrio | 2 | 2 | 1 | 1 |
| Eos squamata squamata | 1 | 0 | 0 | 1 |
| Eos squamata riciniata | 1 | 1 | 0 | 1 |
| Chalcopsitta sintilata | 2 | 1 | 0 | 2 |
| Chalcopsitta atra | 1 | 1 | 0 | 1 |
| Charmosyna papou | 2 | 2 | 0 | 2 |
| Charmosyna pulchella | 1 | 1 | 0 | 1 |
| Chalcopsitta cardinalis | 1 | 0 | 0 | 1 |
| Glossopsitta concinna | 1 | 1 | 1 | 0 |
| Lorius lory | 2 | 1 | 0 | 2 |
| Lorius garrulus flavopalliatus | 1 | 1 | 0 | 1 |
| Lorius chlorocercus | 1 | 1 | 1 | 0 |
| Loriculus philippensis | 1 | 0 | 0 | 1 |
| Oreopsittacus arfaki | 2 | 0 | 0 | 2 |
| Trichoglossus haematodus deplanchii | 1 | 1 | 1 | 0 |
| Trichoglossus haematodus capistratus | 3 | 3 | 0 | 3 |
| Trichoglossus euteles | 1 | 1 | 0 | 1 |
| Trichoglossus haematodus rosenbergii | 2 | 0 | 0 | 2 |
| Pseudeos fuscata | 2 | 1 | 1 | 1 |
| Vini australis | 1 | 1 | 0 | 1 |
| Lorys total: | 29 | 19 | Success rate: 65.5% | |
| Eclectus roratus roratus | 1 | 1 | 0 | 1 |
| Eclectus roratus vosmaeri | 1 | 1 | 0 | 1 |
| Eclectus roratus salomonensis | 6 | 6 | 2 | 4 |
| Eclectus roratus polychloros | 13 | 12 | 13 | 0 |
| Eclectus roratus aruensis | 1 | 1 | 1 | 0 |
| Tanygnathus lucionensis | 4 | 4 | 1 | 3 |
| Tanygnathus sumatranus | 3 | 3 | 1 | 2 |
| Tanygnathus/Eclectus total: | 29 | 28 | Success rate: 96.6% | |
| Agapornis lilianae | 1 | 1 | 0 | 1 |
| Agapornis taranta | 2 | 1 | 0 | 2 |
| Agapornis roseicollis | 1 | 0 | 0 | 1 |
| Alisterus amboinensis amboinensis | 1 | 1 | 0 | 1 |
| Alisterus c. moszkowski | 1 | 1 | 0 | 1 |
| Aratinga pertinax pertinax | 2 | 1 | 1 | 1 |
| Aratinga pertinax surinama | 1 | 1 | 1 | 0 |
| Aratinga solstitialis | 1 | 1 | 0 | 1 |
| Aratinga cactorum | 1 | 1 | 0 | 1 |
| Aratinga wagleri minor | 3 | 1 | 0 | 3 |
| Aratinga rubritorquis | 1 | 0 | 0 | 1 |
| Aratinga erythrogenys | 1 | 0 | 0 | 1 |
| Aratinga holochlora | 1 | 0 | 0 | 1 |
| Aratinga acuticaudata | 2 | 0 | 1 | 1 |
| Aratinga leucopthalmus | 1 | 0 | 0 | 1 |
| Aratinga wagleri | 1 | 0 | 0 | 1 |
| Barnardius zonarius semitorquatus | 1 | 1 | 0 | 1 |
| Brotogeris chrysopterus tuipara | 1 | 1 | 0 | 1 |
| Brotogeris cyonoptera gustavi | 1 | 1 | 1 | 0 |
| Brotogeris cyanoptera | 1 | 0 | 0 | 1 |
| Caracopsis nigra | 1 | 0 | 0 | 1 |
| Cyanoliseus patagonus | 1 | 1 | 0 | 1 |
| Deroptyrus accipitrinus | 2 | 1 | 0 | 2 |
| Forpus conspicitatus | 1 | 0 | 0 | 1 |
| Guaruba guarouba | 2 | 1 | 0 | 2 |
| Nestor notabilis | 2 | 2 | 0 | 2 |
| Psittacula chaltorpae | 3 | 2 | 0 | 3 |
| Psittacula derbiana | 1 | 1 | 0 | 1 |
| Psittacula himalayana | 1 | 1 | 0 | 1 |
| Psittacula krameri krameri | 1 | 1 | 0 | 1 |
| Psittacula eupatria siamensis | 1 | 0 | 0 | 1 |
| Psittacula alexandrii | 2 | 0 | 0 | 2 |
| Psittacus erithacus erithacus | 5 | 0 | 0 | 5 |
| Psittacus timneh | 9 | 4 | 4 | 5 |
| Pionopsitta pileata | 7 | 5 | 0 | 7 |
| Pionus maximiliani | 3 | 0 | 0 | 3 |
| Pionus menstruus | 1 | 0 | 0 | 1 |
| Pionitis leucogaster leucogaster | 2 | 0 | 0 | 2 |
| Platycercus elegans | 1 | 0 | 0 | 1 |
| Platycercus adelaide | 1 | 1 | 0 | 1 |
| Poicephalus robustus fuscicollis | 1 | 1 | 0 | 1 |
| Poicephalus meyeri | 1 | 1 | 1 | 0 |
| Poicephalus senegalus mesotypus | 1 | 1 | 0 | 1 |
| Poicephalus rueppeli | 2 | 1 | 0 | 2 |
| Poicephalus rufiventris | 3 | 0 | 0 | 3 |
| Poicephalus senegalus | 1 | 0 | 0 | 1 |
| Poicephalus guielmi | 1 | 0 | 0 | 1 |
| Psephotus h. haematogaster | 1 | 1 | 0 | 1 |
| Psittrichas fulgidus | 1 | 1 | 0 | 1 |
| Purpureicephalus spurius | 1 | 0 | 1 | 0 |
| Pyrrhura cruentata | 2 | 1 | 0 | 2 |
| Pyrrhura picta roseifrons | 1 | 1 | 0 | 1 |
| Pyrrhura leucotis emma | 1 | 1 | 0 | 1 |
| Pyrrhura hoffmanni gaudens | 2 | 2 | 0 | 2 |
| Pyrrhura melanura souancei | 2 | 2 | 1 | 1 |
| Pyrrhura rupicola rupicola | 1 | 1 | 0 | 1 |
| Pyrrhura perlata perlata | 1 | 1 | 0 | 1 |
| Pyrrhura molinae restricta | 1 | 0 | 1 | 0 |
| Rhynchopsitta pachyrhyncha | 1 | 0 | 0 | 1 |
| Triclaria malachitacea | 1 | 0 | 0 | 1 |
| Others total: | 98 | 47 | Success rate: 48.0% | |
| (total species: 151) | (total n = 288) | (total n = 230) | (total success rate 79.9%) | |
Semen evaluation
A complete semen examination was only possible in 81 samples due to the limited volume or the need for artificial insemination of the samples. The results of semen analysis differed between psittacines families, subfamilies or tribes. Volumes and sperm concentrations of Eclectus/Tanygnathus and Cockatoos were the highest, whereas the Amazons and Macaws resulted in lower values (see Tab. 2). Birds from the same family, subfamily, tribe or species exhibited comparable semen quality.
Table 2. Semen parameters of selected families, subfamilies or tribes.
| Group | volume (μl) | sperms/μl | total sperm number | L-D | motility | Forward motility |
|---|---|---|---|---|---|---|
| Cockatoos (n = 21) | 12.4 | 427704 | 5183354 | 90.3 | 77.9 | 71.5 |
| (1.8–54) | (850–4 500 000) | (10540–69300000) | (71–6.5) | (40–85) | (30–80) | |
| Macaws (n = 6) | 7.4 | 70068 | 435750 | 87.1 | 75.8 | 70 |
| (3–19.5) | (27 500–150 000) | (41300–806664) | (81–2.5) | (70–85) | (60–80) | |
| Amazons (n = 11) | 8.5 | 77532 | 675050 | 87.9 | 80 | 75 |
| (2.8–18.4) | (8750–231 000) | (101050–2263800) | (83.5–91) | (all 80) | (all 75) | |
| Eclectus ssp./Tanygnathus spp. (n = 26) | 11.4 | 3781285 | 35828804 | 94.6 | 87.3 | 82.3 |
| (1–29) | (187 000–16 000 000) | (550000–157200000) | (87–99) | (60–95) | (55–90) | |
| Loris (n = 12) | 6.3 | 1534965 | 9806674 | 95.9 | 86.8 | 81.4 |
| (1.3–12.6) | (29583–5050000) | (82832–41265000) | (92–99.5) | (70–95) | (60–95) | |
| Others (n = 21) | 7.1 | 1317526 | 5318745 | 89 | 80.7 | 75 |
| (1.5–29.8) | (26500–10 450 000) | (53000–33440000) | (55–99) | (55–95) | (50–90) |
L-D: Live-Death rate; Figures in bold are the mean value, figures in brackets are the total range
Artificial insemination
In total, 64 inseminations were performed, which included repeated inseminations of the same female (12 cases). 15 samples were inseminated directly after intracapillary examination without a detailed analysis (see Table 3). After artificial insemination, 25 fertile eggs were obtained out of the 36 eggs laid (69.4%) by eleven females (see Table 4). Altogether, in nine out of 25 species, fertilised eggs were produced by artificial insemination (see Tab. 4).
Table 3. Semen parameters in samples used for artificial insemination: Volumes, sperm-concentrations and total sperm cells used for AI as well as fertility of the obtained clutches. Only clutches with successful or likely successful artificial insemination are listed. Each row represents one female and each semen data one artificial insemination.
| Species | AI before first oviposition | AI after first oviposition | AI after second oviposition | Total eggs/potentially fertilised by AI/fertilised |
|---|---|---|---|---|
| Cacatua haematuropygia | 1.) 5.3 μl (volume) | 2/2/2 | ||
| +++ (concentration) | ||||
| 2.). 5.1 μl | ||||
| +++ | ||||
| Ara chloroptera | 19.2 μl | 18.2 μl | 2/2/1 | |
| 9000/μl | + | Second egg fertilised | ||
| 172800 (total sperms) | ||||
| Amazona pretrei | 8.3 μl | 10.3 μl | 4/3/2 | |
| ++ | + | Second and third egg fertilised | ||
| Amazona pretrei | 2 μl | 5/3/0 | ||
| + | AI 1 day after first egg, second egg not potentially fertilised with AI | |||
| Amazona xantholora | 6 μl | 8 μl | 4/3/3 | |
| +++ | 25000/μl | |||
| 200000 | ||||
| Amazona finschii | 7 μl | 4/3/1 | ||
| 140000/μl | Second egg fertilised | |||
| 980000 | ||||
| Tanygnathus lucionensis | 3.1 μl | 3.8 μl | 3/3/2 | |
| 16000000/μl | 14 840000/μl | Second and third egg fertile | ||
| 49600000 | 56392000 | |||
| Eclectus roratus polychlorus | 1.) 7 μl | 2/2/2 | ||
| 2725000/μl | ||||
| 19075000 | ||||
| 2.) 6.5 μl | ||||
| 1590000/μl | ||||
| 10335000 | ||||
| Eclectus roratus polychlorus | 5 μl | 5 μl | 3/3/2 | |
| 2725000/μl | 1590000/μl | Second and third egg fertile | ||
| 13625000 | 7950000 | |||
| Eclectus roratus polychlorus | 1.) 10 μl | 2/2/2 | ||
| 6000000/μl | ||||
| 60000000 | ||||
| 2.) 5 μl | ||||
| 1590000/μl | ||||
| 7950000 |
Second to fourth column: volumes and concentrations used for AI (1. = first insemination before first egg; 2. = second insemination before first egg. If semen was only examined within the capillary, the concentration is graded like described in the text (sporadic (+), low (++), middle (+++), high (++++) and extremely high (+++++)). Fifth column: 4/3/2 = In a clutch with a total of 4 eggs, 3 eggs were laid after AI (potentially fertilised) and 2 were effectively fertilised.
Table 4. Success after artificial insemination.
| Species | No. eggs after AI | Fertile eggs | Fertility rate (%) | Genetic tests |
|---|---|---|---|---|
| Cacatua haematuropygia | 2 | 2 | 100 | 1 juvenile result of AI |
| Calyptorhynchus funereus latirostris | 2 | 2 | 100 | Semen of own partner was used - no test |
| Ara chloroptera | 2 | 1 | 50 | Two females were partners – no test necessary |
| Amazona pretrei | 6 | 2 | 33.3 | No result: partner male and semen donor brothers |
| Amazona xantholora | 3 | 3 | 100 | No result: Semen donor son of inseminated mother |
| Amazona finschii | 3 | 1 | 33.3 | No result: partner male and semen donor brothers |
| Amazona guildingii | 2 | 0 | 0 | |
| Amazona viridigenalis | 1 | 1 | 100 | Not possible due to very early embryonic death |
| Tanygnathus lucionensis | 3 | 2 | 66.3 | No test, eggs were eaten by parents |
| Eclectus roratus polychlorus | 7 | 6 | 85.7 | Juveniles result of AI |
| Pionopsitta pileata | 5 | 5 | 100 | One egg eaten, partner male is the father of all juveniles |
| total | 36 | 25 | 69.4 |
AI = Artificial insemination.
A successful fertilisation by artificial insemination was confirmed by DNA fingerprinting in all Eclectus and one of the two Cacatua haematuropygia cases investigated. The Pionopsitta pileata juveniles had derived from natural mating of the females with their respective partners. In the other cases, both males (partner male and semen donor) were too closely related to achieve an unambiguous paternity test (see Table 4). However, as clutches prior to and after the study of those pairs remained infertile it seems likely that the technique had been successful even in those unresolved cases.
Discussion
For the first time, a technique for semen collection in psittacines was successful in a larger number of different species. This allowed the comparison of semen parameters between species as well as assisted reproduction techniques in species conservation programs. Using this technique, semen collection was frequently more successful in some parrot groups or families than in others (see Table 1). This could be related to the corresponding breeding seasons because the study was only performed during three months in the spring. Psittacines have a relatively short time of sexual activity14. As semen volume and sperm concentrations are significantly affected by the season15, the timing of successful semen collection is extremely important. It is unlikely that the differing success rates are related to the method itself, as it was generally successful in all families. Individual failure of the technique might also be explained in cases where males copulated with their partners immediately prior to the semen collection procedure, as this has been previously described as a potential reason for failure in semen collection16. However, this needs to be evaluated in further studies. In general, this novel technique did not result in any case of macroscopic physical irritations or negative changes of behaviour.
In many breeding pairs, especially in Eclectus spp. and Tanygnathus spp., it was possible to collect high concentration semen samples from nearly all of the males, although most clutches of those species were infertile. Certain factors besides male infertility (e.g. housing system) may be responsible for this problem. Eclectus parrots (Eclectus roratus) have a particular mating system compared to other parrots17, in that a female is naturally paired with several males. In addition, we assume that polygamy is the reason for the high sperm count in those species, because the semen of different males has to compete for the same follicle. Similar observations have been previously described, demonstrating that testicular size is significantly larger in species that breed in colonies compared to species that breed solitarily18. However, several semen samples were not investigated in detail, as the volume was too low or the sample was used for artificial insemination. Future studies should therefore focus on a more detailed semen analysis in single species to establish reference parameters, which would also allow a better comparison between species. Additionally, spermatozoa survival studies using this technique should be performed. Also, the possibility that semen quality might be reduced due to the electrical technique cannot be excluded. However, as no other technique for semen collection in large psittacines is routinely available to date, this cannot be investigated in the species used here at present.
Using the collected semen for artificial insemination, fertilised eggs were obtained, even when samples of low sperm concentration were transferred: for example the Green winged Macaw (Ara chloropterus) was inseminated with 19.2 μl semen (concentration of 9000 sperm/μl and a total sperm number of 172,800) prior to first oviposition and 18.2 μl semen [containing sporadic sperm cells (+)] after the first egg. In this case, the second of the two eggs was fertile. The number of sperm cells was much lower than normally required for successful insemination (i.e., 20,000,000 spermatozoa) in free-ranging birds19 or chickens20. This should be kept in mind when working in a species conservation program with a limited number of available males of reduced semen quality. Obviously they still could be genetically included in the population.
Importantly, divided semen samples (e.g. Red-sided Eclectus Parrot (Eclectus roratus polychlorus)) resulted in offspring from different females. In particular, one sample of 17.1 μl (1,590,000 sperm/μl) formed three aliquots (two 5 μl and one 6.5 μl), fertilising the eggs of three different females. This is a very important result, as this will allow the representation of genetically valuable males in an endangered population to be increased. However, it must be taken into account that egg-infertility after artificial insemination might be caused by poor semen quality, as well as by the time of insemination. The fertility period of the hen and each egg to be laid, as well as the average spermatozoon survival time in the oviduct, vary greatly between species21 and are unknown in the psittacine species used in this study. Therefore, more experience must be collected and further studies performed to investigate the optimal time and frequency of insemination to achieve the best fertility results. Therefore, the values provided for the semen samples (see Table 3) which did not lead to a fertile egg are just for information purposes. The quality of those samples cannot be contemplated the reason for infertility, as many other reasons must also be considered (see above). Most importantly, the values of those semen samples which lead to a fertile egg should be used as a guideline until more data are available. However, it is worth noting that semen collected with the novel electrical-based technique is able to fertilise eggs.
Overall, although our novel approach was highly successful, it must be kept in mind that semen samples are able to transmit different viral22,23 and bacterial24,25 diseases. Therefore, it is very important to examine the health status of a semen donor prior to semen collection.
It must be noted that 27 out of 64 artificial inseminations did not lead to oviposition. It can be speculated that the insemination procedure had interrupted breeding behaviour. In these cases, the females had been selected at the beginning of the study according to their expected breeding condition. After gaining some experience, we selected females for insemination only after breeding behaviour occurred or after a first egg was laid; as a consequence, clutches were always completed in these cases. However, it cannot be completely excluded that artificial insemination does not interfere with breeding activity.
While it has not been possible to obtain repeated semen samples from larger psittacine species in the past, the newly developed technique allows semen collection and evaluation in Psittaciformes for the first time. The novel technique is based on electro-stimulation, which has previously been described in waterfowl26,27. The significant alterations in probe design and electrode placements enabled the success of this technique in psittacine birds. However, it must be kept in mind that electrical stimulation for semen collection might affect the semen quality and only the proof to enable fertilisation described the success of a collection method. This was the case in the present study. This novel technique enables the precise and non-invasive assessment of the fertility of males as a prerequisite for successful artificial insemination. In the past, a biopsy of the testes was often the main method for the investigation of male fertility28. Furthermore, it initially enables the development of semen reference values and research about cryopreservation in large psittacines, especially in highly endangered species. However, bearing in mind that semen was obtained from Spix's macaws (Cyanopsitta spixii), this method is a major breakthrough in species conservation projects and might be a valuable method to assist the survival of this species. It allows the inclusion of surplus males into a breeding collection, but also the inclusion of surplus females which are present in many species conservation breeding programs (e.g. the Spix's Macaw). Such females can be paired with a sterilised partner of a closely related species being artificially inseminated with semen of her own species29.
To best of our knowledge, this study resulted in the world's first assisted reproduction macaw (Ara chloropterus) as well as Pionopsitta pileata, Cacatua haematuropygia and species of Psittaculinae.
Methods
Birds
In this study, 280 (243 males, 37 females) psittacines of 151 species and subspecies of different families were included (see Table 1). They were housed in aviaries as pairs, groups or single birds, receiving feed (seed mixture, fruit and vegetables) and fresh drinking water twice a day. Females which had repeatedly produced infertile eggs were selected for artificial insemination, and there was also a pure female pair of Green winged Macaws (Ara chloroptera). Males included for semen collection were divided into two groups: Group 1: Paired and sexually active males with frequently fertile clutches in the past; Group 2: Single males, sexually inactive males, or those with problems in reproduction in the past.
Semen collection method
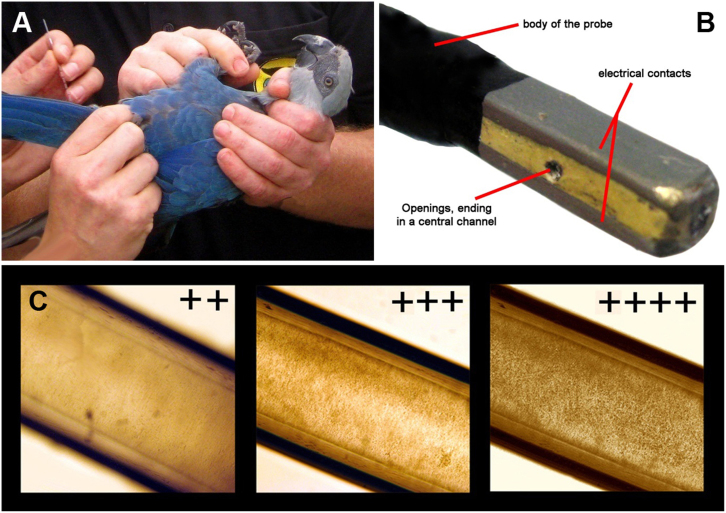
The birds were caught directly prior to semen collection and immediately placed on their back. No sedation or anaesthesia was applied. Assisted by a second person, the cloaca was visualised (see Fig. 1A). The semen collection was carried out by an electrical stimulation procedure with a newly developed bipolar probe (Patent pending). The probe consists of non-conducting plastic, where two electrical contacts are placed at the sides. Two small openings in the head of the probe end in a central channel (see Fig. 1B), through which a glass micro-capillary can be inserted from behind. The ejaculate sequesters automatically in the capillary after semen disposal. The size of the probe correlated with the size of the respective bird. First, the probe was introduced into the cloaca until both electrodes were completely inside; this ensured contact with the cloacal wall. Importantly, the probe should not be inserted as far as the rectum and should be specifically located with the majority in the urodeum. A slight electric current (between 1 and 14 V) was applied in short 1–2 seconds intervals (with breaks between 2 and 5 sec, slightly raising the voltage) until contractions of the cloaca and the muscles of the tail were observed and ejaculations had occurred. In most individuals, 6 V was ideal, with 5–8 intervals necessary to reach ejaculations, depending on the individual. The amperage depends physically on the electric resistance of each individual and can therefore not be provided as a general rule. The complete procedure of semen collection was usually completed within 3–5 minutes. In cases where 10 intervals within 5 minutes did not lead to ejaculation, the attempts were counted as failures. After the removal of the capillary from the probe, the semen was evaluated macroscopically.
Figure 1. Semen collection and artificial insemination.
(A) Fixation of the male during semen collection process in a Spix's macaw (Cyanopsitta spixii). (B) The head of the probe used for the semen collection. A micro-glass capillary has to be inserted from behind. (C) Micro capillary filled with semen classified ++ (low), +++ (middle) and ++++ (high) in concentration (400× magnification).
Semen evaluation
In the capillary, sperm-concentration, motility and contamination of the semen sample were evaluated. The sperm concentration was graded as sporadic (+), low (++), middle (+++), high (++++) and extremely high (+++++) (see Fig. 1C) using a microscopic 100× magnification. Motility was graded as motile and immobile. The contaminations were assessed macroscopically and graded as low (+), middle (++) and high (+++). Afterwards, the semen samples were used for artificial insemination and/or for further evaluation. In many samples used for artificial insemination, a more detailed examination was not possible due to the limited amount available. A more detailed examination would have required the complete amount, not leaving any for the insemination procedure. In the semen samples investigated (>5 μl), the volume and pH of ejaculates, as well as motility, vitality, morphology, concentration and total number of spermatozoa were investigated11,12,30. Volume was measured via scaled capillaries and pH using commercial indicator paper. Motility was estimated within 5 fields of view on a pre-warmed slide (microscopic magnification 100×). Sperm concentration and total sperm count was assessed using a Neubauer's counting chamber. Eosin B (2%) was used as live/dead stain for vitality analysis and for assessment of sperm morphology as well11,30.
Artificial insemination
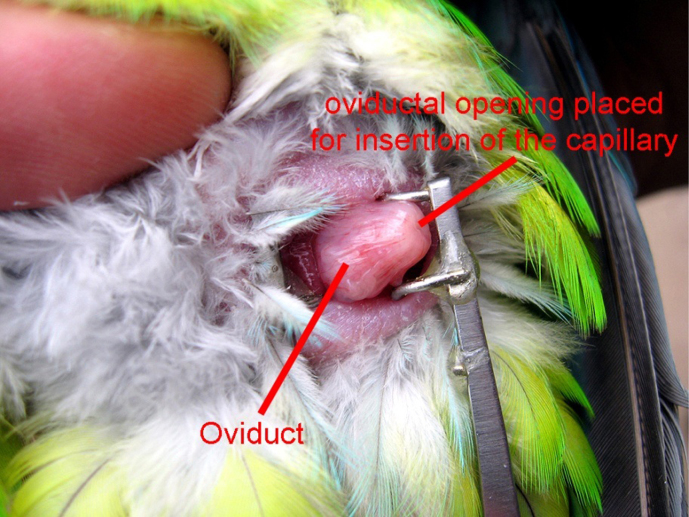
Insemination was performed in different species (Table 3), using semen without contamination and with motile spermatozoa 1–2 days prior to the expected oviposition or directly (within 2 hours) after the first egg was laid. Oviposition was expected due to observed breeding behaviour or typical abdominal extension. If possible, females were inseminated with semen from males other than their partners. The females were turned on their back and the oviduct opening was everted within the cloaca, followed by semen deposition into the oviduct (see Fig. 2). For this, the semen was pushed directly from the capillary into the oviduct by a plunger as previously described11. After 8–10 days of incubation in eggs which were laid after insemination, fertilisation was assessed by candling. Eggs with visual embryonic development were classified as fertile.
Figure 2. Visualisation of the oviduct opening in Pionopsitta pileata.
Assessment of parentage by DNA fingerprinting
Dead in shell embryos or hatched chicks were subjected to a paternity test by multilocus DNA fingerprinting, to exclude the female's partner as a father and to prove successful insemination.
Total DNA was isolated from 100 μl of blood using standard proteinase K (Merck, Darmstadt) and phenol/chloroform procedures31. Multilocus DNA fingerprinting was carried out to investigate the genetic relationships among and between the sampled birds following standard protocols32. The evaluation of the DNA fingerprints followed some fundamental rules33 by trying to assign all bands of the nestlings to the putative parents. The informative (i.e. polymorphic) bands of the DNA fingerprint (usually between 10 and 20) were visually scored into a data matrix as either absent (“0”) or present (“1”), which was then used to calculate the band-sharing coefficients (BSC) as BSC = C × 2/(A + B), with C being the number of shared bands, and A and B the number of bands in the profiles of individual A and B, respectively34.
Author Contributions
M.L. and D.N. developed the technique, M.L. planned the study, wrote the manuscript and participated in the field work, D.N. contributed to the study design, the manuscript preparation and performed the field work, H.M. and M.R. assisted in the field work and the selection of the birds, M.W. contributed to the manuscript and performed all laboratory tests for the paternity analysis.
Additional Information
Herewith the authors declare, that the study (manuscript no: SREP-12-03370A) was done in accordance with the national laws and that the method used in this manuscript was approved for use in birds by the Regierungspräsidium (Regional council) Giessen, Germany, with the permission number V54-19C 20-15 (1) GI18/9 Nr. 77/2009.
Acknowledgments
The authors thank the Loro Parque Fundacion, Tenerife, Spain, for the direct as well as financial support of the project.
References
- Caparroz R., Miyaki C. Y., Bampi M. I. & Wajntal A. Analysis of the genetic variability in a sample of the remaining group of Spix's Macaw (Cyanopsitta spixii, Psittaciformes: Aves) by DNA fingerprinting. Biol. Cons. 99, 307–311 (2001). [Google Scholar]
- Russelo M., Calcagnotto D., Desalle R. & Amato G. Characterisation of microsatellite loci in the endangered St. Vincent Parrot, Amazona guildingii. Mol. Ecol. Notes 1(3), 162–164 (2002). [Google Scholar]
- Watson R. Spix's Macaw fact file http://awwp.alwabra.com/index.php/content/view/171/51/ (28.10.2009).
- Lierz M. Reproductive disease, incubation and artificial insemination. In: Chitty J., Lierz M., eds. Manual of Raptors, Pigeons and Passerine Birds. BSAVA Publisher, Gloucestershire, 235–49 (2008). [Google Scholar]
- Christensen V. L. & Bagley L. G. Efficacy of fertilisation in artificially inseminated turkey hens. Poultry Sci. 68(5), 724–9 (1989). [DOI] [PubMed] [Google Scholar]
- Bourne D. Use and limitations of artificial insemination in cranes (Gruidae). European Association of Zoo and Wildlife Veterinarians (EAZWV). Second scientific meeting. Chester (1998). [Google Scholar]
- Berens von Rautenfeld D., Bley G. & Hickel E. M. Zur Technik der künstlichen Besamung und Sterilisation bei der Taube (Columbia livia). Prakt. Tierarzt 12(1), 103–5 (1979). [Google Scholar]
- Harrison G. J. & Wasmut D. Preliminary studies of electrostimulation to facilitate manual semen collection in psittacines. Proc. Conf. AAV. 2, 207–213 (1983). [Google Scholar]
- Brock M. Semen collection and artificial insemination in the Hispaniolan parrot (Amazona ventralis). J. Zoo Wild. Med. 22(1), 107–114 (1991). [Google Scholar]
- Joyner K. L. Theriogenology. In: Ritchie B. W., Harrison G. J., Harrison L. R., eds. Avian Medicine: Principles and Application. Wingers Publ. Inc. Lake Worth 748–804 (1994). [Google Scholar]
- Neumann D., Kaleta E. F. & Lierz M. Semen collection and artificial insemination in cockatiels (Nymphicus hollandicus) – A potential model for Psittacines. Tierärztl Prax (K) 41(2), 101–105 (2013). [PubMed] [Google Scholar]
- Samour J. H. The reproductive biology of the Budgerigar (Melopsittacus undulatus): Semen preservation techniques and artificial insemination procedures. J. Avian Med. Surg. 16(1), 39–49 (2002). [Google Scholar]
- Department of Conservation (DOC) kakapo recovery team. Successful artificial insemination a world first. http://www.KAKAPORECOVERY.ORG.NZ (28.10.2009).
- Blanco J. M., Wildt D. E., Höfle U., Voelker W. & Donoghue A. M. Implementing artificial insemination as an effective tool for ex situ conservation of endangered avian species. Theriogenology 71, 200–213 (2009). [DOI] [PubMed] [Google Scholar]
- Santiago-Moreno J. et al. Use of the hypo-osmotic swelling test and aniline blue staining to improve the evaluation of seasonal sperm variation in native Spanish free-range poultry. Poult. Sci. 88, 2661–2669 (2009). [DOI] [PubMed] [Google Scholar]
- Birkhead T. R. & Fletcher F. Depletion determines sperm numbers in male zebra finches. Anim. Behav. 49, 451 (1995). [Google Scholar]
- Heinsohn N. R. Ecology and evolution of the enigmatic Eclectus Parrot (Eclectus roratus). J. Avian Med. Surg. 22(2), 146–150 (2008). [DOI] [PubMed] [Google Scholar]
- Pitcher T. E., Dunn P. O. & Whittingham L. A. Sperm competition and the evolution of testes size in birds. J. Evolution Biol. 18, 557–567 (2005). [DOI] [PubMed] [Google Scholar]
- Blanco J. M., Gee G. F., Wildt D. E. & Donoghue A. M. Producing progeny from endangered birds of prey: Treatment of urine-contaminated semen and a novel intramagnal insemination approach. J. Zoo Wild. Med. 33(1), 1–7 (2002). [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay U. K., Chaudhuri D. & Johari D. C. Determination of minimum insemination dose of spermatozoa for optimum fertility and profitability in White Leghorn chicken using artificial insemination. Indian J. Poult. Sci. 41(1), 95–97 (2006). [Google Scholar]
- Holt W. V. Mechanisms of sperm storage in the female reproductive tract: an interspecies comparison. Reprod. Domest. Anim. 46(Suppl 2), 68–74 (2011) [DOI] [PubMed] [Google Scholar]
- Hoop R. K. Transmission of chicken anaemia virus with semen. Vet. Rec. 27(133), 551–2 (1993). [DOI] [PubMed] [Google Scholar]
- Metz A. L., Hatcher L., Newman J. A. & Halvorson D. A. Venereal pox in breeder turkeys in Minnesota. Avian Dis. 29, 850–3 (1993). [PubMed] [Google Scholar]
- Lierz M. & Hafez H. M. Occurrence of mycoplasmas in semen samples of birds of prey. Avian Pathol. 37(5), 495–497 (2008). [DOI] [PubMed] [Google Scholar]
- Blanco J. M. & Höfle U. Bacterial and fungal contaminants in raptor ejaculates and their survival to sperm cryopreservation protocols. Proceedings 6th conference of the European Wildlife Disease Association (2004). [Google Scholar]
- Samour J. H., Spratt D. M. J., Hutton R. E. & Jones D. M. Studies on semen collection in waterfowl by electrical stimulation. Brit. Vet. J. 141, 265–268 (1985). [DOI] [PubMed] [Google Scholar]
- Watanabe M. An improved technique of the artificial insemination in ducks. J. Fac. Fish Anim. Husb. 1, 363–370 (1957). [Google Scholar]
- Crosta L., Bürkle M., Gerlach H. & Timossi L. Testicular biopsy in psittacine birds: technique and histological findings. Proc. Conf. Europ. Assoc. Avian Vet. 113–116 (2003). [Google Scholar]
- Lierz M. Endoscopy, biopsy and endosurgery. In: Chitty J., & Lierz M., eds. BSAVA Manual of Raptors, Pigeons and Passerine birds. BSAVA Publishing, Gloucester, UK, 139–42 (2008). [Google Scholar]
- Stelzer G. Spermagewinnung, -untersuchung und -flüssigkonservierung bei verschiedenen Papageienvögeln (Psittaciformes). Vet. Med. Diss. Leipzig (2004). [Google Scholar]
- Sambrook J., Fritsch E. F. & Maniatis T. Molecular Cloning: A Laboratory Manual (1989), New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Swatschek I., Ristow D. & Wink M. Mate fidelity and parentage in Cory's shearwater Calonectris diomedea - field studies and DNA fingerprinting. Mol. Ecol. 3, 259–262 (1994). [Google Scholar]
- Westaneat D. F. Genetic parentage in the indigo bunting: A study using DNA fingerprinting. Behav. Ecol. Sociobiol. 27(1), 67–76 (1990). [Google Scholar]
- Wetton J. H. et al. Demographic study of a wild house sparrow population by DNA fingerprinting. Nature 327, 147–149 (1987). [DOI] [PubMed] [Google Scholar]