Abstract
Beta-cyclodextrins (β-CDs) can form inclusion complexes with cholesterol, and are commonly used to manipulate cholesterol levels of biomembranes. In this work, we have used multiscale molecular dynamics simulations to provide a detailed view on the interaction between β-CDs and lipid model membranes. We show that cholesterol can be extracted efficiently upon adsorption of β-CD dimers at the membrane/water interface. However, extraction is only observed to occur spontaneously in membranes with high cholesterol levels. Free energy calculations reveal the presence of a kinetic barrier for cholesterol extraction in the case of low cholesterol content. Cholesterol uptake is facilitated in case of (poly)unsaturated lipid membranes, which increases the free energy of the membrane bound state of cholesterol. Comparing lipid/cholesterol compositions typical of liquid-disordered (Ld) and liquid-order (Lo) domains, we furthermore show that cholesterol is preferentially extracted from the disordered regions, in line with recent experimental data.
Biomembranes are heterogeneous lipid assemblies in which preferential association of certain lipids, sterols, and proteins can lead to the formation of nanodomains, so-called rafts1. Such rafts, enriched in cholesterol and saturated lipids, display physicochemical properties different from those of their disordered fluid surroundings, and are believed to play an important role in the self-assembly of membrane proteins into functional platforms2. Traditionally, detergents were used as the main criterion for raft association; raft constituents were defined simply as the detergent-resistant membrane (DRM) remaining after non-ionic detergent solubilization. This criterion was usually combined with the use of methyl-β-cyclodextrin to extract cholesterol from cell membranes. Thus, DRMs solubilization after cyclodextrin treatment was indicative of it being part of a raft component. In addition, if a biological process in living cells was disrupted by cyclodextrin treatment, the process was considered to be raft-based3,4,5,6,7,8,9. From the chemical point of view, cyclodextrins (CD) are composed of several glucose units, linked together by α-1,4-glucosidic bonds. The unique properties of these macrocycles result from their characteristic cylindrical structure with a hydrophilic exterior and hydrophobic central cavity10. The latter serves as a suitable microenvironment for lipolytic compounds. The limiting parameter in the complexation process is the inner diameter of the cyclodextrin cone; for β-CD, consisting of seven glucose monomers, the diameter measures 7.8 Å and is perfect for complexation of cholesterol.
To characterize the ability of β-CD to uptake cholesterol from biomembranes, many studies have been devoted to model membranes6,8,9,11,12,13,14,15,16. Ohvo and Slotte12 demonstrated that the desorption rates of various sterols from the surface film to β-CD solution depend on the relative polarity of sterols and increase considerably with increasing surface pressure. Moreover, sterol desorption from cholesterol/phospholipid mixed systems was found to be notably retarded in comparison to the extraction from pure cholesterol monolayers. More recently, Sanchez et al.17 have shown that β-CDs preferentially remove cholesterol from liquid-disordered (Ld) instead of liquid-ordered (Lo) membrane domains. With the notion that Lo domains supposedly resemble raft domains or DRMs, this finding casts doubt on the use of β-CDs to characterize the cholesterol content in vivo.
In order to interpret the experimental data, a clear molecular understanding of how β-CDs extract cholesterol from lipid membranes is needed. To provide such a detailed view, we resort to multiscale molecular dynamics (MD) simulations. Nowadays, MD simulation is a powerful tool (denoted “computational microscopy”18,19) that can be used to complement experimental techniques. Using atomistic resolution, we investigated the interaction between β-CD and cholesterol/lipid membrane models, systematically exploring the effect of lipid type and cholesterol content on the extraction process. At a coarse-grain level, we show the preferential extraction of cholesterol from Ld domains in phase separated Ld/Lo membranes. Our results provide a molecular view on β-CD mediated cholesterol depletion from lipid membranes that may aid in interpreting experimental data. We expect it will also contribute to the design of more effective cyclodextrin derivatives for medical treatment of lipid metabolism pathologies, like for example in the treatment of Niemann-Pick type C disease20.
Results
Spontaneous extraction of cholesterol from mixed PC/CHOL monolayer by β-CD dimers
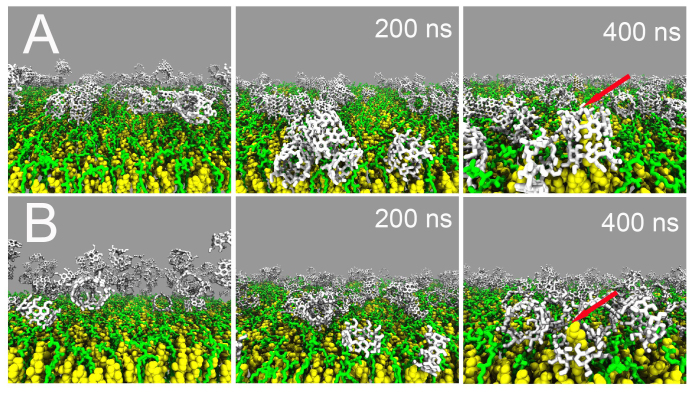
To provide a molecular view on β-CD mediated cholesterol extraction from model membranes, we simulated monolayers of diC16-PC/CHOL at a 1:3 ratio in the presence of β-CD dimers. Dimers, rather than monomers, were previously shown to be the dominant aggregation state of β-CDs in solution21. We observe that nearly each of the twelve β-CD dimers ends up bound to the monolayer surface with a binding time scale between 50 and 150 ns, governed by their random diffusion (Figure 1A). Some of the β-CDs (about 30%) aggregate on the monolayer forming stacked barrels, mostly tilted by 90° with respect to the monolayer surface normal. In this conformation extraction of cholesterol is not possible. The majority of the dimers, however, adopt a perpendicular orientation suitable for the extraction process to occur, stabilized in this position by adjacent β-CDs. Indeed cholesterols are extracted from the monolayer by these β-CD dimers (Figure 1A, final snapshot). Two cholesterol molecules are completely extracted during the 500 ns length of the simulation. A repeat simulation shows extraction of three cholesterols. The averaged, normalized extraction rate is 0.0002 ns−1 per β-CD. Similar rates were obtained in smaller test systems in which β-CDs were pre-adsorbed on the membrane surface (Supplementary Table S1). The fact that only a small number of β-CDs are able to extract cholesterol on the time scale of the simulation points to a kinetic barrier opposing extraction. As will become apparent later, this barrier reflects the formation of the encounter complex between β-CD and the cholesterol head. Once β-CD is positioned on top of the cholesterol, cholesterol is extracted rapidly (typically within 5 ns).
Figure 1. Spontaneous extraction of cholesterol from a diC16-PC/cholesterol 1:3 monolayer by cyclodextrin.
(A) Set-up with 12 β-CD dimers. Initially placed in the aqueous solution (top-left), the β-CDs adsorb at the lipid/water interface (middle) and adopt a number of configurations, either tilted or untilted with respect to the surface normal or forming stacked barrels. Dimers present in the untilted orientation are capable to extract cholesterol (top-right, red arrow). (B) Set-up with 24 β-CD monomers. Initially dispersed in solution (bottom-left), β-CD monomers are also able to bind to the surface of the monolayer (middle), however, only partial extraction of cholesterol is observed (bottom-right, red arrow). Color code: diC16-PC, green, cholesterol, yellow, β-CDs, white. Water is not depicted for clarity.
We also studied the ability of β-CD monomers to extract cholesterol using the same set-up, i.e. with 24 monomers placed in random initial positions in the aqueous solution (Figure 1B). Like the dimers, also the monomers have a high affinity for the monolayer surface, and binding is observed to occur on a similar timescale (within 200 ns). Under the conditions of the simulations, the β-CDs do not have enough time to form dimers, and bind the interface as monomers. Once adsorbed, diffusion is too slow and no further aggregation takes place on the time scale of the simulation (500 ns). In their monomeric form, the β-CDs are able to capture the cholesterol head group (Figure 1B, final snapshot), but are unable to completely extract cholesterol out of the monolayer in line with results from simulations performed on pure cholesterol monolayers21. The same qualitative behavior was observed in three independent simulations.
Based on the results described in this section, we conclude that spontaneous cholesterol extraction from mixed lipid/cholesterol monolayers occurs on a sub-microsecond time scale, requiring a suitably oriented and interfacially embedded β-CD dimer.
Cholesterol content drastically affects extraction kinetics and thermodynamics
Varying the molar lipid-cholesterol ratio in the membrane has a dramatic effect on the cyclodextrin extraction activity. In the case of a diC16-PC/CHOL 1:1 molar ratio monolayer with nine β-CD dimers pre-adsorbed at the interface, no cholesterol is complexed during three independent 200 ns simulations. To understand the apparent large effect of the lipid/cholesterol ratio on the ability of β-CD to extract cholesterol, we analyzed the equilibrium configuration of the CD dimer at the monolayer interface. Snapshots of these configurations are shown in Figure 2A–C at an increasing PC/cholesterol ratio from 0:1, 1:3, to 1:1. In the case of a pure cholesterol monolayer, in fact the energetically most favorable configuration for a single β-CD dimer is a tilted one (Figure 2A). Lipid head groups appear to stabilize an upright orientation by formation of hydrogen bonds between the hydroxyl groups of the cyclodextrins and the phosphate groups of PC, clearly seen in the 1:3 mixture (Figure 2B). Comparison of Figure 2B and 2C, however, shows that the presence of too many lipid head groups does not allow the β-CDs to interact directly with the cholesterol molecules anymore. In the case of the 1:1 mixture, most cholesterols are shielded by the lipid head groups explaining the lack of spontaneous extraction events in our simulations. Spontaneous extraction was also not observed by increasing the amount of cholesterol to a lipid/cholesterol 1:2 molar ratio. As shown above, increasing the amount of cholesterol in the monolayer even further (diC16-PC/CHOL 1:3) facilitates the cholesterol extraction due to the presence of enough space between the phospholipid head groups for the proper positioning of the β-CDs on the monolayer surface.
Figure 2. Close-ups of cyclodextrin membrane interaction.
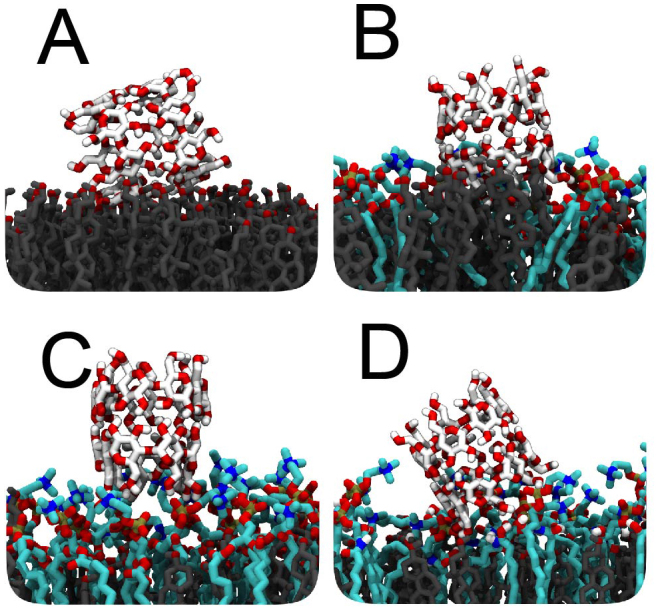
(A) Pure cholesterol monolayers, favoring the tilted conformation of the β-CD dimer. (B) Adding diC16-PC lipids (1:3 PC/CHOL ratio), a straight conformation is stabilized through the formation of hydrogen bonds between the lipid head groups and the hydroxyl groups of the β-CDs. (C) Adding more diC16-PC lipids (1:1 PC/CHOL), β-CDs are no longer able to contact directly with cholesterols. (D) Replacing diC16-PC with SM (1:1 SM/CHOL), results in stronger binding of β-CD.
To get more insight into the inaccessibility of cholesterol by β-CD, we calculated the cholesterol-CD radial distribution function (RDF). The results are shown in Supplementary Fig. S1 as a function of cholesterol-lipid molar ratio. The probability of finding β-CD molecules within a distance of 0.2 nm to the cholesterol hydroxyl group (approximate distance for making a hydrogen bond) increases as the amount of phospholipid decreases. These results show that an important requirement for the spontaneous extraction is the proximity between cyclodextrins and cholesterol, facilitated at high cholesterol levels. To further quantify the extraction process, we calculated the potential of mean force (PMF) for the extraction of cholesterol from the membrane into a β-CD dimer. An example is shown in Figure 3 for the diC16-PC/CHOL 1:2 system. The PMF shows an overall favorable extraction energy, with a stabilization of 40 ± 2 kJ mol−1 in the complexed state. A clear barrier of about 20 ± 2 kJ mol−1 is also visible. As argued above, this barrier is due to the presence of lipid head groups that shield the cholesterol from being extracted. PMFs for other membrane compositions show an overall similar profile (Fig. S2, Table S2). The extraction free energy and barrier obtained from these PMFs are shown in the inset of Figure 3 as function of cholesterol fraction. In each case the final cholesterol-CD complex is energetically more favorable than the cholesterol-lipid association in the monolayer. The energy barrier for cholesterol desorption decreases with a decreasing amount of phospholipid present in the monolayers. Thus we conclude that for all these conditions cholesterol extraction is favorable, but that a kinetic barrier prevents rapid (sub-microsecond) extraction at high lipid/cholesterol ratios when cholesterol is shielded by surrounding lipids.
Figure 3. Energetic characterization of cholesterol extraction.
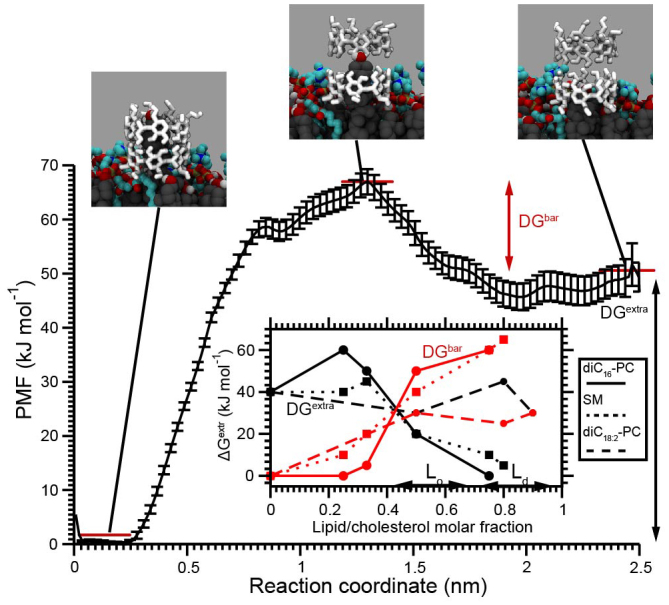
Potential of mean force (PMF) for β-CD mediated cholesterol extraction from a diC16-PC/CHOL 1:2 monolayer. The reaction coordinate represents the distance between the center-of-mass of the extracted cholesterol and the β-CD dimer. A distance of 0 nm corresponds to the cholesterol complexed with β-CD, the largest distance indicates full embedding of cholesterol in the monolayer. Red bars indicate the various states used to calculate the extraction free energy ΔGextr and barrier free energy ΔGbar. The inset shows the ΔGextr and ΔGbar as a function of the lipid molar fraction for diC16-PC, SM, and diC18:2-PC/CHOL monolayers. The error bars on these data points do not exceed 5 kJ mol−1. Typical composition ranges for Lo and Ld membranes are indicated. Additional PMFs and a supporting table with free energy data are given in Fig. S2 and Table S2.
Extraction process in presence of sphingomyelin and unsaturated lipids is similar
To evaluate whether or not the type of lipid effects the extraction process, monolayers containing sphingomyelin (SM), a ceramide based lipid, were also considered. The results are found to be similar to those obtained with diC16-PC monolayers. At a SM/CHOL 1:3 molar ratio, the simulations show spontaneous cholesterol extraction at a rate comparable to the PC/CHOL system (cf. Table S1). When the concentration of SM increases, the extraction rate drops, with no spontaneous events observed for the SM/CHOL 1:1 monolayer. Comparing the binding modes of β-CD to PC and SM containing monolayers (Figure 2C, D), a stronger binding of SM is noticeable due to the presence of the amide group in the ceramide linker. Analysis of the cholesterol-CD RDFs (Fig. S1) and PMFs (Fig. S2) confirm that the extraction process is very similar to that of PCs. Interestingly, and in agreement with experimental evidence6, the barrier in the SM system seems to disappear already at a 1:2 ratio whereas for PC this is observed only at a 1:3 ratio. Consistent with these data is the occurrence of spontaneous cholesterol extraction in SM/CHOL, but not PC/CHOL, 1:2 monolayers (Supplementary Fig. S3).
In addition, we looked at the effect of substituting the fully saturated lipid tails for (poly)unsaturated lipid tails. PMFs for 4:1 and 1:1 mixtures of diC18:2-PC/CHOL monolayers are shown in Supplementary Fig. S2. The energy profiles again look similar to those of the saturated lipids, with a net gain in free energy for extraction of cholesterol by the β-CD dimer but a barrier against spontaneous extraction. The associated extraction and barrier free energies are shown in the inset of Figure 3. Due to the poor packing of unsaturated lipids and cholesterol, the free energy gain is larger compared to the saturated lipid case at high lipid/cholesterol ratio, e.g. ΔGextr = 0 kJ mol−1 for diC16-PC/CHOL 4:1 versus ΔGextr = 40 kJ mol−1 for diC18:2-PC/CHOL 4:1. Remarkably, this difference vanishes at a lipid/CHOL ratio of 1:1, with ΔGextr ~ 20 kJ mol−1 irrespective of lipid type.
Based on our results on different lipid types, we conclude that substituting either glycerol based (diC16-PC) for ceramide based (SM) lipids or saturated for unsaturated lipids does not change the extraction process on a qualitative level. Subtle changes in the binding mode of β-CDs due to the sphingosine moiety and less efficient packing of cholesterol with unsaturated lipids, however, do modulate the free energy landscape and result in substantially different extraction kinetics.
Extraction of cholesterol from bilayers is similar to monolayers
Most of the conclusions drawn so far are based on simulations of lipid model monolayers. In order to verify our results for biologically more relevant systems, we repeated some of the PMF calculations for lipid bilayers. The PMFs for extracting a single cholesterol into a β-CD dimer from either mono- or bilayer are compared in Supplementary Fig. S4. In case of diC16-PC/CHOL 3:1, the free energy barrier as well as the final states are nearly identical between mono- and bilayer systems. Replacing diC16-PC by SM, the profiles still look similar but a notable difference (about 20 kJ mol−1) shows up in the relative free energy of the membrane bound state. Apparently cholesterol is tighter bound in the SM bilayer with respect to the monolayer. For the diC18:2-PC/CHOL 10:1 mixture, the PMFs are again nearly identical between the monolayer and bilayer membranes. Together, the data in Fig. S4 point to a very similar extraction mechanism between mono- and bilayers, allowing us to extrapolate most of our conclusions from monolayers to bilayers.
Cholesterol is preferentially extracted from liquid-disordered membranes
Finally, we turn to the question whether cholesterol is more easily extracted from Ld or Lo membranes. This question can be answered by a comparison of the PMFs among the different lipid compositions (Fig. S2) for compositions mimicking those of Lo (cholesterol fraction 0.3–0.6) and Ld (cholesterol fraction below 0.25) domains. Although the PMF profiles are qualitatively similar, the end states show a larger free energy difference in the case of our Ld mimic. Consequently, the energy barrier between the two states is reduced. Thus, complex formation in the Ld phases is favored by two different effects: on the one hand, the formation of a more stable complex and on the other hand the decrease of the activation energy, see Figure 3. Efficient extraction also depends on the affinity of β-CD for either Ld or Lo phase. To measure this, we calculated the binding free energy of a β-CD dimer on membranes of different compositions (Fig. S5). This binding energy is found to be favorable by 35 ± 5 kJ mol−1 for both Ld and Lo mimicking membranes, suggesting that β-CDs will distribute quite evenly across a heterogeneous membrane.
We thus expect that cholesterol is extracted more easily from a liquid-disordered phase, both from a kinetic and from a thermodynamic point of view. To test this prediction directly, we resort to a coarse-grained (CG) approach. Our CG model system is either a planar lipid bilayer or a small liposome, both composed of a ternary mixture of SM, diC18:2-PC, and CHOL at approximately 1:1:1 ratio. Consistent with the experimental ternary phase diagram of comparable systems22, the membranes show coexisting Ld and Lo domains, with lipid/cholesterol compositions close to 10:1 for the Ld and 1:1 for the Lo domain. CD dimers were placed at a concentration of 0.04 M in the surrounding aqueous solution. Due to the rapid diffusion of the β-CDs, the lipid-water interface is covered by the carbohydrate in a matter of 10 s of nanoseconds, consistent with our atomistic resolution simulations. The final configuration of the systems are shown in Fig. 4. In agreement with our calculated binding free energy calculations, β-CDs are found adsorbed in roughly equal amounts at the Ld and Lo domains in both planar and vesicular membranes. In case of the planar membrane we observe the formation of 11 β-CD/cholesterol complexes during a total of 0.1 ms simulation time (Fig. 4, insert). Analyzing the type of lipids contacting a cholesterol at the moment of extraction, we can assign extraction from either ordered or disordered domains. We find that spontaneous extractions take place both from the Ld and Lo domains, with 8 and 3 occurrences respectively. Expressed as rate per β-CD monomer, this corresponds to 2 × 10−6 ns−1 cholesterol extractions for the Ld and 7.5 × 10−7 ns−1 for the Lo region. Given the about tenfold lower cholesterol concentration in the Ld domain, it appears that cholesterol is extracted more efficiently from disordered membrane domains, supporting our predictions based on the atomistic PMFs. In the vesicular system, the overall extraction process is the same as for the planar bilayer. Again 11 cholesterol molecules were extracted, however, during a much shorter simulation time of 20 μs. We explain the higher extraction efficiency by considering the high curvature of the simulated vesicle which increases the accessibility of cholesterols. Also in the vesicular system most cholesterols were extracted from the Ld domain, but as the domains are less well defined further quantification proved difficult.
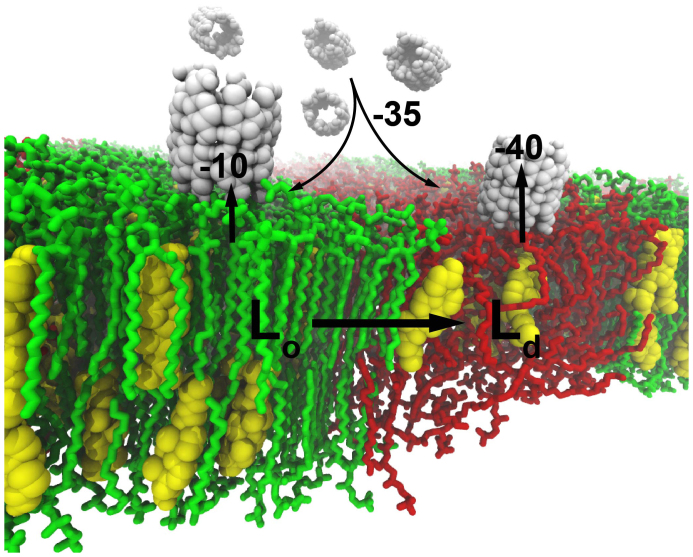
Figure 4. Cholesterol extraction from coexisting liquid-ordered Lo and liquid-disordered Ld domains.
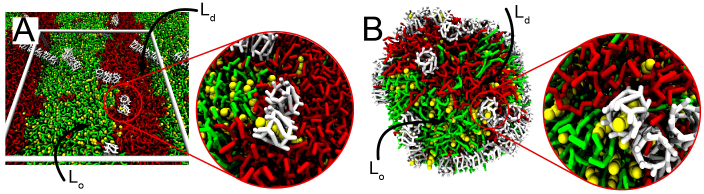
Systems consist of SM/diC18:2-PC/CHOL 35:35:30 molar ratio with 0.04 M β-CD dimers in solution, and were simulated at a CG level. Snapshots are shown after 10 μs simulation for both planar (A) and vesicular (B) membranes. β-CDs interact with the lipids from both Ld and Lo domains, and spontaneous cholesterol extraction is observed in both systems. Preferential extraction takes place form the disordered phase. The zoomed view highlights some of the CD-CHOL complexes formed. Color code: SM, light green; diC18:2-PC, red; cholesterol, yellow; β-CD dimer; white. Water molecules are not depicted for clarity.
Discussion
Our computational microscopy approach provides a molecular view on β-CD mediated cholesterol extraction from model membranes. We show that a β-CD dimer can efficiently extract cholesterol on a submicrosecond time scale, at least for membranes in which the cholesterol is not too strongly embedded. This occurs most easily in the presence of unsaturated lipids and/or at high cholesterol levels. The effect of unsaturation can be explained by considering the chemical potential of cholesterol. Bennett et al.23 showed that a free energy of nearly 80–90 kJ mol−1 is required for the extraction of a single cholesterol molecule out of a diC16-PC/CHOL bilayer (in the range 0–40% cholesterol), however, this energy drops by about 10–20 kJ mol−1 in case of unsaturated PCs. The facilitated extraction at high cholesterol levels is caused by the ability of the β-CDs to directly interact with the cholesterols without being hampered by lipid head groups. Based on our PMF analysis, a large kinetic barrier appears for lipid fractions exceeding 0.3 (cf. Figure 3). This barrier steeply increases with increasing lipid fraction. Our results explain the experimental observations made by Ohvo et al.12 and recently by Flasinski et al.6. Based on lipid monolayer experiments, a strong correlation was found between a reduced cholesterol content and a diminished ability of cyclodextrins to extract cholesterol. Our data also support the model of Lange and Steck24, in which the ease of extraction of cholesterol from disordered membranes is explained by an increase in cholesterol activity. According to this model, cholesterols form stoichiometric complexes with saturated phospholipids in particular. These complexes prevent the projection of cholesterol into the aqueous phase, in contrast with activated (non-complexed) cholesterols that undergo constant bobbing motions easing their capturing by extracellular acceptors such as CD. The model thus predicts facilitated uptake under conditions where complex formation is reduced, i.e. in the presence of unsaturated lipids or at high cholesterol levels, in line with our observations.
In regard to the ability of β-CD to extract cholesterol from either Ld or Lo domains, the two effects mentioned above appear to counter each other. The presence of unsaturated lipids favors extraction from Ld domains, whereas a higher cholesterol fraction favors extraction from Lo domains. Whether cholesterol is primarily extracted from Ld or Lo domains will therefore critically depend on lipid composition as well as extraction conditions. Based on the data shown in Fig. 3, however, it becomes clear that cholesterol is preferentially extracted from the Ld phase. Assuming a typical lipid/cholesterol ratio of 5:1 for the Ld phase, and 2:1 for the Lo phase, the free energy gain upon complexation of cholesterol by β-CD is about 40 kJ mol−1 and 10 kJ mol−1 for Ld and Lo phase respectively, a difference of 30 kJ mol−1. In addition, the barrier along the extraction pathway increases from 30 kJ mol−1 to 60 kJ mol−1, again a difference of 30 kJ mol−1. The use of slightly different compositions for the Ld and Lo phases changes these numbers but not the overall conclusion. Our CG simulations show indeed a preferential extraction of cholesterol from the Ld domain of a 1:1:1 SM/diC18:2-PC/CHOL mixture, despite the low concentration (0.1 molar ratio) of cholesterol in the Ld domain. Recent experimental data by Sanchez et al. support our calculations17. The experiment correlates the changes in Laurdan GP (generalized polarization) value with changes in cholesterol content in the membrane. The measured GP value decreases in the Ld phase as a result of the addition of β-CD. According to the authors, this change results from the removal of cholesterol from the Ld phase, followed by a rapid re-equilibration of cholesterol from the Lo to the Ld. In another study by Jablin et al.9, neutron reflectometry was used to characterize membranes of various compositions before and after β-CD was added to the subphase. Only the structure of bilayers with molar cholesterol ratios mimicking the Ld phase was changed, indicating that β-CD is capable to remove non-complexed cholesterol.
Finally we turn the discussion to the relevance of our data to the in vivo situation. A first point of concern is the temperature at which our simulations are performed, at 288 K considerably lower than physiological temperature. The lower temperature was chosen as it has experimentally been shown to be optimal for CD mediated cholesterol extraction from cholesterol monolayers12. To probe the effect of temperature, we recalculated the PMFs of cholesterol extraction from both Lo and Ld mimetic bilayers at 310 K. The resulting PMFs (Fig. S6) show that the qualitative features do not change upon the increase in temperature. Changes of the order of 10 kJ mol−1 in the extraction free energy and height of the barrier can be appreciated, both being reduced at higher temperature irrespective of membrane composition. These data point to an increased cholesterol desorption rate at higher temperature, but other factors such as cyclodextrin monomer-dimer equilibrium and membrane adsorption propensity are also temperature dependent and could potentially lead to less efficient extraction. Another important difference between our simulated systems and real membranes is composition. Most of our results pertain artificial model membrane systems, in particular monolayers rich in cholesterol. Actually, no spontaneous cholesterol extraction was observed unless the phospholipid to cholesterol ratio was 1:3 or sphingomyelin to cholesterol ratio was higher than 1:1 of which the first is an impossibility for a bilayer and the second contains more cholesterol than reported for any biological membrane. Furthermore, the lipid mix of SM and diC18:2-PC, chosen to illustrate the coexistence of Lo and Ld phases, results in phases with a big difference in cholesterol content and associated membrane order. In the plasma membrane of real cells the difference in order between the two phases is not as large as in artificial well-separated phases25. Direct simulation of cholesterol extraction from more realistic mixtures is, however, hampered by two main reasons. First, simulation of phase separation using more realistic mixtures requires much larger system sizes as the width of the domain boundary will exponentially increase with reduced line tension between the domains. Second, as we have shown, the spontaneous extraction process from more realistic membranes becomes too slow to be sampled on the accessible microsecond time scale. In order to extrapolate our data to more realistic conditions, we therefore resorted to an indirect approach through computation of the energetic factors involved during cholesterol extraction. As discussed above, our energetic data (cf. Fig. 3) suggest that also at physiological composition cholesterol extraction occurs primarily from the Ld phase. Based on recent in vivo studies involving T cells, Mahammad and Parmryd26 also challenge the widespread assumption that β-CD preferentially targets cholesterol in lipid rafts and that sensitivity to β-CD is proof of lipid raft involvement in a cellular process. Nevertheless, in real cell membranes situation might be more complicated than the picture emerging based on model systems.
Keeping these considerations in mind, we summarize the main conclusion of our work in Fig. 5. Adding of β-CDs to a membrane which contains both Ld and Lo domains results in the following processes: i) rapid interfacial binding of β-CD dimers uniformly across the membrane, ii) cholesterol extraction mainly from the disordered domain by suitably oriented β-CD dimers, iii) re-equilibration of cholesterol between the two domains, notably the migration of cholesterol from the ordered to the disordered domains, and iv) disappearance of the domains eventually once the cholesterol fraction becomes too small. The whole extraction process slows down as the overall cholesterol content decreases. Furthermore, due to the favorable adsorption free energy of β-CDs on the membrane, the desorption rate of the CD-cholesterol complex from the membrane into the aqueous phase is predicted to be very slow. Our results should be considered when interpreting experimental assays that use β-CDs to manipulate cholesterol content in membranes, both in vitro and in vivo, and open the way to the rational design of more efficient β-CD based cholesterol carriers.
Figure 5. Proposed model for cyclodextrin-mediated cholesterol extraction from lipid model membranes.
β-CDs form dimers which bind to a membrane surface with high affinity of 35 kJ mol−1, irrespective of the membrane phase (either Lo or Ld). Extraction, however, is more favorable from the Ld phase, with a gain in free energy of 40 kJ mol−1 compared to 10 kJ mol−1 from the Lo domain. The cholesterol extraction from Ld domains will cause a redistribution of cholesterol between the domains, and lastly result in the disruption of the phase coexistence.
Methods
System set-up
For the unbiased atomistic level extraction simulations, a monolayer consisting of 300 cholesterol molecules, 24 β-CDs, and 100 diC16-PC molecules was set-up. The β-CDs were initially placed at a distance of 1.0 nm away from the monolayer surface either in monomeric or dimeric conformations. A number of smaller scale systems, containing 9 β-CDs pre-assembled on the monolayer, were simulated to probe the effect of lipid composition on the extraction process. For the free energy calculations, systems consisting of a single β-CD dimer in direct contact with either a monolayer or bilayer were used. The CG lipid bilayer consisted of a ternary mixture (SM/diC18:2-PC/CHOL 35:35:30 molar ratio), phase separated into coexisting Lo and Ld domains. The initial configuration was taken from our previous work27, replacing diC16-PC with SM. The bilayer contains 2000 lipids and is fully solvated with 30000 CG water beads. We added 20 β-CD dimers to achieve an overall concentration of 0.04 M. Additionally, a ternary vesicle was prepared using the same composition as the planar bilayer. Details of the system set-up are given in the Supplementary Materials and Methods. Table S1 provides an overview of all simulations performed.
Computational details
Simulations were performed using the GROMACS molecular dynamics package28, version 4.0. The parameter set for the atomistic simulations of β-CD was based on the GROMOS force field for carbohydrates29. The parameters for cholesterol were taken from Holtje et. al30. Lipids were modeled using an in-house force field (de Vries et al., unpublished), based on the set of parameters for aliphatic hydrocarbons which are part of the GROMOS 53a6 force field31. The SPC model32 was used for the solvent. The temperature of all systems was maintained at 288 K by weak coupling to a bath. A surface-tension coupling scheme was used to maintain a constant surface pressure of 33 mN m−1 for the monolayers. The values for temperature as well as for surface pressure used here have been shown to be optimal for cyclodextrin mediated cholesterol extraction from cholesterol monolayers12. Parameters for the simulation of the CG lipids and solvent were taken from the MARTINI model33,34. The β-CD model was based on the MARTINI carbohydrate model35 and refined to reproduce the thermodynamics of the atomistic simulations. The temperature of both planar and vesicular membrane systems is maintained at 300 K. In case of the planar system semi-isotropic pressure scaling is used. More details of the computations can be found in the Supplementary Materials and Methods, including a description of the method to compute the PMFs and parameterization of the CG β-CDs.
Author Contributions
C.A.L., A.H.V. & S.J.M. designed research, C.A.L. performed the simulations, all authors contributed to writing of the manuscript.
Supplementary Material
Supplementary information
References
- Simons K. & Ikonen E. Functional rafts in cell membranes. Nature 387, 569–572 (1997). [DOI] [PubMed] [Google Scholar]
- Simons K. & Gerl M. J. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell. Biol. 11, 688–699 (2010). [DOI] [PubMed] [Google Scholar]
- Atger V. M. et al. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 99, 773–780 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow R. & Zhang B. Cholesterol recognition and binding by cyclodextrin dimers. J. Am. Chem. Soc. 118, 8495–8496 (1996). [Google Scholar]
- Castagne D. et al. Study of the cholesterol extraction capacity of beta-cyclodextrin and its derivatives, relationships with their effects on endothelial cell viability and on membrane models. J. Inclusion Phenom. Macrocyclic Chem. 63, 225–231 (2009). [Google Scholar]
- Flasinski M., Broniatowski M., Majewski J. & Dynarowicz-Latka P. X-ray grazing incidence diffraction and langmuir monolayer studies of the interaction of beta-cyclodextrin with model lipid membranes. J. Colloid. Interface Sci. 348, 511–521 (2010). [DOI] [PubMed] [Google Scholar]
- Frijlink H. et al. The effect of parenterally administered cyclodextrins on cholesterol levels in the rat. Pharm. Res. 8, 9–16 (1991). [DOI] [PubMed] [Google Scholar]
- Grauby-Heywang C. & Turlet J. M. Study of the interaction of beta-cyclodextrin with phospholipid monolayers by surface pressure measurements and fluorescence microscopy. J. Colloid. Interface Sci. 322, 73–78 (2008). [DOI] [PubMed] [Google Scholar]
- Jablin M. S. et al. Effects of beta-cyclodextrin on the structure of sphingomyelin/cholesterol model membranes. Biophys. J. 99, 1475–1481 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel C., Saenger W., Hingerty B. E. & Brown G. M. Topography of cyclodextrin in- clusion complexes, part 20. circular and flip-flop hydrogen bonding in beta-cyclodextrin undecahydrate: a neutron diffraction study. J. Am. Chem. Soc. 106, 7545–7557 (1984). [Google Scholar]
- Mascetti J., Castano C., Cavagna t. D. & Desbat B. Organization of beta-cyclodextrin under pure cholesterol, dmpc, or dmpg and mixed cholesterol/phospholipid monolayers. Langmuir 24, 9616–9622 (2008). [DOI] [PubMed] [Google Scholar]
- Ohvo H. & Slotte J. P. Cyclodextrin-mediated removal of sterols from monolayers: effects of sterol structure and phospholipids on desorption rate. Biochemistry 24, 8018–8024 (1996). [DOI] [PubMed] [Google Scholar]
- Ohvo-Rekila H., Akerlund B. & Slotte J. P. Cyclodextrin-catalyzed extraction of fluorescent sterols from monolayer membranes and small unilamellar vesicles. Chem. Phys. Lipids 105, 167–178 (2000). [DOI] [PubMed] [Google Scholar]
- Puglisi G., Fresta M. & Ventura C. Interaction of natural and modified beta- cyclodextrins with a biological membrane model of dipalmitoylphosphatidylcholine. J. Colloid. Interface Sci. 180, 542–547 (1996). [Google Scholar]
- Tsamaloukas A., Szadkowska H., Slotte P. J. & Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys. J. 89, 1109–1119 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsamaloukas A., Szadkowska H. & Heerklotz H. Thermodynamic comparison of the interactions of cholesterol with unsaturated phospholipid and sphingomyelins. Biophys. J. 90, 4479–4487 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez S. A., Gunther G., Tricerri M. A. & Gratton E. Methyl-beta-cyclodextrins preferentially remove cholesterol from the liquid disordered phase in giant unilamellar vesicles. J. Membr. Biol. 241, 1–10 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. H. et al. Discovery through the computational microscope. Structure 17, 1295–1306 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror R. O. et al. Biomolecular simulation: a computational microscope for molecular biology. Annu Rev. Biophys. 41, 429–452 (2012). [DOI] [PubMed] [Google Scholar]
- Rosenbaum A. I. & Maxfield F. R. Niemann-pick type c disease: molecular mecha- nisms and potential therapeutic approaches. J. Neurochem. 116, 789–795 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- López C. A., de Vries A. H. & Marrink S. J. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput. Biol. 7, 1–11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D. in Handbook of lipid bilayers (CRC Press), 1174 (2013). [Google Scholar]
- Bennett W. F. et al. Molecular view of cholesterol flip-flop and chemical potential in different membrane environments. J. Am. Chem. Soc. 131, 12714–12720 (2009). [DOI] [PubMed] [Google Scholar]
- Lange Y. & Steck T. L. Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Progress Lip. Res. 47, 319–332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H. J. et al. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 106, 16645–16650 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahammad S. & Parmryd I. Cholesterol homeostasis in T cells. Methyl-beta-cyclodextrin treatment results in equal loss of cholesterol from Triton X-100 soluble and insoluble fractions. Biophys. Biochim. Acta 1778, 1251–1258 (2008). [DOI] [PubMed] [Google Scholar]
- Risselada H. J. & Marrink S. J. The molecular face of lipid rafts in model membranes. Proc. Natl. Acad. Sci. USA 105, 17367–17372 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Kutzner C., van der Spoel D. & Lindahl E. Gromacs 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008). [DOI] [PubMed] [Google Scholar]
- Lins R. D. & Hünenberger P. H. A new gromos force field for hexopyranose-based carbohydrates. J. Comput. Chem. 26, 1400–1412 (2005). [DOI] [PubMed] [Google Scholar]
- Höltje M. et al. Molecular dynamics simulations of stratum corneum lipid models: fatty acids and cholesterol. Biochim. Biophys. Acta 1511, 156–167 (2001). [DOI] [PubMed] [Google Scholar]
- Oostenbrink C., Villa A., Mark A. E. & van Gunsteren W. F. A biomolecular force field based on the free enthalpy of hydration and solvation: The gromos force-field parameter sets 53a5 and 53a6. J. Comput. Chem. 25, 1656–1676 (2004). [DOI] [PubMed] [Google Scholar]
- Berendsen H. J. C., Postma J. P. M., van Gunsteren W. F. & Hermans J. Interaction models for water in relation to protein hydration. Inter. forces 11, 331–342 (1981). [Google Scholar]
- Marrink S. J. et al. The martini force field: coarse grained model for biomolecular simulations. J. Phys. Chem. B 111, 7812–7824 (2007). [DOI] [PubMed] [Google Scholar]
- Marrink S. J. & Tieleman D. P. Perspective on the Martini model. Chem. Soc. Rev. in press, (2013). 10.1039/C3CS60093A. [DOI] [PubMed] [Google Scholar]
- López C. A. et al. Martini coarse-grained force field: Extension to carbohydrates. J. Chem. Theory. Comput. 5, 3195–3210 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information