Abstract
Objective
To estimate the cost-effectiveness of Balloon Kyphoplasty (BKP) for the treatment of patients hospitalised with acute Osteoporotic Vertebral Compression Fracture (OVCF) compared to Percutaneous Vertebroplasty (PVP) and Non-Surgical Management (NSM) in the UK.
Methods
A Markov simulation model was developed to evaluate treatment with BKP, NSM and PVP in patients with symptomatic OVCF. Data on health related quality of life (HRQoL) with acute OVCF were derived from the FREE and VERTOS II Randomised Clinical Trials (RCTs) and normalized to the NSM arm in the FREE trial. Estimated differences in mortality among the treatments and costs for NSM were obtained from the literature whereas procedure costs for BKP and PVP were obtained from three NHS hospitals. It was assumed that BKP and PVP reduced hospital length of stay by six days compared to NSM.
Results
The incremental cost-effectiveness ratio (ICER) was estimated at GBP 2,706 per QALY and GBP 15,982 per QALY compared to NSM and PVP respectively. Sensitivity analysis showed that the cost-effectiveness of BKP vs. NSM was robust when mortality and HRQoL benefits with BKP were varied. The cost-effectiveness of BKP compared to PVP was particularly sensitive to changes in the mortality benefit.
Conclusion
BKP may be a cost-effective strategy for the treatment of patients hospitalised with acute OVCF in the UK compared to NSM and PVP. Additional RCT data on the benefits of BKP and PVP compared to simulated sham-surgery and further data on the mortality benefits with BKP compared to NSM and PVP would reduce uncertainty.
Keywords: HTA, Markov, Osteoporosis, QALY, United Kingdom, Spinal Fracture
INTRODUCTION
Osteoporotic Vertebral Compression Fracture (OVCF) is a significant health problem with more than 1,400,000 vertebral fractures occurring annually in Europe [1]. Circa 30% of all morphometric fractures come to clinical attention. It has been estimated that c. 65,000 clinical OVCF occur per annum in the UK [2], of which c. 10% (ie c. 6,500) lead to hospitalisation [3,4].
Vertebral compression fracture present as sudden back pain, often followed by deformity, loss of height, and chronic pain. Even if the acute pain of an OVCF subsides, many patients will have developed irreversible spinal deformity (increased kyphosis) associated with several significant health consequences including decreased physical functioning and diminished health related quality of life (HRQoL) [5-7], increased future fracture risk [8,9], chronic back pain [10], impaired balance, and increased incidence of falls [11]. Additionally, vertebral fractures can decrease the volume of the thoracic and abdominal cavities resulting in reduced lung functioning [12-14] and gastrointestinal issues [15].
Excess mortality associated with OVCF has been well documented [16]. Mortality risk may be linked to spinal deformity and a 1.14 fold increased risk of death for each age-adjusted standard deviation (SD) increase in kyphotic angle has been reported [17].
The clinical consequences translate into significant burden to patients, the healthcare system and society. An important proportion of the burden is incurred by patients requiring hospitalisation and institutional care; the annual cost of hospital care for OVCF in Europe has been estimated at EUR 377 million, with an average cost per fracture of EUR 3,892 [18-20].
The most common first line treatment strategy for hospitalised OVCF is conservative treatment, i.e. bed rest, physiotherapy, bracing, and analgesics. Surgical intervention – Balloon Kyphoplasty (BKP) or Percutaneous Vertebroplasty (PVP) – is reserved upon failure of conservative therapy indicated by clinical symptoms and radiographic evidence. Clinical guidelines recommend that percutaneous vertebral augmentation (BKP or PVP) should be offered when non-surgical methods have not provided pain relief or where the pain is substantially impacting the patient’s lifestyle [21].
BKP and PVP are two minimally invasive surgical options, both relying on percutaneous injection of bone cement. Whereas the cement is injected directly into the fractured vertebrae in a PVP procedure, BKP utilises inflatable bone tamps to reduce the fracture, restoring vertebral anatomy, and control cement injection. Whilst both are minimally invasive surgical approaches they target different treatment outcomes: PVP aims to achieve spinal stabilisation and pain relief, whereas BKP additionally aims to correct and prevent spinal deformity. Compared to Non-Surgical Management (NSM), both BKP and PVP have been shown in different extents to result in greater pain relief, vertebral body height restoration, decreased hyperkyphosis, improved physical functioning and HRQoL [22]. In a recent review, only one prospective randomised study directly comparing BKP to PVP was found [23]. Liu et al (2010) randomly assigned 100 patients with OVCF to either BKP or PVP and followed them for six months. The main results from the study was that there was little difference between BKP and PVP in terms of pain reduction, but that BKP increased vertebral body height and reduced kyphotic wedge angle more than PVP. [24]. Meta-analysis and systematic reviews though somewhat conflicting suggest that the methods provide similar pain relief and restoration of physical function while BKP shows a lower incidence of clinically symptomatic complications and greater improvement in deformity correction. The direction and size of effect on subsequent fracture risk remains unclear [22,25,26]. Recently, a US large claims database analysis showed that BKP was associated with a significant reduction in mortality compared to both NSM and PVP [27].
BKP and PVP were shown to result in substantial gains compared to NSM in the two largest RCTs published to date. FREE [28,29] (BKP) and VERTOS II [30] (PVP) were two prospective multicenter randomised controlled clinical trials with durations of two (FREE) and one year (VERTOS II). It has been argued that the results from these trials may be skewed due to the lack of blinding after two randomised studies comparing PVP to simulated sham-surgery showed little or no benefit with PVP over simulated sham-surgery [31,32]. These results have caused considerable debate and concerns around the sham-controlled studies include the size of the trials, the amount of cement used, a high crossover rate from the sham arm to the treatment arm in one study, the use of analgesic as part of sham-surgery in one of the studies and lack of standardization of treatment [33-35]. The investigators have refuted the critique pertaining to trial design, stating that the trials were sufficiently powered for their primary objective; that participation rates were acceptable by usual trial standards; and that the cross-over occurred after measurement of primary outcome [36].
The most important difference between FREE and VERTOS II and the simulated sham-controlled studies were that the former two enrolled only acute fractures whereas the latter also enrolled choric fractures [37]. Given that percutaneous vertebral augmentation procedures appear to have better effect in acute fractures, the difference in patient selection may be the reason for the diverging results [38]. However, individual patient data meta-analysis of the two simulated sham-controlled studies found no difference between PVP and simulated sham-surgery in acute fractures [39]. A new study, VERTOS IV aims to address this issue and is currently recruiting patients. Until further evidence emerges caution is needed when interpreting the evidence from the existing studies.
Cost-effectiveness of both BKP and PVP compared to NSM has been estimated using mainly efficacy data on HRQoL from RCTs. However, to our knowledge no economic analysis has so far compared BKP, PVP and NSM.
This study is based on a re-designed version of a previously published cost-effectiveness model designed to compare BKP to NSM for the treatment of hospitalised acute OVCF in a UK setting [40]. The new model has been constructed to evaluate NSM, BKP and PVP in the principal dimensions relevant to health care policy: Costs, HRQoL and mortality. The procedures may also result in different rates of adverse events with probable important economic impact but given the dearth of available data, adverse events were not incorporated into the model. The objective of the model is to estimate the cost effectiveness of BKP compared to NSM and PVP in patients hospitalised with acute OVCF.
METHODS
Outcomes and Perspective
The economic analysis was performed from a healthcare perspective and uses Quality Adjusted Life Years (QALYs) for measuring health effects as recommended by NICE [41]. The main findings are reported as Incremental Cost-Effectiveness Ratios (ICERs). Costs reflect 2009 values and were inflated as appropriate using the UK Consumer Price Index (CPI) [42]. Both costs and effects were discounted at 3.5% reflecting NICE guidance [41].
Modelling Strategy
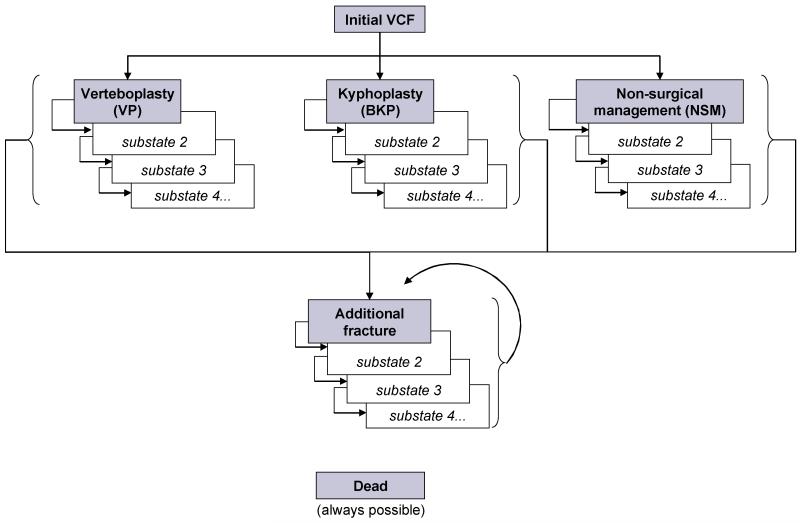
The model is a re-designed version of a recently published Markov Tunnel model with a life-time horizon and six months cycle length [40]. Tunnel Models allow for transition probabilities, costs and health utilities to reflect the actual duration of a patient’s stay in a specific health state. Patients entering the model experience an OVCF, are treated with NSM, BKP or PVP, and then placed in the first sub-state of the relevant treatment arm. In every 6 month cycle, patients may die, fracture anew, or remain free of new fracture. Patients who die move to the “dead” health state and remain there for the remainder of the simulation. Patients who fracture anew move to the first state of the additional OVCF treatment arm. It is assumed that all additional OVCF are treated with NSM. Patients already in the additional OVCF treatment arm who fracture anew move back to the first additional OVCF sub-state.
The model structure is illustrated graphically in Figure 1 below.
Figure 1. State transition diagram.
DATA
Health Related Quality of Life
A recent systematic review of prospective controlled studies comparing the efficacy and safety of BKP, PVP and NSM found four randomised studies reporting data on health utility: the FREE trial comparing BKP to NSM [28,29], the VERTOS II trial comparing PVP to NSM [30], and two studies comparing PVP to simulated sham-surgery [31,32]. Given that the present model estimates the cost-effectiveness in patients with acute fracture (inclusion criteria in FREE and VERTOS II trials) whereas the trials with simulated sham-surgery enrolled both acute and chronic fractures, health utility (EQ-5D) were derived from FREE and VERTOS II. In order to consider the evidence from the simulated sham-controlled studies, sensitivity analyses were conducted where the HRQoL gain with BKP and PVP compared to NSM varied.
In the 24-month FREE trial HRQoL data were collected at 1, 3, 6, 12 and 24 months, enabling calculation of health utilities for NSM and BKP for cycles 1 and 2. The health utility at 18-months was derived by linear interpolation between the 12 and 24 months estimates, enabling the calculation of health utilities for cycles 3 and 4. The published data from VERTOS II presented the health utility difference between PVP and NSM at baseline and QALY gains at 1 and 12 months. The 6 months QALY difference between PVP and NSM was derived by linear interpolation between the baseline and 12 month values. Given that the QALY difference at 6 and 12 months represent the gain in health utility with PVP compared to NSM for these periods, the gains were added to the health utilities observed in the NSM arm in the FREE trial to derive PVP health utilities for cycles 1 and 2. The PVP health utilities at 18 and 24 months were derived by applying the percentage change in health utility with BKP between 12 and 18 months and 12 and 24 months observed in the FREE trial to the inferred PVP health utility at 12 months. The resulting health utilities for BKP, NSM and PVP are summarised – along with other efficacy data – in Table 1.
Table 1. Effectiveness data in the model.
| BKP | PVP | NSM | |
|---|---|---|---|
| Health utility cycle 1 | 0.276 | 0.273 | 0.219 |
| Health utility cycle 2 | 0.311 | 0.309 | 0.255 |
| Health utility cycle 3 | 0.307 | 0.305 | 0.260 |
| Health utility cycle 4 | 0.307 | 0.305 | 0.265 |
| Health utility cycle 5 | 0.292 | 0.291 | 0.264 |
| Health utility cycle 6 | 0.278 | 0.277 | 0.264 |
| Health utility cycle 7 | 0.263 | 0.263 | 0.263 |
| Relative risk of death cycles 1 to 8 | 0.56 | 0.76 | 1.00 |
| Relative risk of fracture | 1.00 | 1.00 | 1.00 |
Given the availability of up to 24 months of health utility data from the trials, assumptions on persistence of effect beyond completion of the trials were made. It was assumed that the difference (compared to NSM) linearly approached zero during another 12 months (1 year offset) and that BKP and PVP had the same offset time. The resulting health utilities are presented in Table 1. After the offset period, it was assumed that patients treated with BKP and PVP had the same health utility as patients treated with NSM. The effect of the offset assumption on the results is explored in the sensitivity analysis.
In order not to overestimate the HRQoL benefits over time, the model accounts for an age-related reduction in HRQoL. The health utilities estimated from the FREE and VERTOS II trials were consequently assumed to decline at the rate observed in the general population [43,44]. This age-related decline was assumed to be the same for all patients in the model, irrespective of treatment.
It was assumed that all additional OVCFs were treated with NSM, incurring the same HRQoL implications as an NSM-treated primary fracture. Thus, irrespective of initial treatment, a patient who suffers an additional OVCF follows the HRQoL trajectory for NSM detailed in Table 1.
Mortality
Following the previous model, relative mortality after a vertebral fracture treated with NSM was derived from Swedish data as a function of sex, age at fracture; whether it was the first or a subsequent vertebral fracture; and time after fracture [40]. The relative risk of death the first cycle after the first (subsequent) fracture for 70- and 80-year old women amounted to c. 2.5 (2.9) and 1.7 (1.5) respectively and declined thereafter. Given that very few BKPs and PVPs are conducted in Sweden, the mortality after fracture pertains to NSM. Based on US Medicare data, Edidin et al published mortality hazard ratios (HRs) for BKP and PVP compared to NSM for the first four years after fracture, controlling for observable baseline differences in patients undergoing treatment with the different procedures [27]. Based on the results of this analysis, four-year mortality HRs for BKP and PVP compared to NSM of 0.56 (95% confidence interval (CI) 0.55-0.57) and 0.76 (95% CI 0.75-0.77) were incorporated in the base case scenario (Table 1). Furthermore, in a sensitivity analysis in Edidin et al, differences in mortality among the three treatments in patients who survived at least one year were analysed, resulting in 3-year mortality HRs for BKP and PVP compared to NSM of 0.76 (CI 0.74-0.77) and 0.93 (CI 0.91-0.95). These mortality HRs were modelled in a sensitivity analysis where the relative risk of death were set at 0.76 and 0.93 for BKP and PVP compared to NSM for years 2, 3 and 4.
Costs
OVCF costs for the initial fracture, excluding procedure related costs, were obtained by updating the costs used in the previous model, originally reported by Puffer et al [45] (GP and referral costs), Stevenson et al [46] (analgesics), Stevenson et al (number of bed days) [47], and Curtis [48] (cost per bed day), resulting in a total 12-month cost of GBP 7,341 comprising costs for hospitalisation, GP visits, referrals, and analgesics of GBP 6,855 (GBP 457 per bed day), GBP 115, GBP 145, and GBP 226 respectively. Materials and surgery costs for BKP and PVP were based on updated reference cost and resource use data from the three National Health Service (NHS) UK hospitals used to derive costs in Strom et al. Furthermore, it was assumed that BKP and PVP were associated with the same reduction in hospital length of stay as BKP in Strom et al (six fewer bed days during the first twelve months). The costs are presented in Table 2 below.
Table 2. Resources and costs (GBP) in the model.
| BKP | PVP | NSM | |
|---|---|---|---|
| Procedure costs | |||
| Devices | 96 | 53 | 0 |
| Consumables | 2,877 | 770 | 0 |
| Other procedure costs | |||
| Preliminary Phase | |||
| Interventional Radiologist | 0 | 107 | 0 |
| Surgeon | 107 | 0 | 0 |
| Nurse | 16 | 18 | 0 |
| Rx Spine | 77 | 77 | 0 |
| MRI | 176 | 176 | 0 |
| ECG | 68 | 68 | 0 |
| Blood Test | 21 | 21 | 0 |
| Drugs | 16 | 16 | 0 |
| Operating Phase | |||
| Anesthetist | 107 | 107 | 0 |
| Nurse – Anesthesia | 12 | 13 | 0 |
| Drugs | 38 | 22 | 0 |
| Radiologist | 0 | 107 | 0 |
| Surgeon | 107 | 0 | 0 |
| Nurse – Operation | 17 | 17 | 0 |
| Cost of operating room | 160 | 160 | 0 |
| Post operative phase | |||
| Nurse | 41 | 41 | 0 |
| Drugs | 27 | 63 | 0 |
| Total Procedure costs | 3,964 | 1,838 | 0 |
| Fracture costs | |||
| Cost GP | 115 | 115 | 115 |
| Cost referral | 145 | 145 | 145 |
| Cost analgesics | 226 | 226 | 226 |
| Total Fracture cost | 486 | 486 | 486 |
| Hospitalisation cost | |||
| Days in hospital | 9 | 9 | 15 |
| Cost per hospital day | 457 | 457 | 457 |
| Total Hospitalisation cost | 4,113 | 4,113 | 6,855 |
| Grand total costs | 8,563 | 6,437 | 7,341 |
It was assumed that additional OVCFs incurred the same costs as NSM treated primary fractures except for hospitalisation costs which were set at 35% of the hospitalisation costs for primary fractures reflecting the rate of hospitalisation for clinically apparent vertebral fractures [47].
Fracture Incidence
UK incidence for a number of fracture types were published in Singer et al. However, the study identified vertebral fractures in a traumatology department, potentially underestimating the incidence of vertebral fractures [49]. Instead, UK incidence of vertebral fractures were imputed by multiplying the incidence of hip fractures from Singer et al. with the ratio of the incidences of vertebral fractures to hip fractures observed in Sweden [50]. This approach has been validated in multiple countries [51] and was used in the previous model. Given that patients who enter the model have an acute vertebral fracture and lower BMD than the general population of the same age, the fracture risk in the model population was adjusted using previously described methods [52]. In short, to reflect the BMD status of the modelled population, the Z-score was derived from the T-score using NHANES III data [53], and thereafter the relative risk compared to the general population associated with the Z-score – derived from Marshall et al [54] – was adjusted for Jensen’s inequality. The relative risk associated with prevalent vertebral fracture compared to the general population was derived by taking the risk of a new vertebral fracture in a patient with a prevalent vertebral fracture compared to a patient without a prevalent vertebral fracture – obtained from Klotzbuecher et al [55] – and down-adjusting the risk for the prevalence of vertebral fracture in the general population. The risk was subsequently down-adjusted 10% to reflect that BMD was not accounted for in the estimate of relative risk associated with prevalent fracture. The composite relative risk was assumed to remain constant for the duration of the model.
The risk of additional OVCF was assumed to be equal among treatment arms. However, given some heterogeneity in the evidence, a sensitivity analysis where BKP was assumed to increase the risk of the first additional OVCF by 50% was run.
As in the previous model, all patients were assumed to be prescribed bisphosphonates the first five years after the initial fracture, reducing vertebral fracture risk by 40%. The yearly cost of bisphosphonates was set at GBP 14 reflecting the lowest available price for generic alendronate available in the NHS Drug Tariff [56]. Acknowledging that not all patients are compliant with treatment or receive medical therapy a sensitivity analysis was run in which patients were modelled not to take any fracture prevention medication.
Model Population
The average age (70) of the all-female population analysed in the base case was derived by weighting the average age in the FREE and VERTOS II trials. Baseline T-score was set at −3.0 reflecting the average T-score in the VERTOS II trial (no information on T-score available from the FREE trial).
RESULTS
Base Case Cost-Effectiveness
The results given the base case assumptions outlined above – 70 year old female with T-Score of −3.0 and acute OVCF requiring hospitalisation – are exhibited in Table 2. BKP was associated with incremental costs of GBP 1,345 and GBP 2,156 compared to NSM and PVP respectively. Compared to NSM and PVP, the respective QALY gains totalled 0.50 and 0.14. Thus BKP had both better outcomes and higher costs compared to NSM and PVP, resulting in incremental cost per QALY ratios of GBP 2,706 and GBP 15,982 respectively.
In the base case analysis, a substantial proportion of patients experienced an additional OVCF or died in the first few years after fracture: Within three (five) years 4.3% (7.0%), 4.4% (7.2%) and 4.4% (7.3%) treated with NSM, PVP and BKP respectively experienced an additional OVCF while 10.3% (18.5%), 7.9% (15.5%) and 5.9% (12.9%) treated with NSM, PVP and BKP respectively died.
Sensitivity Analysis
Mortality, HRQoL, relative risk of fracture with treatment, use of fracture prevention medication, costs, age, and discount rate were tested in one way sensitivity analysis – with and without the base case mortality benefit with BKP and PVP compared to NSM. Furthermore, Probabilistic Sensitivity Analysis (PSA) was undertaken to assess the joint uncertainty of mortality, HRQoL, and costs in six scenarios.
In one way sensitivity analysis of mortality, the mortality HRs observed in the Medicare claims database study was varied between 100% (full mortality reduction) to 0% (no mortality reduction) for BKP alone and for BKP and PVP jointly. The results are presented in Table 4. When BKP was compared to NSM, the ICER remained at comparatively low levels without mortality reduction. However, when BKP was compared to PVP the ICER was sensitive to reduction in the mortality benefit – both when the mortality reduction with PVP was held constant and when it was varied alongside the BKP mortality reduction. When modelling the 3-year HRs for one year survivors, the ICER increased to c. GBP 4,000 compared to NSM and to c. GBP 26,000 compared to PVP.
Table 4. Sensitivity analysis.
| ICER (GBP/QALY) | |||
|---|---|---|---|
|
| |||
| Scenarios assessing proportion of HRQoL and mortality benefit | BKP vs NSM | BKP vs PVP - PVP effect constant |
BKP vs PVP - PVP effect varies with BKP effect |
| Mortality benefit varied | |||
| 75% mortality reduction | 3,104 | 32,419 | 20,879 |
| 50% mortality reduction | 3,646 | Dominated | 29,970 |
| 25% mortality reduction | 4,431 | Dominated | 52,665 |
| No mortality reduction | 5,667 | Dominated | 210,188 |
| HRQoL benefit varied | |||
| 75% HRQoL benefit | 3,059 | 27,764 | 16,330 |
| 50% HRQoL benefit | 3,517 | 105,638 | 16,697 |
| 25% HRQoL benefit | 4,136 | Dominated | 17,084 |
| No mortality reduction - HRQoL benefit varied | |||
| 75% HRQoL benefit | 7,556 | Dominated | 278,837 |
| 50% HRQoL benefit | 11,334 | Dominated | 415,947 |
| 25% HRQoL benefit | 22,668 | Dominated | 826,858 |
| Other Scenarios | BKP vs NSM | BKP vs PVP |
|---|---|---|
| With full mortality reduction with BKP and PVP | ||
| No offset time | 2,919 | 16,249 |
| No bisphosphonate treatment | 2,764 | 16,305 |
| Increased fracture risk with BKP | 3,503 | 24,904 |
| PVP price set at 0% of BKP price | 2,706 | 29,606 |
| PVP price set at 50% of BKP price | 2,706 | 14,914 |
| PVP price set at 75% of BKP price | 2,706 | 7,568 |
| 0 days reduction with BKP and PVP in length of stay | 8,132 | 15,982 |
| 3 days reduction with BKP and PVP in length of stay | 5,419 | 15,982 |
| 9 days reduction with BKP and PVP in length of stay | Cost saving | 15,982 |
| 0% discount rate (with mortality reduction) | 2,224 | 11,922 |
| 7% discount rate (with mortality reduction) | 3,193 | 20,661 |
| 60 year old patients (with mortality reduction) | 2,912 | 19,249 |
| 80 year old patients (with mortality reduction) | 2,373 | 12,038 |
| Without mortality reduction with BKP and PVP | ||
| No offset time | 6,705 | 250,997 |
| No bisphosphonate treatment | 5,743 | 212,001 |
| Increased fracture risk with BKP | 7,904 | Dominated |
| PVP price set at 0% of BKP price | 5,667 | 391,868 |
| PVP price set at 50% of BKP price | 5,667 | 195,939 |
| PVP price set at 75% of BKP price | 5,667 | 97,969 |
| 0 days reduction with BKP and PVP in length of stay | 17,557 | 210,188 |
| 3 days reduction with BKP and PVP in length of stay | 11,612 | 210,188 |
| 9 days reduction with BKP and PVP in length of stay | Dominating | 210,188 |
| 0% Discount rate | 5,392 | 203,712 |
| 7% Discount rate | 5,949 | 216,711 |
| 60 year old patients | 5,507 | 206,515 |
| 80 year old patients | 5,905 | 215,739 |
In one way sensitivity analysis of the HRQoL benefit, the health utility benefit with BKP and PVP compared to NSM in the base case scenario was varied between 100% (full health utility benefit) and 25% (a quarter of the health utility benefit) for BKP alone and for BKP and PVP jointly. The results are presented in Table 4. When BKP was compared to NSM, the ICER remained at comparatively low levels with a quarter of the health utility benefit. When BKP was compared to PVP the ICER was sensitive to reduction in the health utility benefit when the benefit with PVP was held constant, but not when it was varied alongside the BKP health utility benefit. The impact of the health utility gain in the absence of any mortality reduction was also assessed. When BKP was compared to NSM, without any mortality reduction and 25% of the base case health utility gain was modelled, the ICER stood at c. GBP 23,000 per QALY.
Another uncertainty around the HRQoL effect of treatment is the duration of the benefit compared to NSM. The FREE and VERTOS II trials show that the benefits of BKP and PVP persist for at least two and one years respectively. Given the similarity in results during the first year in the FREE and VERTOS II trials, it was assumed that PVP had the same proportional benefit in year two relative to year one as BKP exhibited in the FREE trial. Furthermore, it was assumed that both treatments had a one year offset. Longer offset time favours BKP and PVP over NSM and BKP over PVP (results not shown). Given the base case settings, removal of offset time did not impact the ICER substantially compared to NSM, even in the absence of any mortality reduction (Table 4).
In the base case, it was assumed that patients took medication reducing the risk of another vertebral fracture by 40% for five years. When including the base case mortality reduction and patients were modelled not to take such medication, the BKP vs. NSM ICER increased to c. 2,800 and the BKP vs. PVP ICER increased to c. 16,300. In the scenario where BKP was assumed to increase the risk of the first additional OVCF by 50%, the BKP vs. NSM ICER increased to c. 3,500 and the BKP vs. PVP ICER increased to c. 25,000 (Table 4).
It was assumed that BKP (and PVP) required six fewer hospital days than NSM. Given the base case inputs, when the number of avoided hospital days in the BKP vs. NSM comparison was varied, the ICER with zero avoided hospital days amounted to c. GBP 8,000 and BKP became cost saving from nine avoided hospital days. In the BKP vs. PVP comparison, where no difference in avoided hospital days was assumed, the costs differences were driven by the procedure cost. When the procedure cost of PVP was set at 0%, 50% and 75% to that of BKP the resulting ICERs (BKP vs. PVP) stood at c. GBP 30,000, c. GBP 15,000 and c. GBP 8,000 (Table 4).
Neither age (+/− 10 years), nor discount rate (+/−3.5 ppts) materially impacted the cost-effectiveness results (Table 4).
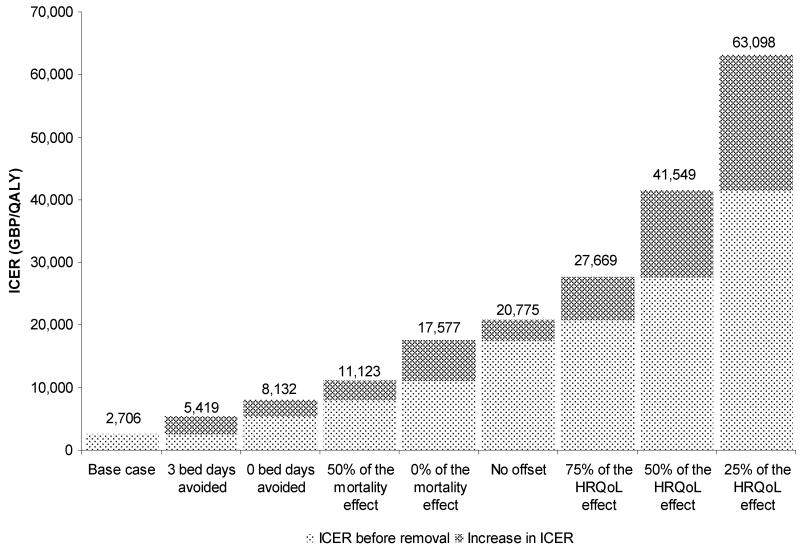
In the context of this model, the benefits of BKP compared to NSM are threefold: Reduced length of stay, improved HRQoL, and reduced mortality. A bridge analysis was conducted to determine the impact on the ICER when benefits were removed sequentially (Figure 2). When disregarding any benefits with BKP compared to NSM beyond those observed in the FREE RCT; i.e. no HRQoL offset time, no mortality reduction and no reduction in hospital length of stay, the ICER stood at c. GBP 21,000.
Figure 2. Impact of removal of BKP benefits on the BKP vs. NSM ICER (GBP/QALY).
Probabilistic Sensitivity Analysis
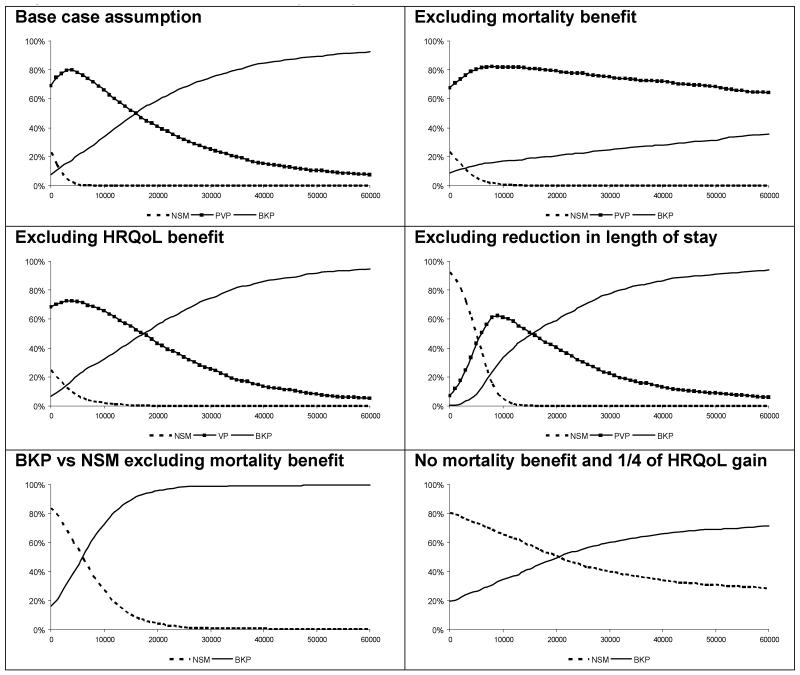
PSA was conducted in order to represent the statistical uncertainty around the model inputs. For HRQoL benefits with BKP, distributions of the gains observed in the FREE trial at 0-6 and 7-12 months were generated using bootstrapping techniques and applied to the mean health utility observed in respective time periods. For months 13-36, it was assumed that the relationship between the mean and the uncertainty observed in months 7-12 remained constant. For PVP the relationship between the mean and uncertainty observed with BKP was used. For the uncertainty of the mortality reduction, the confidence intervals around the mortality HRs with BKP and PVP compared to NSM observed in Edidin et al were used. Reflecting the previous model, reduction in length of hospital stay associated with BKP (and PVP) compared to NSM was assumed to be normally distributed with the standard error conservatively set at 50% of the mean (i.e., 3 days). It is important to note that PSA measures the effect of the joint parameter uncertainty around the input variables and does not consider other types of uncertainty. In order to reflect such uncertainty, six PSA scenarios were run: One with the base case assumptions detailed above; one where the mortality benefits with BKP and PVP were removed; one where the HRQoL benefits with BKP and PVP were removed; one where reductions of length of stay with BKP and PVP were removed; one in which BKP was compared to NSM alone and where the mortality benefits with BKP were removed; and one in which BKP was compared to NSM alone and where the mortality and three quarters of the health utility benefits with BKP were removed.
PSA (1,000 simulations) was conducted. The acceptability curves presented in Figure 3 show the proportion of simulations which fell below given values of Willingness To Pay (WTP) for an incremental QALY for each modelled treatment in the six scenarios. The PSA indicated that, given the base case inputs, BKP had a probability of c 60% to be the optimal intervention at a WTP threshold of GBP 20,000 and a probability of c. 75% at a WTP threshold of GBP 30,000 per QALY. Three of the remaining five scenarios produced similar results. However, in the scenario where mortality reduction was removed, PVP had the highest probability of being the optimal intervention given generally accepted cost-effectiveness thresholds. Furthermore, in the scenario where BKP was compared to NSM alone and where the mortality and three quarters of the health utility benefits with BKP from the base case were removed, there was considerable uncertainty around the median ICER of c. GBP 23,000. Across the six scenarios, the maximum difference between the deterministic and mean probabilistic ICERs was 6%.
Figure 3. Probabilistic sensitivity analyses.
DISCUSSION
This study indicates that BKP may be a cost-effective intervention for the treatment of acute hospitalised OVCF compared to NSM and PVP in a UK setting. The base case ICERs of BKP compared to NSM and PVP of GBP 2,706 and GBP 15,982 respectively fall below the willingness-to-pay (WTP) threshold range of GBP 20,000-30,000 per QALY gained. In addition, the PSA showed that, given the base case settings, BKP had a 60-75% probability to be the optimal intervention given a WTP threshold range of GBP 20,000-30,000. Furthermore, the small variation between the probabilistic and deterministic ICERs suggests that the model is reasonably linear and therefore that the deterministic result is a reliable estimates of the true ICER. Sensitivity analysis showed that the cost-effectiveness of BKP vs. NSM was robust when either HRQoL or mortality benefits with BKP were set at low levels compared to the base case. Given the model framework, should no HRQoL or mortality benefit with BKP compared to NSM be modelled, the decision problem would become one of cost-minimisation and the resulting recommendation would be that the least expensive option, ie NSM, should be implemented. The cost-effectiveness of BKP compared to PVP was particularly sensitive to changes in the mortality benefit.
The aim of decision analytical modelling is to synthesise the best available evidence to estimate the cost-effectiveness of an intervention. Subsequently, the results are sensitised to reflect uncertainty. Whilst we have strived to do this, two important caveats exist: The mortality reduction with BKP and PVP were not obtained from a randomised trial and the HRQoL benefits with BKP and PVP were obtained from NSM-rather than simulated sham-controlled trials. The two caveats are discussed in turn below.
The incorporation of mortality reduction from a US retrospective study in a UK cost-effectiveness model necessitates caution when interpreting the model results. Firstly, the retrospective nature of the data means that there may be residual confounding. Secondly, BKP is mainly conducted as an inpatient procedure in the UK whereas it is conducted in both inpatient and outpatient settings in the US. Thirdly, the characteristics of patients undergoing the procedures in the two countries may differ. However, little information on the patients treated with the procedure in the UK exists, making comparisons between characteristics of patients treated in the UK and the US difficult. Nevertheless, the available evidence supports a mortality reduction with BKP compared to NSM and PVP, and a plausible clinical argument to the source of the effect exists. Concerns with mortality reduction were addressed in sensitivity analyses by varying the magnitude of mortality reductions (from 100% of the reductions observed in the US retrospective study to 0%) and modelling the 3-year mortality HRs for one year survivors. Whilst, the cost-effectiveness of BKP vs. NSM was robust with an ICER of c. GBP 6,000 in the absence of any mortality reduction; the cost-effectiveness of BKP vs. PVP was sensitive to the modelled mortality reduction, with the ICER increasing from c. GBP 16,000 with the full mortality reduction to c. GBP 210,000 in the absence of any mortality reduction with BKP or PVP.
The HRQoL effects modelled were obtained from trials comparing BKP and PVP to NSM rather than to simulated sham-surgery, potentially raising the concern that the observed effects were placebo-driven. Indeed, two randomised trials with simulated sham-controls indicated that PVP and sham-surgery were equally effective [31,32]. These results have caused considerable debate [23]. The most important difference between FREE and VERTOS II and the simulated sham-controlled studies were that both acute and chronic fractures were included in the sham-controlled studies whereas the NSM controlled studies only enrolled acute fractures [37]. However, a recent individual patient data meta-analysis of the two simulated sham-controlled trials found no advantage of PVP over simulated sham surgery for patients with recent onset (acute) fracture (≤ 6 weeks) or severe pain (VAS ≥ 8). It should be noted that 24 participants were reported to be needed in each treatment group to show a 2.5 unit advantage in pain score (assuming a SD of 3.0, significance level of 5%, and 80% power); and while 25 of 106 PVP patients in the meta-analysis had onset of pain before 6 weeks, it is not known if all these patients had severe pain at baseline, or if they were a mix of patients with mild, moderate or severe pain. Furthermore, at one month a trend towards a higher proportion of the PVP group achieving at least 30% improvement in pain scores was observed (relative risk 1.32, 0.98-1.76, P=0.07) [39]. Given that the cost-effectiveness analysis pertained to acute hospitalised fractures and the heterogeneity in the sham trials patient populations, we chose to omit the sham-controlled trials from the base case analysis and considered their evidence in sensitivity analysis. Solely based on the evidence from the simulated sham-controlled studies, PVP (and potentially BKP) is no more effective than sham-procedure in improving outcomes after OVCF, and would not be cost-effective compared to sham-intervention if it was associated with higher costs. Once results from the VERTOS IV trial are reported, the relative merits of PVP and sham-intervention may be clearer, allowing for improved cost-effectiveness analysis.
The HRQoL data for BKP and PVP were derived from separate trials using NSM as a common comparator. Whilst most baseline characteristics in the two trials were reasonably similar, the baseline health utility (measured with EQ-5D) differed (0.17 in FREE and 0.33 in VERTOS II) indicating the existence of differences in the trial populations which potentially render the results difficult to compare. One potential source for this discrepancy is that the UK tariff was used to transform EQ-5D scores to health utilities in the FREE trial and the Dutch tariff was used for the same purpose in the VERTOS II trial.
Since UK costs associated with the treatments were not published in either the FREE or the VERTOS II trials, costs had to be obtained elsewhere. Costs for NSM-treated hospitalised fractures were obtained from the literature; BKP and PVP procedure costs were obtained from three NHS hospitals; and it was assumed that BKP and PVP were associated with a net reduction in hospital length of stay of six days compared to NSM. The reason for considering hospitalised fractures are twofold: Firstly, the FREE trial – providing heath utility data for NSM and BKP – was conducted on hospitalised patients and it is not evident that the health utility gains would be the same in an outpatient setting. Secondly, BKP is predominately an inpatient procedure in the UK. Whilst several studies supports a reduction in hospital length of stay of six days or more for hospitalised fractures [57-60], a recent Austrian study indicates that the reduction may be shorter.
When comparing the base case results to the previous UK study, costs remain similar but QALYs increase in both the BKP and the NSM arm. Two main reasons for the difference in QALYs exist. Firstly, the present study draws on two year data from the FREE trial whereas the previous study used the one year data. Secondly, the cohort in the current model consists solely of women whereas the cohort in the previous model included 23% men, resulting in increased longevity in the current study. Furthermore, in the BKP arm the increase in the QALY gain also reflect the modelled mortality reduction.
An inherent limitation of this study is that data from multiple sources with varying degrees of validity were used. Decision analytical modelling aims to bring together the best quality data available to allow estimation of the cost effectiveness of an intervention. Nevertheless, as detailed above uncertainty exists for the comparison of the treatments, especially acknowledging that no RCTs have incorporated all three treatments and compared those to sham-intervention and that the modelled mortality benefit with BKP comes from retrospective analysis.
Another limitation is that the comparisons of BKP to NSM and PVP are conducted on a hypothetical average patient. It may very well be that specific patient segments have better response to one of the procedures than the others. Whilst the difference in baseline HRQoL between the FREE and VERTOS II noted above stem from trial settings, it may reflect clinical practice: A recent medical practice survey [61] and another observation [62], suggest that BKP is potentially reserved for acute and more debilitating OVCF while PVP is more generally used for symptomatic, chronic fractures. Other factors that may be important include age at fracture, degree of height loss, deformity, severity and ability of the patient to lie in the prone position to complete one of the minimally invasive procedures. A recent study on a cohort of 91 patients with vertebral compression fracture, found that one third of patients technically suitable patients for PVP responded beneficially to local anaesthetic and steroid facet joint injection for very short term pain relief – 2 weeks – suggesting that those patients suffer from pain arising from paravertebral structures rather than the vertebral fracture itself and may not benefit from percutaneous injection of bone cement to the same extent, an hypothesis which partially may explain the findings from the trials comparing PVP to simulated sham surgery [63,64].
In summary, the results from the study indicate that BKP has a 60 to 75% probability of being a cost-effective intervention compared to NSM and PVP for acute hospitalised OVCFs in a UK setting with uncertainty predominately coming from the lack of sham-control in the FREE and VERTOS II studies and the non-randomised nature of the data underlying the mortality differences between the treatments. More research is needed to increase model validity and identify the patient segments who respond better to specific treatments: specifically, standardised assessment of pain and a large randomised controlled clinical trial incorporating BKP, PVP, NSM with adequately simulated sham-surgery would be useful. However, further data are also needed on down-stream fracture risk, adverse events, mortality, long-term HRQoL consequences and both inpatient and outpatient resource utilisation. Once such data are available, further cost-effectiveness analysis should be conducted.
Table 3. Base case results.
| NSM | PVP | BKP | |
|---|---|---|---|
| Costs in treatment tunnel | |||
| Procedure Cost | 0 | 1,838 | 3,964 |
| Cost GP | 113 | 113 | 113 |
| Cost referral | 142 | 142 | 143 |
| Cost analgesics | 221 | 222 | 222 |
| Cost hospitalisation | 6,712 | 4,036 | 4,043 |
| Cost bisphosphonates | 60 | 61 | 62 |
| Total costs treatment tunnel | 7,247 | 6,412 | 8,547 |
| Costs in additional OVCF tunnel | |||
| Costs GP | 29 | 30 | 31 |
| Cost referral | 36 | 37 | 39 |
| Cost analgesics | 57 | 58 | 60 |
| Cost hospitalisation | 600 | 620 | 637 |
| Total cost additional OVCF tunnel | 722 | 746 | 766 |
| Total costs | 7,969 | 7,157 | 9,313 |
| QALYs in treatment tunnel | 4.318 | 4.659 | 4.776 |
| QALYs in additional OVCF tunnels | 0.658 | 0.679 | 0.697 |
| Total QALYs | 4.976 | 5.338 | 5.473 |
| BKP cost / QALY gained vs. | 2,706 | 15,982 |
Note: Costs in GBP
Acknowledgments
AS has received funding from several companies involved in the marketing of products for treatment of osteoporosis. LA is an employee in the Spine & Biologics unit of Medtronic International Trading SARL. CC has received consultancy/advisory committee fees from Servier, Proctor and Gamble/Alliance, Eli Lilly, Merck, Sharp and Dohme, GSK/Roche, Amgen, Novartis and Medtronic. DM has received consultancy/advisory committee fees from Amgen, Novartis, Servier, Striker Biotech and Medtronic. OS has received funding from several companies involved in the marketing of products for treatment of osteoporosis.
Footnotes
Disclosure of Conflicts of Interest:
Financial support was obtained via an unrestricted grant from Medtronic.
Reference List
- 1.Incidence of vertebral fracture in europe: results from the European Prospective Osteoporosis Study (EPOS) J Bone Miner Res. 2002;17:716–24. doi: 10.1359/jbmr.2002.17.4.716. [DOI] [PubMed] [Google Scholar]
- 2.Strom O, Borgstrom F, Kanis JA, Compston J, Cooper C, McKloskey EV, Jonsson B. Osteoporosis; Burden, health care provision and opportunities in the EU - A report prepared in collaboration with the International Osteoporosis Foundation (IOF), the European Federation of Pharmaceutical Industry Associations (EFPIA), and Amgen. 2011. [DOI] [PubMed] [Google Scholar]
- 3.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ., III Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985-1989. J Bone Miner Res. 1992;7:221–27. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 4.Gehlbach SH, Bigelow C, Heimisdottir M, May S, Walker M, Kirkwood JR. Recognition of vertebral fracture in a clinical setting. Osteoporos Int. 2000;11:577–82. doi: 10.1007/s001980070078. [DOI] [PubMed] [Google Scholar]
- 5.Hallberg I, Rosenqvist AM, Kartous L, Lofman O, Wahlstrom O, Toss G. Health-related quality of life after osteoporotic fractures. Osteoporos Int. 2004;15:834–41. doi: 10.1007/s00198-004-1622-5. [DOI] [PubMed] [Google Scholar]
- 6.Kado DM, Huang MH, Nguyen CB, Barrett-Connor E, Greendale GA. Hyperkyphotic posture and risk of injurious falls in older persons: the Rancho Bernardo Study. J Gerontol A Biol Sci Med Sci. 2007;62:652–57. doi: 10.1093/gerona/62.6.652. [DOI] [PubMed] [Google Scholar]
- 7.Silverman SL, Minshall ME, Shen W, Harper KD, Xie S. The relationship of health-related quality of life to prevalent and incident vertebral fractures in postmenopausal women with osteoporosis: results from the Multiple Outcomes of Raloxifene Evaluation Study. Arthritis Rheum. 2001;44:2611–19. doi: 10.1002/1529-0131(200111)44:11<2611::aid-art441>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 8.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, Stracke H, Seeman E. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay R, Burge RT, Strauss DM. One year outcomes and costs following a vertebral fracture. Osteoporos Int. 2005;16:78–85. doi: 10.1007/s00198-004-1646-x. [DOI] [PubMed] [Google Scholar]
- 10.Nevitt MC, Ettinger B, Black DM, Stone K, Jamal SA, Ensrud K, Segal M, Genant HK, Cummings SR. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128:793–800. doi: 10.7326/0003-4819-128-10-199805150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR. Balance disorder and increased risk of falls in osteoporosis and kyphosis: significance of kyphotic posture and muscle strength. Osteoporos Int. 2005;16:1004–10. doi: 10.1007/s00198-004-1791-2. [DOI] [PubMed] [Google Scholar]
- 12.Culham EG, Jimenez HA, King CE. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine (Phila Pa 1976) 1994;19:1250–1255. doi: 10.1097/00007632-199405310-00010. [DOI] [PubMed] [Google Scholar]
- 13.Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. 1990;141:68–71. doi: 10.1164/ajrccm/141.1.68. [DOI] [PubMed] [Google Scholar]
- 14.Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, Ziegler R, Leidig-Bruckner G. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261–67. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 15.Miyakoshi N, Kasukawa Y, Sasaki H, Kamo K, Shimada Y. Impact of spinal kyphosis on gastroesophageal reflux disease symptoms in patients with osteoporosis. Osteoporos Int. 2009;20:1193–98. doi: 10.1007/s00198-008-0777-x. [DOI] [PubMed] [Google Scholar]
- 16.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR, Study of Osteoporotic Fractures Research Group Vertebral fractures and mortality in older women: a prospective study. Arch Intern Med. 1999;159:1215–20. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 17.Kado DM, Lui LY, Ensrud KE, Fink HA, Karlamangla AS, Cummings SR. Hyperkyphosis predicts mortality independent of vertebral osteoporosis in older women. Ann Intern Med. 2009;150:681–87. doi: 10.7326/0003-4819-150-10-200905190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borgstrom F, Zethraeus N, Johnell O, Lidgren L, Ponzer S, Svensson O, Abdon P, Ornstein E, Lunsjo K, Thorngren KG, Sernbo I, Rehnberg C, Jonsson B. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int. 2006;17:637–50. doi: 10.1007/s00198-005-0015-8. [DOI] [PubMed] [Google Scholar]
- 19.Finnern HW, Sykes DP. The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int. 2003;14:429–36. doi: 10.1007/s00198-003-1395-2. [DOI] [PubMed] [Google Scholar]
- 20.Gehlbach SH, Burge RT, Puleo E, Klar J. Hospital care of osteoporosis-related vertebral fractures. Osteoporos Int. 2003;14:53–60. doi: 10.1007/s00198-002-1313-z. [DOI] [PubMed] [Google Scholar]
- 21.Jensen ME, McGraw JK, Cardella JF, Hirsch JA. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the American Society of Interventional and Therapeutic Neuroradiology, Society of Interventional Radiology, American Association of Neurological Surgeons/Congress of Neurological Surgeons, and American Society of Spine Radiology. J Vasc Interv Radiol. 2007;18:325–30. doi: 10.1016/j.jvir.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Taylor RS, Fritzell P, Taylor RJ. Balloon kyphoplasty in the management of vertebral compression fractures: an updated systematic review and meta-analysis. Eur Spine J. 2007;16:1085–100. doi: 10.1007/s00586-007-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boonen S, Wahl DA, Nauroy L, Brandi ML, Bouxsein ML, Goldhahn J, Lewiecki EM, Lyritis GP, Marsh D, Obrant K, Silverman S, Siris E, Akesson K. Balloon kyphoplasty and vertebroplasty in the management of vertebral compression fractures. Osteoporos Int. 2011;22:2915–34. doi: 10.1007/s00198-011-1639-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, Lin TB. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21:359–64. doi: 10.1007/s00198-009-0952-8. [DOI] [PubMed] [Google Scholar]
- 25.Eck JC, Nachtigall D, Humphreys SC, Hodges SD. Comparison of vertebroplasty and balloon kyphoplasty for treatment of vertebral compression fractures: a meta-analysis of the literature. Spine J. 2008;8:488–97. doi: 10.1016/j.spinee.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Felder-Puig R, Piso B, Guba B, Gartlehner G. Kyphoplasty and vertebroplasty for the management of osteoporotic vertebral compression fractures: a systematic review. Orthopade. 2009;38:606–15. doi: 10.1007/s00132-009-1446-2. [DOI] [PubMed] [Google Scholar]
- 27.Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and non-operated vertebral fracture patients in the medicare population. J Bone Miner Res. 2011 doi: 10.1002/jbmr.353. [DOI] [PubMed] [Google Scholar]
- 28.Boonen S, Van MJ, Bastian L, Cummings SR, Ranstam J, Tillman JB, Eastell R, Talmadge K, Wardlaw D. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res. 2011 doi: 10.1002/jbmr.364. [DOI] [PubMed] [Google Scholar]
- 29.Wardlaw D, Cummings SR, Van MJ, Bastian L, Tillman JB, Ranstam J, Eastell R, Shabe P, Talmadge K, Boonen S. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016–24. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 30.Klazen CA, Lohle PN, de VJ, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, van Rooij WJ, Schoemaker MC, Juttmann JR, Lo TH, Verhaar HJ, van der Graaf Y, van Everdingen KJ, Muller AF, Elgersma OE, Halkema DR, Fransen H, Janssens X, Buskens E, Mali WP. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet. 2010;376:1085–92. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 31.Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, Graves S, Staples MP, Murphy B. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361:557–68. doi: 10.1056/NEJMoa0900429. [DOI] [PubMed] [Google Scholar]
- 32.Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, Edwards R, Gray LA, Stout L, Owen S, Hollingworth W, Ghdoke B, nnesley-Williams DJ, Ralston SH, Jarvik JG. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gangi A, Clark WA. Have recent vertebroplasty trials changed the indications for vertebroplasty? Cardiovasc Intervent Radiol. 2010;33:677–80. doi: 10.1007/s00270-010-9901-3. [DOI] [PubMed] [Google Scholar]
- 34.Baerlocher MO, Munk PL, Liu DM, Tomlinson G, Badii M, Kee ST, Loh CT, Hardy BW, Murphy KJ. Clinical utility of vertebroplasty: need for better evidence. Radiology. 2010;255:669–74. doi: 10.1148/radiol.10092107. [DOI] [PubMed] [Google Scholar]
- 35.Clark W, Goh AC. Vertebroplasty for acute osteoporotic spinal fractures-best evidence? J Vasc Interv Radiol. 2010;21:1330–1333. doi: 10.1016/j.jvir.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Kallmes DF, Jarvik JG, Osborne RH, Comstock BA, Staples MP, Heagerty PJ, Turner JA, Buchbinder R. Clinical utility of vertebroplasty: elevating the evidence. Radiology. 2010;255:675–80. doi: 10.1148/radiol.10100425. [DOI] [PubMed] [Google Scholar]
- 37.Firanescu C, Lohle PN, de VJ, Klazen CA, Juttmann JR, Clark W, van Rooij WJ. A randomised sham controlled trial of vertebroplasty for painful acute osteoporotic vertebral fractures (VERTOS IV) Trials. 2011;12:93. doi: 10.1186/1745-6215-12-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark WA, Burnes JP, Lyon SM. Vertebroplasty for acute osteoporotic fractures - position statement from the Interventional Radiology Society of Australasia. J Med Imaging Radiat Oncol. 2011;55:1–3. doi: 10.1111/j.1754-9485.2010.02237.x. [DOI] [PubMed] [Google Scholar]
- 39.Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Heagerty PJ, Buchbinder R. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ. 2011;343:d3952. doi: 10.1136/bmj.d3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strom O, Leonard C, Marsh D, Cooper C. Cost-effectiveness of balloon kyphoplasty in patients with symptomatic vertebral compression fractures in a UK setting. Osteoporos Int. 2009 doi: 10.1007/s00198-009-1096-6. [DOI] [PubMed] [Google Scholar]
- 41.NICE . Technology Appraisal Guide. 2008. Guide to the methods of technology appraisal. [Google Scholar]
- 42.OECD . Consumer Prices - Annual inflation. 2011. [Google Scholar]
- 43.Ara R, Tumur I, Pandor A, Duenas A, Williams R, Wilkinson A, Paisley S, Chilcott J. Ezetimibe for the treatment of hypercholesterolaemia: a systematic review and economic evaluation. Health Technol Assess. 2008;12:iii, xi–iii, 212. doi: 10.3310/hta12210. [DOI] [PubMed] [Google Scholar]
- 44.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316:736–41. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puffer S, Torgerson DJ, Sykes D, Brown P, Cooper C. Health care costs of women with symptomatic vertebral fractures. Bone. 2004;35:383–86. doi: 10.1016/j.bone.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 46.Stevenson M, Davis S. Analyses of the cost-effectiveness of pooled alendronate and risedronate, compared with strontium ranelate, raloxifene, etidronate and teriparatide. 2006. [Google Scholar]
- 47.Stevenson MD, Davis SE, Kanis JA. The hospitalisation costs and out-patient costs of fragility fractures. Women-s Health medicine. 2006;3:145–89. [Google Scholar]
- 48.Curtis L. Unit Costs of Health and Social Care. 2008. [Google Scholar]
- 49.Singer BR, McLauchlan GJ, Robinson CM, Christie J. Epidemiology of fractures in 15,000 adults: the influence of age and gender. J Bone Joint Surg Br. 1998;80:243–48. doi: 10.1302/0301-620x.80b2.7762. [DOI] [PubMed] [Google Scholar]
- 50.Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–74. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 51.Borgstrom F, Strom O, Kleman M, McCloskey E, Johansson H, Oden A, Kanis JA. Cost-effectiveness of bazedoxifene incorporating the FRAX(R) algorithm in a European perspective. Osteoporos Int. 2011;22:955–65. doi: 10.1007/s00198-010-1291-5. [DOI] [PubMed] [Google Scholar]
- 52.Borgstrom F, Jonsson B, Strom O, Kanis JA. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int. 2006;17:1781–93. doi: 10.1007/s00198-006-0193-z. [DOI] [PubMed] [Google Scholar]
- 53.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Jr., Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 54.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–59. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 56.NHS Prescription Services . Drug Tariff. 2011. [Google Scholar]
- 57.Atalay B, Caner H, Gokce C, Altinors N. Kyphoplasty: 2 years of experience in a neurosurgery department. Surg Neurol. 2005;64(Suppl 2):S72–S76. doi: 10.1016/j.surneu.2005.07.039. [DOI] [PubMed] [Google Scholar]
- 58.Dudeney S, Lieberman IH, Reinhardt MK, Hussein M. Kyphoplasty in the treatment of osteolytic vertebral compression fractures as a result of multiple myeloma. J Clin Oncol. 2002;20:2382–87. doi: 10.1200/JCO.2002.09.097. [DOI] [PubMed] [Google Scholar]
- 59.Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila Pa 1976) 2006;31:57–64. doi: 10.1097/01.brs.0000192687.07392.f1. [DOI] [PubMed] [Google Scholar]
- 60.Lieberman IH, Dudeney S, Reinhardt MK, Bell G. Initial outcome and efficacy of “kyphoplasty” in the treatment of painful osteoporotic vertebral compression fractures. Spine (Phila Pa 1976) 2001;26:1631–38. doi: 10.1097/00007632-200107150-00026. [DOI] [PubMed] [Google Scholar]
- 61.Rollinghoff M, Zarghooni K, Schluter-Brust K, Sobottke R, Schlegel U, Eysel P, Delank KS. Indications and contraindications for vertebroplasty and kyphoplasty. Arch Orthop Trauma Surg. 2010 doi: 10.1007/s00402-010-1083-6. [DOI] [PubMed] [Google Scholar]
- 62.Strom O, Bianchi ML, Dimai HP, Lekander I, Svedbom A, Thoamas T, Borgstron F. Characteristics of patients with vertebral fractures stratified by treatment type (non-surgical management, balloon kyphoplasty and vertebroplasty)—results from the Austrian, French and Italian ICUROS substudies. Osteoporos Int. 2011:22. [Google Scholar]
- 63.Wilson DJ, Owen S, Corkill RA. Facet joint injections as a means of reducing the need for vertebroplasty in insufficiency fractures of the spine. Eur Radiol. 2011;21:1772–78. doi: 10.1007/s00330-011-2115-5. [DOI] [PubMed] [Google Scholar]
- 64.Wilson DJ. Vertebroplasty for vertebral fracture. BMJ. 2011;343:d3470. doi: 10.1136/bmj.d3470. [DOI] [PubMed] [Google Scholar]