Abstract
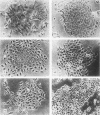
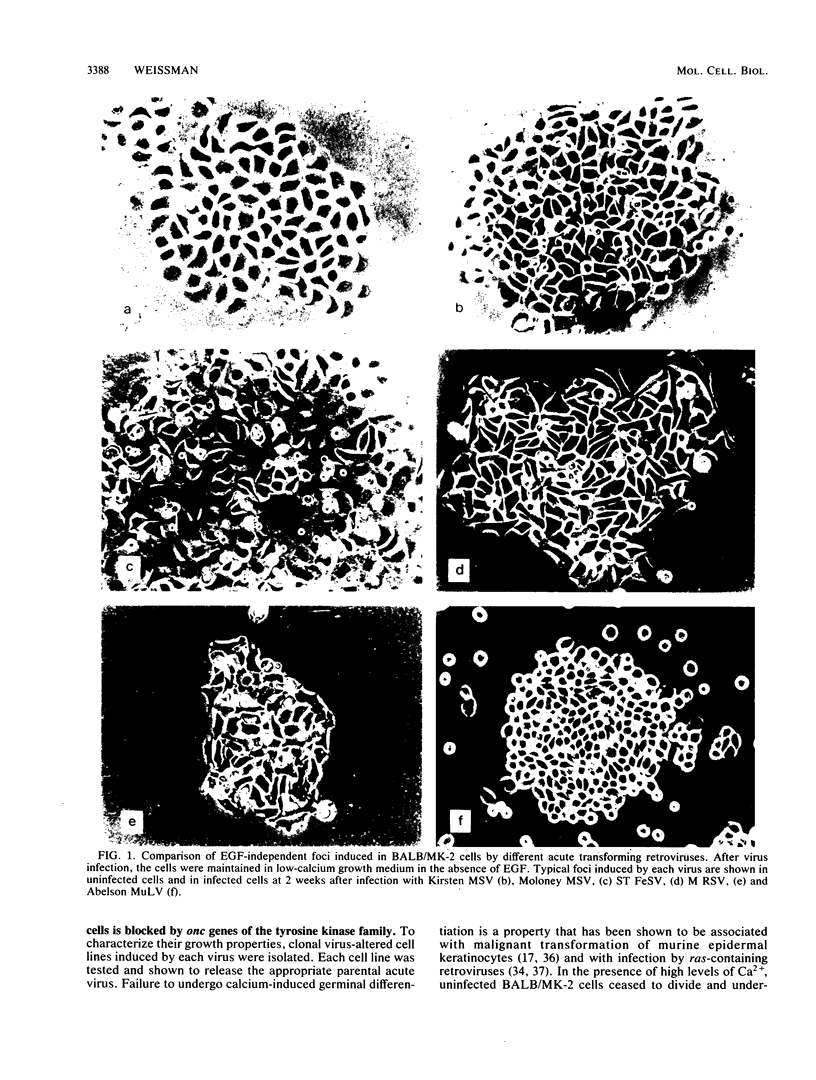
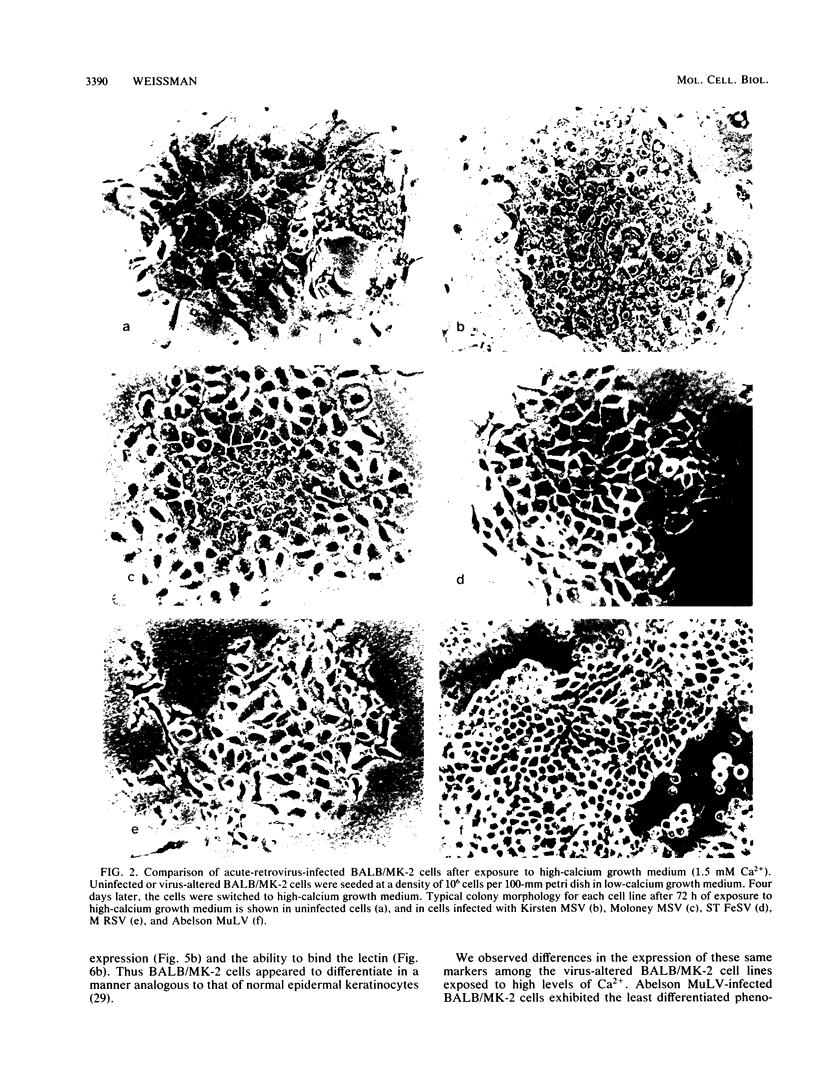
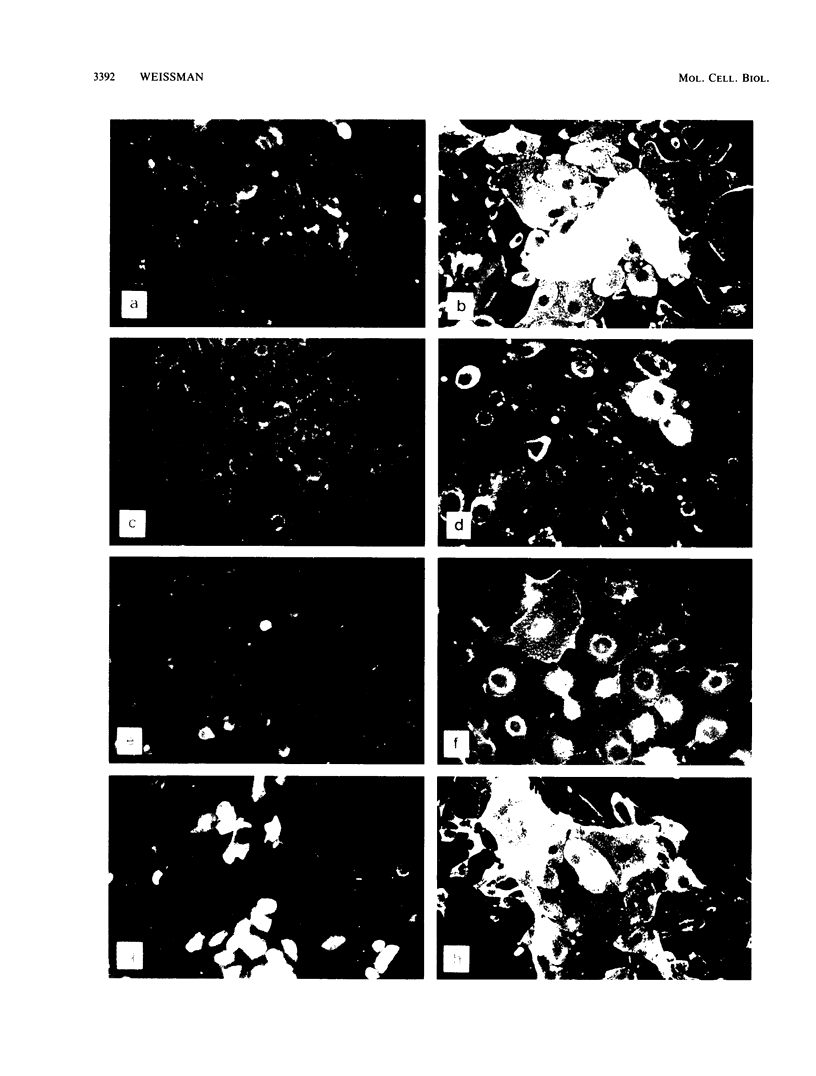
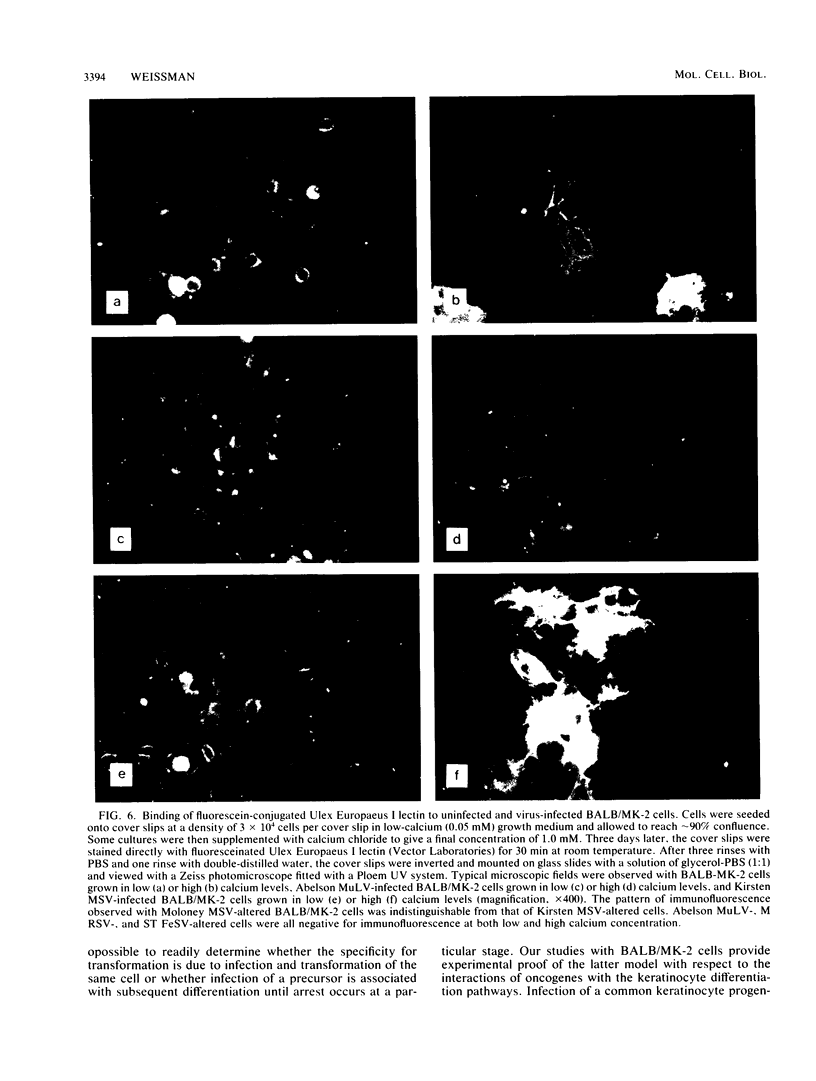
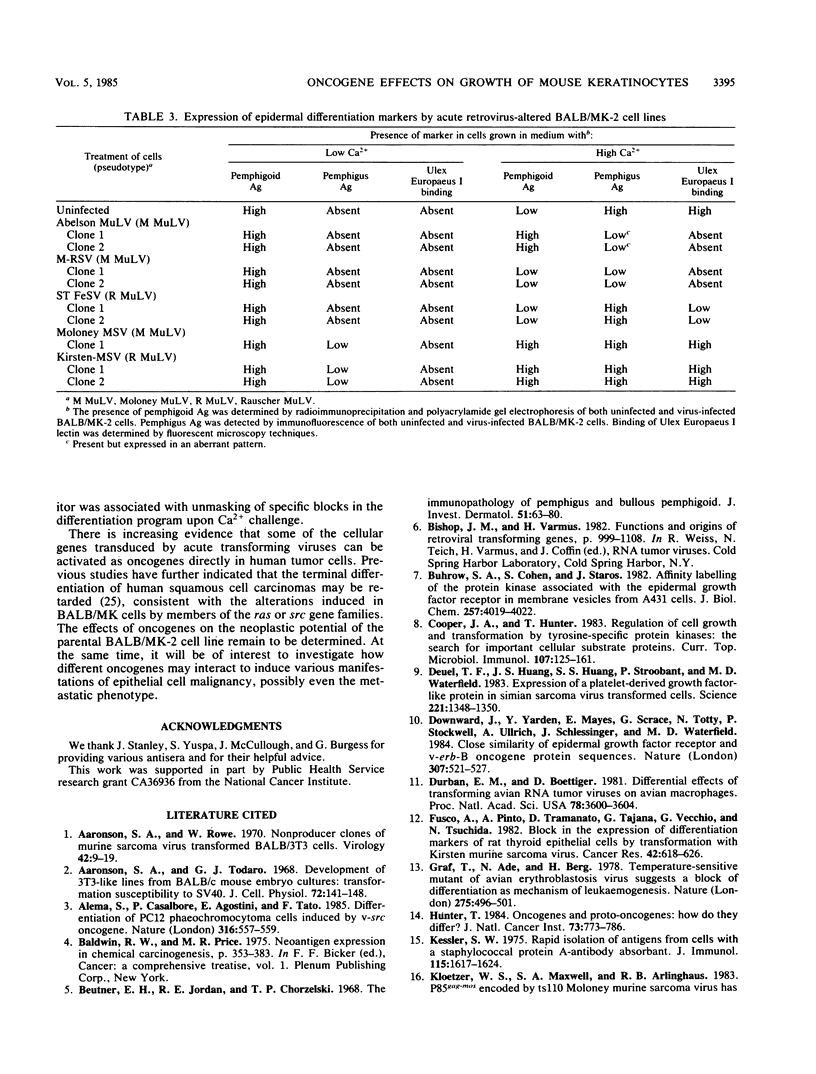
BALB-/MK-2 mouse epidermal keratinocytes required epidermal growth factor for proliferation and terminally differentiated in response to high Ca2+ concentration. Infection with retroviruses containing transforming genes of the src and ras oncogene families led to rapid loss of epidermal growth factor dependence, in some cases, accompanied by alterations in cellular morphology. The virus-altered cells continued to proliferate in the presence of high levels of extracellular calcium but exhibited alterations in normal keratinocyte terminal differentiation that appear to be specific to the particular oncogene. These alterations bore similarities to abnormalities in differentiation observed in naturally occurring squamous epithelial malignancies.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Rowe S. P. Nonproducer clones of murine sarcoma virus transformed BALB-3T3 cells. Virology. 1970 Sep;42(1):9–19. doi: 10.1016/0042-6822(70)90233-3. [DOI] [PubMed] [Google Scholar]
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Alemà S., Casalbore P., Agostini E., Tatò F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985 Aug 8;316(6028):557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Beutner E. H., Jordon R. E., Chorzelski T. P. The immunopathology of pemphigus and bullous pemphigoid. J Invest Dermatol. 1968 Aug;51(2):63–80. [PubMed] [Google Scholar]
- Buhrow S. A., Cohen S., Staros J. V. Affinity labeling of the protein kinase associated with the epidermal growth factor receptor in membrane vesicles from A431 cells. J Biol Chem. 1982 Apr 25;257(8):4019–4022. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Regulation of cell growth and transformation by tyrosine-specific protein kinases: the search for important cellular substrate proteins. Curr Top Microbiol Immunol. 1983;107:125–161. doi: 10.1007/978-3-642-69075-4_4. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco A., Pinto A., Tramontano D., Tajana G., Vecchio G., Tsuchida N. Block in the expression of differentiation markers of rat thyroid epithelial cells by transformation with Kirsten murine sarcoma virus. Cancer Res. 1982 Feb;42(2):618–626. [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. Oncogenes and proto-oncogenes: how do they differ? J Natl Cancer Inst. 1984 Oct;73(4):773–786. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kloetzer W. S., Maxwell S. A., Arlinghaus R. B. P85gag-mos encoded by ts110 Moloney murine sarcoma virus has an associated protein kinase activity. Proc Natl Acad Sci U S A. 1983 Jan;80(2):412–416. doi: 10.1073/pnas.80.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesz-Martin M. F., Koehler B., Hennings H., Yuspa S. H. Quantitative assay for carcinogen altered differentiation in mouse epidermal cells. Carcinogenesis. 1980;1(12):995–1006. doi: 10.1093/carcin/1.12.995. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Wagner E. F. Differentiation of F9 teratocarcinoma stem cells after transfer of c-fos proto-oncogenes. Nature. 1984 Oct 4;311(5985):438–442. doi: 10.1038/311438a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Nigg E. A., Hunter T. The transforming protein of Moloney murine sarcoma virus is a soluble cytoplasmic protein. Cell. 1983 May;33(1):161–172. doi: 10.1016/0092-8674(83)90345-8. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. BALB- and Harvey-murine sarcoma virus transformation of a novel lymphoid progenitor cell. J Exp Med. 1982 Sep 1;156(3):873–887. doi: 10.1084/jem.156.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. In vitro transformation of murine pre-B lymphoid cells by Snyder-Theilen feline sarcoma virus. J Virol. 1983 Jun;46(3):993–1002. doi: 10.1128/jvi.46.3.993-1002.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reano A., Faure M., Jacques Y., Reichert U., Schaefer H., Thivolet J. Lectins as markers of human epidermal cell differentiation. Differentiation. 1982;22(3):205–210. doi: 10.1111/j.1432-0436.1982.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Dunn C. Y., Aaronson S. A. Different lymphoid cell targets by transformation by replication-competent Moloney and Rauscher mouse leukemia viruses. Cell. 1980 Mar;19(3):663–669. doi: 10.1016/s0092-8674(80)80043-2. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G. Human squamous cell carcinoma in culture: a defect in terminal differentiation and its relation to malignancy. Natl Cancer Inst Monogr. 1982;60:133–138. [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Hawley-Nelson P., Cheng C. K., Yuspa S. H. Expression of keratin genes in mouse epidermis and normal and malignantly transformed epidermal cells in culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):144s–149s. doi: 10.1111/1523-1747.ep12540939. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J. R., Yuspa S. H. Specific epidermal protein markers are modulated during calcium-induced terminal differentiation. J Cell Biol. 1983 Jun;96(6):1809–1814. doi: 10.1083/jcb.96.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A. Characterization of temperature-sensitive mutants of murine leukemia virus. Virology. 1973 Jul;54(1):53–59. doi: 10.1016/0042-6822(73)90113-x. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976 Dec;9(4 Pt 1):511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Fryling C., Johnson P. A., Sporn M. B. Transforming growth factors (TGFs): properties and possible mechanisms of action. J Supramol Struct Cell Biochem. 1981;15(3):287–301. doi: 10.1002/jsscb.1981.380150306. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Aaronson S. A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell. 1983 Feb;32(2):599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Stanley J., Lichti U. Keratinocytes blocked in phorbol ester-responsive early stage of terminal differentiation by sarcoma viruses. Nature. 1985 Apr 4;314(6010):459–462. doi: 10.1038/314459a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Morgan D. L. Mouse skin cells resistant to terminal differentiation associated with initiation of carcinogenesis. Nature. 1981 Sep 3;293(5827):72–74. doi: 10.1038/293072a0. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Vass W., Scolnick E. Altered growth and differentiation of cultured mouse epidermal cells infected with oncogenic retrovirus: contrasting effects of viruses and chemicals. Cancer Res. 1983 Dec;43(12 Pt 1):6021–6030. [PubMed] [Google Scholar]