Abstract
Many organic hazardous pollutants, including 2,4-dinitrophenol (2,4-DNP), which are water soluble, toxic, and not easily biodegradable make concerns for environmental pollution worldwide. In the present study, degradation of nitrophenols-contained effluents by using laccase immobilized on the nano-porous silica beads was evaluated. 2,4-DNP was selected as the main constituent of industrial effluents containing nitrophenols. The performance of the system was characterized as a function of pH, contact time, temperature, pollutant, and mediator concentrations. The laccase-silica beads were employed in a mixed-batch reactor to determine the degradation efficiency after 12 h of enzyme treatment. The obtained data showed that the immobilized laccase degraded more than 90% of 2,4-DNP within 12 h treatment. The immobilization process improved the activity and sustainability of laccase for degradation of the pollutant. Temperatures more than 50°C reduced the enzyme activity to about 60%. However, pH and the mediator concentration could not affect the enzyme activity. The degradation kinetic was in accordance with a Michaelis–Menten equation with Vmax and Km obtained as 0.25–0.38 μmoles/min and 0.13–0.017 mM, respectively. The stability of the immobilized enzyme was maintained for more than 85% of its initial activity after 30 days. Based on the results, it can be concluded that high resistibility and reusability of immobilized laccase on CPC-silica beads make it considerable choice for wastewater treatment.
Keywords: Degradation; Laccase; Immobilization; Nano-porous silica beads; 2,4-dinitrophenol
Introduction
Nitrophenols, categorized as priority pollutants, are one of the main common components which release from industrial effluents and deteriorate the quality of water resources. There are six possible dinitrophenol (DNP) forms and 2,4-Dinitrophenol (2,4-DNP) is the most important toxic and refractory pollutant [1]. 2,4-DNP, a yellowish crystalline solid, has been used in manufacturing of pesticides, pharmaceuticals, production of dyes, explosive materials, and as an indicator for the detection of potassium and ammonium ions. Its entrance into the environment may occur from industrial wastewaters, accidental spills, or as an intermediate metabolite due to degradation of pesticides containing 2,4-DNP [2]. For instance, wastewater from a dye manufacturing plant contained 3.2 mg/L DNP. Groundwater from a waste site that was once occupied by a factory that used DNP contained 30.6 mg DNP/L of water [3].
Several physical and chemical methods which have been used for the treatment of the nitrophenols pollutants are adsorption processes, chemical oxidation, precipitation, evaporation, and incineration. However, due to their problems in the application and economic issues, other relevant treatment methods have been studied. The ability of biological processes on the degradation of organic pollutants, due to their effective and safe performance in compare with chemical and physical treatment techniques, has been considered [4,5]. Among them, the performance of white rots fungi for degradation a wide range of refractory organic pollutants, specially the phenolic compounds, via lignin-modifying enzymes, such as manganese (II)-dependent peroxidase, lignin peroxidase, and laccase (phenol oxidase) have been studied [6-8]. Although biological processes are efficient at low pollutant concentrations, their sensitivity to shock loads, require long hydraulic retention times and forming large amounts of solid residues make their feasible application with challenges [9]. In an enzyme based process, however, some of these disadvantages can be omitted since enzymes can be applied to persistent materials, high and low contaminant concentration over a wide pH, temperature, and salinity range. The most recent research in this area has focused on the enzymatic process for the treatment of wastewater [10,11].
Laccase (EC 1.10.3.2), a multi-copper oxidase enzyme, catalyzes the oxidation of variety of aromatic and inorganic substrates, mostly phenols, with simultaneous reduction of oxygen to water [12-14]. Among advantages of laccase application such as high efficiency, the main disadvantage is that it is often easily inactivated in oxidation process due to the wide range of process conditions (temperature, pH, etc.) and also its separation procedure, for reuse proposes, from the reaction system is difficult which limits the further industrial applications of laccase. An effective method for reuse and improving its stability is using enzyme immobilization technology [15]. Studies showed that several types of supporters could be used in enzyme immobilization which included activated carbon, chitosan microspheres [16], polymeric carrier [17], polyacrylonitrile beads [18] and magnetic chitosan nanoparticles [19].
Although many previous studies used immobilized laccase to degrade organic pollutants such as chlorophenols [20], dyes [21], Poly Aromatic Hydrocarbons (PAHs) [22], however there has not been a reliable research on oxidation of nitrophenol compounds by immobilized laccase. The aim of this study is to investigate the feasibility of 2,4-DNP degradation in effluents by Trametes versicolor laccase immobilized on the controlled porosity carrier (CPC) silica beads.
Materials and methods
Chemicals
T. versicolor laccase, pre-silanized [with 3-aminopropyltriethoxysilane (APTES)] CPC silica beads, Glutaraldehyde solution (25%), and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and 2,4-DNP, acetonitrile and methanol (HPLC grade) were from Merck (Darmstadt, Germany).
Laccase immobilization on CPC-silica beads
Laccase was immobilized on pre-silanized silica beads according to the study of Champagne and Ramsay [6]. An amount of 4 g of pre-silanized CPC-silica beads (355–600 mm in diameter, an average surface area of 42.1 m2/g, and a pore size of 37.5 nm) were immersed in degassed 2.5% glutaraldehyde (2.0 bar vacuum pressure for 2 h) in 0.1 M KH2PO4, pH 5.0, then placed in an enzyme solution (≈ 2.0 U/mL in 0.1 M KH2PO4 at pH 5.0) for more than 36 h at 4°C. Thereafter, beads were purred on a paper filter and washed three times with distilled water and twice with phosphate buffer (0.1 M KH2PO4, pH 5.0).
Enzyme assay
Laccase activity was measured at 420 nm by generation of ABTS˚ˉ radicals from the enzymatic oxidation of ABTS at 25°C using CECIL 8600 spectrophotometer. The assay mixture contained 0.2 mM ABTS, 100 mM sodium acetate buffer (pH 5.0), and the enzyme-containing sample [18]. One unit of laccase activity (U) was defined as the amount of enzyme that formed 1 μmol ABTS per min. Protein concentration was measured as the absorbance at 280 nm and corrected for scattering effects with absorbance readings at 320 nm [6].
Degradation of 2,4-DNP by immobilized laccase
For determining the performance of immobilized enzyme on 2,4-DNP degradation, 0.5 g of CPC-silica beads with 50 ± 3.8 U of laccase/g (i.e. 1.18 ± 0.09 U/m2) were used in a batch reactor (Erlenmeyer 50 mL). Synthetic effluent consisted of 2,4-DNP with concentrations of 0.05, 0.1, and 0.15 M (in 0.1 M phosphate buffer, pH 4–6) and ABTS (as a mediator) of 1, 2, and 3 mM was added to each reactor. Temperature had been adjusted in range of 40–60°C in the shaker incubator (150 rpm). The sampling procedure (1 mL for every 2 h up to 12 h retention time) was done, and then equal volume of methanol (HPLC grade) was added in order to end the reaction process between enzyme and the pollutant. Samples were then filtered by 0.2 μm PTFE (Polytetrafluoroethylene) filters and filled in 5 mL dark vessels (sealed by parafilm tape) in 4°C before analysis.
Analysis and measurements
The concentration of 2,4-DNP in the reaction mixture was measured by high performance liquid chromatography (HPLC, CECIL 4100, USA) equipped with an UV–Visible Diode Array detector. Separation of compounds was obtained with a Nucleodur Sphinx RP (25.0 cm × 4.6 mm) column (MZ-1 PerfectSil, Germany) at a flow-rate of 1 ml/min. The chromatographic determination was performed by using a gradient in 10 min acetonitrile/ 0.5% acetic acid (50:50, v/v). All assays were carried out in triplicate and gave standard deviations lower than 5%.
Results and discussion
Characterization of CPC-silica beads
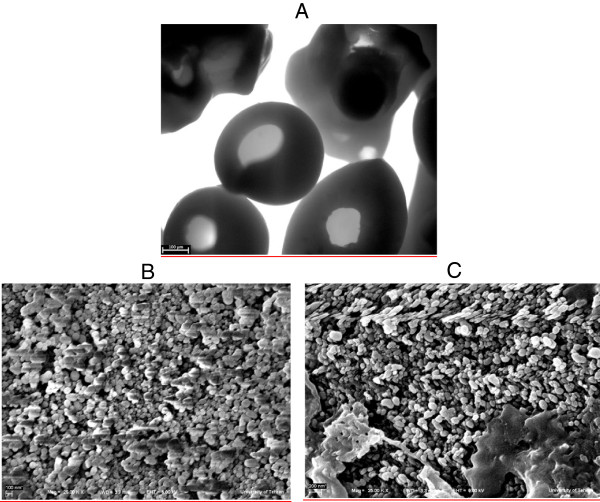
The morphology of CPC-silica beads which was acquired using SEM is shown in Figure 1. It must be noted that the beads were purchased and no additional activity was done for preparing that. As demonstrated in Figure 1A, most silica beads had regular convoluted structure at the micron scale. However, the surface of silica beads had many pores which most of them had diameters less than 100 nm. The nitrogen adsorption–desorption isotherm data indicate that the surface area of silica A is about 40 ± 5 m2/g.
Figure 1.
SEM imaging of nanoporous cpc-silica beads, A) overall shape of beads, B) the surface of beads without immobilized enzyme, C) the surface of beads with immobilized enzyme.
As shown in Figure 1C, it could be observed that the laccase enzyme was effectively immobilized on the surface of the beads due to high surface area which the beads provided. In particular the nano-scale pores and the surface properties provided the suitable prerequisites for enzyme immobilization [6]. By comparison of Figure 1B and C, the immobilized laccase was obviously detectable.
Characteristics of free and immobilized laccase
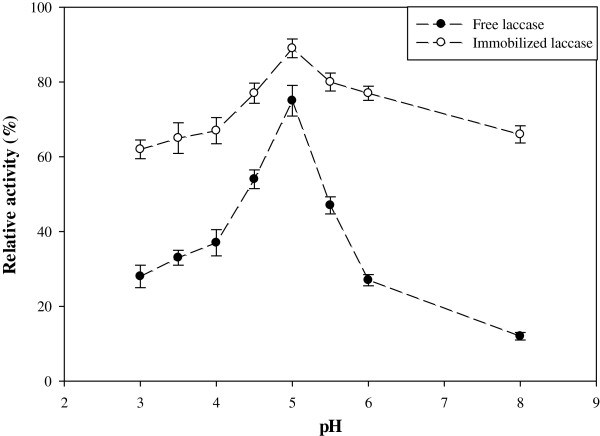
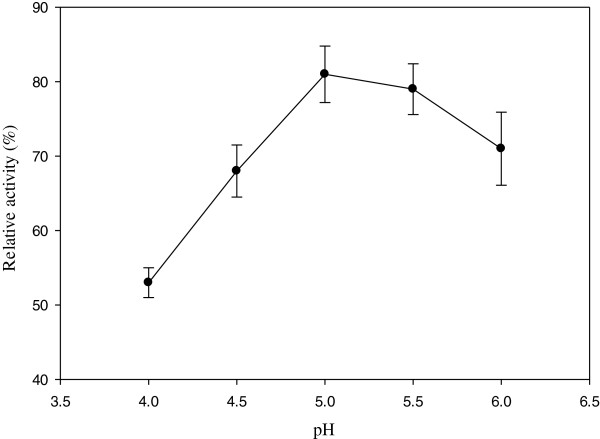
Activity dependence of immobilized laccase on temperature and pH values was determined and compared with the free enzyme. The oxidation reaction for immobilized laccase had the maximum activity at pH 5.0. As demonstrated in Figure 2, the pH trend of immobilized laccase was more extended in comparison with the free form, which showed that the immobilized laccase was more resisting to pH variations. In the pH range of 4–6, the immobilized laccase retained its activity for more than 86% of its maximum activity, however for the free form it was 36%. Moreover, pre-test analysis in pH 3.0 showed that the activity of immobilized enzyme versus free type retained 62% and 28%, respectively, and in alkaline pH (pH ≈ 8.0) the immobilized laccase kept its activity about 60–70% of its maximum activity, while free laccase was almost inactive. Catapane et al. also showed that the optimal pH for free and immobilized laccase on PAN (Poly Acrylo Nitrile) was about 5, however the activity of immobilized enzyme in pH range of 3 to 7 was retained significantly, in compare with free enzyme [23].
Figure 2.
Relative activity of free and immobilized laccase in different pH [ABTS] = 1 mM; [temperature] = 40°C.
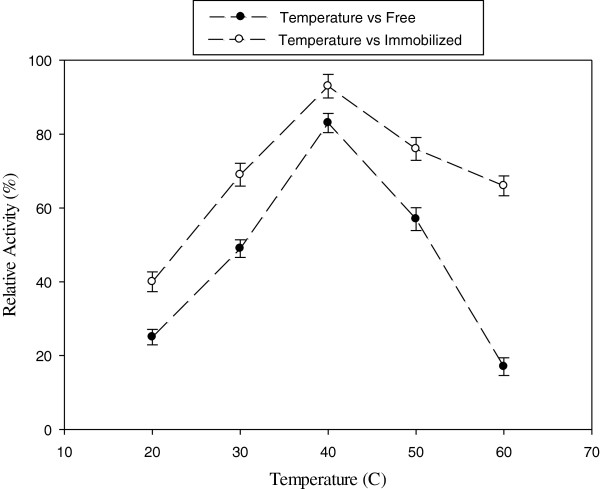
For determining the effect of temperature on laccase, the activity of free and immobilized enzyme was assayed in a solution containing 1 mM of ABTS, a laccase substrate, in 0.1 M phosphate buffer at pH 5.0 in the temperature range 20–60°C. As it can be seen in Figure 3, the maximum activity of both free and immobilized laccase was at 40°C. Moreover, the activity of the immobilized laccase was greater in high temperatures (50–60°C) compared with the free counterpart. Several studies have shown the temperature resistance of immobilized enzyme in contrast with free form [6,18,24]. Results demonstrate that the activity of immobilized enzyme in 60°C retains about 70% of its maximal activity, while the free enzyme is inactivated. Nicolucci et al. also showed that the relative activity of immobilized laccase was about 80% at 70°C while the free laccase was approximately inactivated [18].
Figure 3.
The effect of temperature on relative activity of free and immobilized laccase [ABTS] = 1 mM; [pH] = 5.
Enzymatic degradation of 2,4-DNP
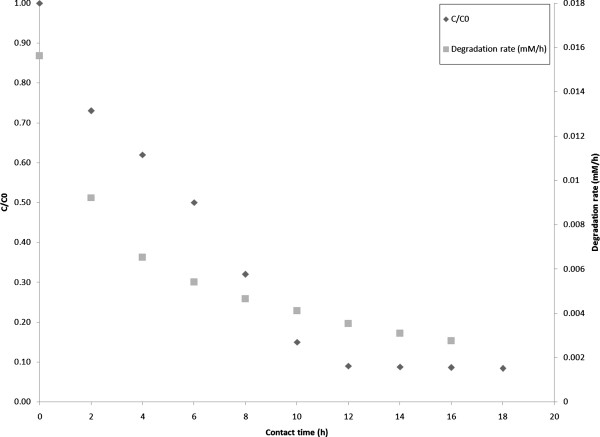
Enzymatic degradation studies of 2,4-DNP were conducted in a batch reactor which CPC-silica beads were easily spread in a reactor by shaking by the rate of 150 rpm. Results showed that the immobilized laccase could effectively break down 2,4-DNP (Figure 4). The maximum degradation of 2,4-DNP was raised to 91% on 12 h contact time at 40°C (pH 5, ABTS 3 mM) which was selected as the optimum condition. It must be noted that rising the contact time over 12 h could not significantly increase the degradation efficiency (Pvalue < 0.05). It is obvious that the contact time plays an important role in pollutant degradation which increases the reaction. Bhattacharya and Banerjee confirmed the positive effect of contact time of enzymatic degradation of 2,4-DCP [25]. However, the degradation rate of the pollutant had decreasing trend from 0.015 to 0.002 mM/h which may due to enzyme inactivation or synthesizing of intermediate compounds during oxidation of 2,4-DNP and therefore laccase involvement to degrade those chemicals [18].
Figure 4.
Residual concentration and degradation rate of 2,4-DNP in different contact time [2,4-DNP] = 0.05 mM; [ABTS] = 1 mM; [temperature] = 40°C; [pH] = 5.
As demonstrated in Figure 5, a bell-shaped dependence on pH was appeared for the plot of 2,4-DNP degradation which is in accordance with the pH profile of laccase activity. This result is consistent with earlier literature reports [26,27]. The optimum pH of 5 was achieved for enzymatic degradation of the pollutant that is in agreement with other researches [26,28]. However, the optimum pH reported by Okazaki et al. was 3 for laccase from Carialus versicalar converting α-phenylenediamine. This difference may be due to differences in the type and concentration of the buffer used and the purity of the enzyme [27,29]. Nicolucci et al., revealed that laccase activity was higher in acidify medium [18]. Moreover, immobilized laccase on silica beads retained its activity in pH 4–6.2 [29].
Figure 5.
Relative activity of the immobilized laccase in different pH for 2,4-DNP [2,4-DNP] = 0.05 mM; [ABTS] = 1 mM; [temperature] = 40°C.
Laccase normally has low redox potential (0.6–0.8 V) which is limited to react with wide range of phenolic compounds [30-32]. However, using proper low molecular weight compounds, known as mediators, can mediate the oxidation of substrates by laccase, so extending the application of laccase on oxidation of wide range of pollutants [6,18]. As shown in Figure 6, the acceptable role of a mediator is to act as an “electron shuttle” between laccase and the substrate [33]. The oxidized mediator by laccase, can strongly oxidizes the substrate due to its higher redox potential (1.8 V for ABTS) [34]. The results in Figure 6 showed that increasing the mediator concentration from 1 to 3 mM could enhance the conversion of 2,4-DNP, however, its effect was calculated as 2%. During the oxidation of 2,4- DNP, the color of the system gradually changed from blue-green to light yellow which indicated ABTS++ was probably present in the systems of this study. Liu showed that increasing ABTS concentration from 10 to 200 μM enhance the conversion of Bisphenol A [29].
Figure 6.
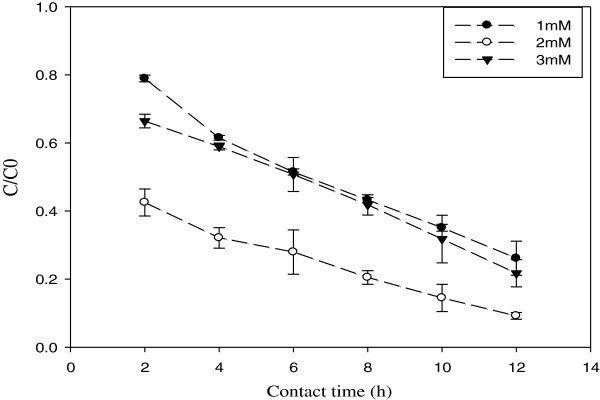
The effect of mediator concentration on the degradation of 2,4-DNP [2,4-DNP] = 0.05 mM; [temperature] = 40°C; [pH] = 5.
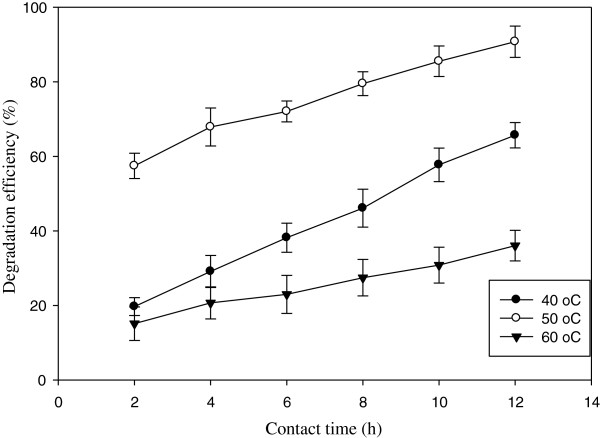
Temperature is an important variable to be considered in laccase-catalyzed reactions and has a double effect on enzymatic systems which are a change in the rate of reaction over time caused by thermal inactivation of the enzyme, and a change in the reaction rate due to Arrhenius effects [8]. Results revealed that by increasing temperature from 40 to 60°C, the pollutant degradation significantly decreased from 91% to only 33%, while other parameters kept constant (optimum condition). In compared with the resistance of free enzyme on temperature, as previously noted, its remained activity could be explained with the immobilization process and improving laccase characteristics [8]. Tavares et al. showed that the activity immobilized laccase on modified silica beads was remained for more than 6 h in 55°C and by increasing the temperature up to 70°C, the enzyme was almost deactivated [35].
As can be seen in Figure 7, the activity of laccase for 2,4-DNP degradation was remained in higher temperature (60°C) in accordance with different studies which have reported the stability of laccase in wide range of high temperatures even in 100°C [29].
Figure 7.
The degradation of 2,4-DNP in different temperatures [2,4-DNP] = 0.05 mM; [ABTS] = 1 mM; [pH] = 5.
Kinetics of 2,4-DNP degradation
The initial reaction rate of 2,4-DNP was measured for determining the kinetic parameters of enzyme reactions. A Michaelis-Menten equation was used for data fitting process. Lineweaver–Burk double reciprocal plots were applied to calculate Km and maximum reaction rate (Vmax) of the immobilized laccase. As shown in Table 1, the Km values of the immobilized laccase for different concentrations of 2,4-DNP (0.05, 0.1, and 0.15 mM) resulted to 0.013, 0.016, and 0.017 mM, respectively. A general increase in the Km values for the immobilized laccase towards every substrate concentration was observed. On the contrary, a decrease in Vmax values was observed. The Vmax for immobilized laccase was reduced from 0.38 to 0.25 μmoles/min by substrate increasing. Due to variation of both Km and Vmax values with substrate concentration, the values of Vmax/Km are also reported in Table 1 for easy comparison of the catalytic efficiency of enzyme-substrate system. The ratio varied between 29.23 and 14.70 for the immobilized laccase. The decreasing trend of the ratio Vmax/Km may be occurred due to inactivation of the enzyme and the production of intermediate compounds during degradation of 2,4-DNP [18].
Table 1.
Kinetic parameters for the immobilized laccase using 2,4-DNP as substrates
| Substrate concentration (mM) | V max (μmoles/min) | Km (mM) | V max/Km (μmoles/min/mM) |
|---|---|---|---|
| 0.05 |
0. 38 |
0.013 |
29.23 |
| 0.10 |
0. 31 |
0.016 |
19.37 |
| 0.15 | 0. 25 | 0.017 | 14.70 |
Stability of immobilized enzyme
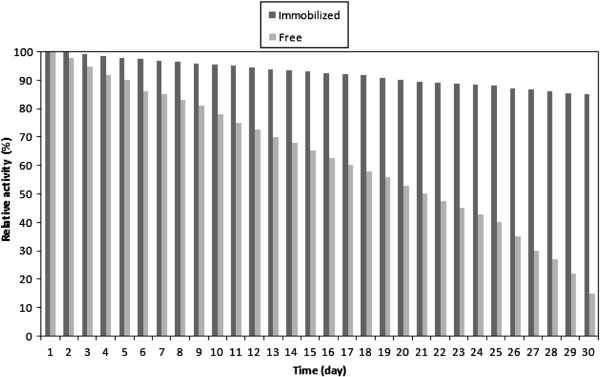
Operational stability is considered as an important factor in order to evaluate the industrial application of enzymes. The immobilized laccase on silica beads were reused every day for 30 successive days. Duration of each experiment was 12 h. The activity of the immobilized enzyme was assayed daily by a spectrophotometrically measurement of color change of the mediator. The experiment condition was the same in the period as 1 mM of ABTS in phosphate buffer solution 0.1 M, pH 5.0 at 25°C. As depicted in Figure 8, the immobilized laccase retained its activity for more than 85%. However, the activity of free laccase remained only 15% of its initial activity. The results confirmed that the immobilized laccase on nano-porous silica could effectively retain its activity and stability [6,23,35].
Figure 8.
Comparison of the stability of free and immobilized enzyme [ABTS] = 1 mM; [temperature] = 40°C; [pH] = 5.
Conclusions
In this study, the performance of the immobilized laccase for degradation of 2,4-DNP in aqueous solutions has been investigated. Results showed that the immobilized enzyme was able to effectively degrade 2,4-DNP as a pollutant in water resources. The optimum condition for the maximum degradation of 2,4-DNP (91%) was achieved on 12 h contact time and pH 5. The immobilized laccase had more resistibility to the pH and temperature changes, in compare with the free form. The improved characteristics of immobilized laccase on CPC-silica beads for stability and reusability could be considered as an advantage in wastewater treatment. Investigation of the enzymatic degradation of other phenolic compounds with several mediators in different pH and Temperature condition could be recommended.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
The overall implementation of this study including design, experiments and data analysis, and manuscript preparation were the results of efforts by corresponding author. All authors have made extensive contribution into the review and finalization of this manuscript. All authors read and approved the final manuscript.
Contributor Information
Emad Dehghanifard, Email: edehghanifard@gmail.com.
Ahmad Jonidi Jafari, Email: a.jonidi@modares.ac.ir.
Roshanak Rezaei Kalantary, Email: r-rezaei@tums.ac.ir.
Amir Hosein Mahvi, Email: ahmahvi@yahoo.com.
Mohammad Ali Faramarzi, Email: faramarz@tums.ac.ir.
Ali Esrafili, Email: a_esrafily@yahoo.com.
Acknowledgements
Authors highly appreciated of Tehran University of Medical Sciences for their financially supports of the study.
References
- Shukla SS, Dorris KL, Chikkaveeraiah BV. Photocatalytic degradation of 2, 4-dinitrophenol. J Hazard Mater. 2009;164(1):310–314. doi: 10.1016/j.jhazmat.2008.08.047. [DOI] [PubMed] [Google Scholar]
- Ahmadi Moghaddam M, Mesdaghinia A, Naddafi K, Nasseri S, Mahvi AH, Vaezi F. Degradation of 2, 4-dinitrophenol by photo fenton process. Asian J Chem. 2010;22(2):1009–1016. [Google Scholar]
- Harris MO, Cocoran J. Toxicological profile for dinitrophenols. Atlanta: Agency for Toxic Substances and Disease Registry; 1995. [PubMed] [Google Scholar]
- Dai R, Chen J, Lin J, Xiao S, Chen S, Deng Y. Reduction of nitro phenols using nitroreductase from E. coli in the presence of NADH. J Hazard Mater. 2009;170(1):141–143. doi: 10.1016/j.jhazmat.2009.04.122. [DOI] [PubMed] [Google Scholar]
- She ZL, Xie T, Li LL, Zhu YJ, Tang GF, Zhao YG. Study on the aerobic degradation kinetics of 2, 4-DNP and 2, 6-DNP. Adv Mater Res. 2012;356:186–189. doi: 10.1016/j.jhazmat.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Champagne PP, Ramsay J. Dye decolorization and detoxification by laccase immobilized on porous glass beads. Bioresource Technol. 2010;101(7):2230–2235. doi: 10.1016/j.biortech.2009.11.066. [DOI] [PubMed] [Google Scholar]
- Hibi M, Hatahira S, Nakatani M, Yokozeki K, Shimizu S, Ogawa J. Extracellular oxidases of Cerrena sp. complementarily functioning in artificial dye decolorization including laccase, manganese peroxidase, and novel versatile peroxidases. Biocatal Agr Biotechnol. 2012;1(3):220–225. [Google Scholar]
- Kurniawati S, Nicell J. Variable stoichiometry during the laccase-catalyzed oxidation of aqueous phenol. Biotechnol Progr. 2007;23(2):389–397. doi: 10.1021/bp060312r. [DOI] [PubMed] [Google Scholar]
- Peralta-Zamora P, Pereira CM, Tiburtius ERL, Moraes SG, Rosa MA, Minussi RC. Decolorization of reactive dyes by immobilized laccase. Appl Catal B-Environ. 2003;42(2):131–144. doi: 10.1016/S0926-3373(02)00220-5. [DOI] [Google Scholar]
- Gholami-Borujeni F, Mahvi AH, Naseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M. Application of immobilized horseradish peroxidase for removal and detoxification of azo dye from aqueous solution. Res J Chem Environ. 2011;15(2):217–222. [Google Scholar]
- Gholami-Borujeni F, Mahvi AH, Nasseri S, Faramarzi MA, Nabizadeh R, Alimohammadi M. Enzymatic treatment and detoxification of acid orange 7 from textile wastewater. Appl Biochem Biotech. 2011;165(5–6):1274–1284. doi: 10.1007/s12010-011-9345-5. [DOI] [PubMed] [Google Scholar]
- Aghaie-Khouzani M, Forootanfar H, Moshfegh M, Khoshayand M, Faramarzi M. Decolorization of some synthetic dyes using optimized culture broth of laccase producing ascomycete Paraconiothyrium variabile. Biochem Eng J. 2011;60:9–15. [Google Scholar]
- Forootanfar H, Movahednia MM, Yaghmaei S, Tabatabaei-Samani M, Rastegar H, Sadighi A. Removal of chlorophenolic derivatives by soil isolated ascomycete of Paraconiothyrium variabile and studying the role of its extracellular laccase. J Hazard Mater. 2012;209–210:199–203. doi: 10.1016/j.jhazmat.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Rekuc A, Jastrzembska B, Liesiene J, Bryjak J. Comparative studies on immobilized laccase behaviour in packed-bed and batch reactors. J Mol Catal B: Enzym. 2009;57(1–4):216–223. [Google Scholar]
- Wang P, Fan X, Cui L, Wang Q, Zhou A. Decolorization of reactive dyes by laccase immobilized in alginate/gelatin blent with PEG. J Environ Sci. 2008;20(12):1519–1522. doi: 10.1016/S1001-0742(08)62559-0. [DOI] [PubMed] [Google Scholar]
- Jiang DS, Long SY, Huang J, Xiao HY, Zhou JY. Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J. 2005;25(1):15–23. doi: 10.1016/j.bej.2005.03.007. [DOI] [Google Scholar]
- Stanescu MD, Gavrilas S, Ludwig R, Haltrich D, Lozinsky VI. Preparation of immobilized Trametes pubescens laccase on a cryogel-type polymeric carrier and application of the biocatalyst to apple juice phenolic compounds oxidation. Eur Food Res Technol. 2012;234(4):655–662. doi: 10.1007/s00217-012-1676-0. [DOI] [Google Scholar]
- Nicolucci C, Rossi S, Menale C, Godjevargova T, Ivanov Y, Bianco M. Biodegradation of bisphenols with immobilized laccase or tyrosinase on polyacrylonitrile beads. Biodegradation. 2011;22(3):673–683. doi: 10.1007/s10532-010-9440-2. [DOI] [PubMed] [Google Scholar]
- Kalkan NA, Aksoy S, Aksoy EA, Hasirci N. Preparation of chitosan coated magnetite nanoparticles and application for immobilization of laccase. J Appl Polym Sci. 2012;123(2):707–716. doi: 10.1002/app.34504. [DOI] [Google Scholar]
- Gaitan IJ, Medina SC, González JC, Rodríguez A, Espejo ÁJ, Osma JF. Evaluation of toxicity and degradation of a chlorophenol mixture by the laccase produced by Trametes pubescens. Bioresource Technol. 2011;102(3):3632–3635. doi: 10.1016/j.biortech.2010.11.040. [DOI] [PubMed] [Google Scholar]
- Mogharabi M, Nassiri-Koopaei N, Bozorgi-Koushalshahi M, Nafissi-Varcheh N, Bagherzadeh G, Faramarzi MA. Immobilization of laccase in alginate-gelatin mixed gel and decolorization of synthetic dyes. Bioinor Chem Appl. 2012;2012:1–6. doi: 10.1155/2012/823830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y, Yin L, Niu J. Laccase-carrying electrospun fibrous membranes for adsorption and degradation of PAHs in shoal soils. Environ Sci Technol. 2011;45(24):10611–10618. doi: 10.1021/es203286e. [DOI] [PubMed] [Google Scholar]
- Catapane M, Nicolucci C, Menale C, Mita L, Rossi S, Mita DG, Diano N. Enzymatic removal of estrogenic activity of nonylphenol and octylphenol aqueous solutions by immobilized laccase from Trametes versicolor. J Hazard Mater. 2013;248–249:337–346. doi: 10.1016/j.jhazmat.2013.01.031. [DOI] [PubMed] [Google Scholar]
- Lante A, Crapisi A, Krastanov A, Spettoli P. Biodegradation of phenols by laccase immobilised in a membrane reactor. Process Biochem. 2000;36:51–58. doi: 10.1016/S0032-9592(00)00180-1. [DOI] [Google Scholar]
- Bhattacharya S, Banerjee R. Laccase mediated biodegradation of 2, 4-dichlorophenol using response surface methodology. Chemosphere. 2008;73(1):81–85. doi: 10.1016/j.chemosphere.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Aktaş N, Tanyolaç A. Reaction conditions for laccase catalyzed polymerization of catechol. Bioresource Technol. 2003;87(3):209–214. doi: 10.1016/S0960-8524(02)00254-7. [DOI] [PubMed] [Google Scholar]
- Okazaki S, Michizoe J, Goto M, Furusaki S, Wariishi H, Tanaka H. Oxidation of bisphenol A catalyzed by laccase hosted in reversed micelles in organic media. Enzyme Microb Tech. 2002;31(3):227–232. doi: 10.1016/S0141-0229(02)00104-7. [DOI] [Google Scholar]
- Rancano G, Lorenzo M, Molares N, Rodriguez Couto S, Sanromán MA. Production of laccase by Trametes versicolor in an airlift fermentor. Process Biochem. 2003;39(4):467–473. doi: 10.1016/S0032-9592(03)00083-9. [DOI] [Google Scholar]
- Liu Y. Laccase-catalyzed oxidation of bisphenol a in a non-aqueous liquid using reverse micelles. Montreal: McGill University, Department of Civil Engineering and Applied Mechanics; 2004. (M.Sc thesis). [Google Scholar]
- Frasconi M, Favero G, Boer H, Koivula A, Mazzei F. Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. BBA-Proteins Proteomics. 2010;1804(4):899–908. doi: 10.1016/j.bbapap.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Hong G, Ivnitski DM, Johnson GR, Atanassov P, Pachter R. Design parameters for tuning the type 1 CU multicopper oxidase redox potential: insight from a combination of first principles and empirical molecular dynamics simulations. J Am Chem Soc. 2011;133(13):4802–4809. doi: 10.1021/ja105586q. [DOI] [PubMed] [Google Scholar]
- Sadhasivam S, Savitha S, Swaminathan K, Lin FH. Production, purification and characterization of mid-redox potential laccase from a newly isolated Trichoderma harzianum WL1. Process Biochem. 2008;43(7):736–742. doi: 10.1016/j.procbio.2008.02.017. [DOI] [Google Scholar]
- Fabbrini M, Galli C, Gentili P. Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B: Enzym. 2002;16(5):231–240. [Google Scholar]
- D’Acunzo F, Galli C, Masci B. Oxidation of phenols by laccase and laccase-mediator systems. Eur J Biochem. 2002;269(21):5330–5335. doi: 10.1046/j.1432-1033.2002.03256.x. [DOI] [PubMed] [Google Scholar]
- Tavares APM, Rodríguez O, Fernández-Fernández M, Domínguez A, Moldes D, Sanromán MA, Macedo EA. Immobilization of laccase on modified silica: stabilization, thermal inactivation and kinetic behaviour in 1-ethyl-3-methylimidazolium ethylsulfate ionic liquid. Bioresource Technol. 2013;131:405–412. doi: 10.1016/j.biortech.2012.12.139. [DOI] [PubMed] [Google Scholar]