Abstract
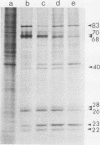
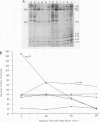
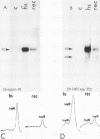
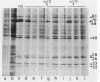
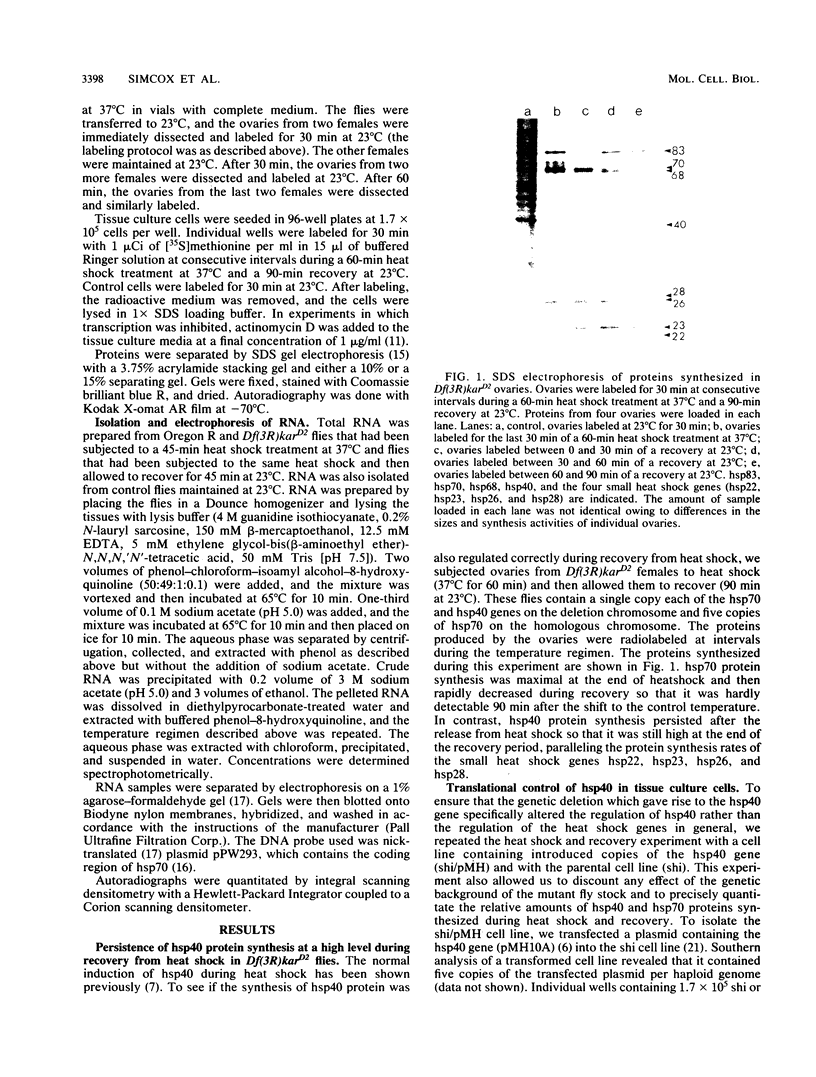
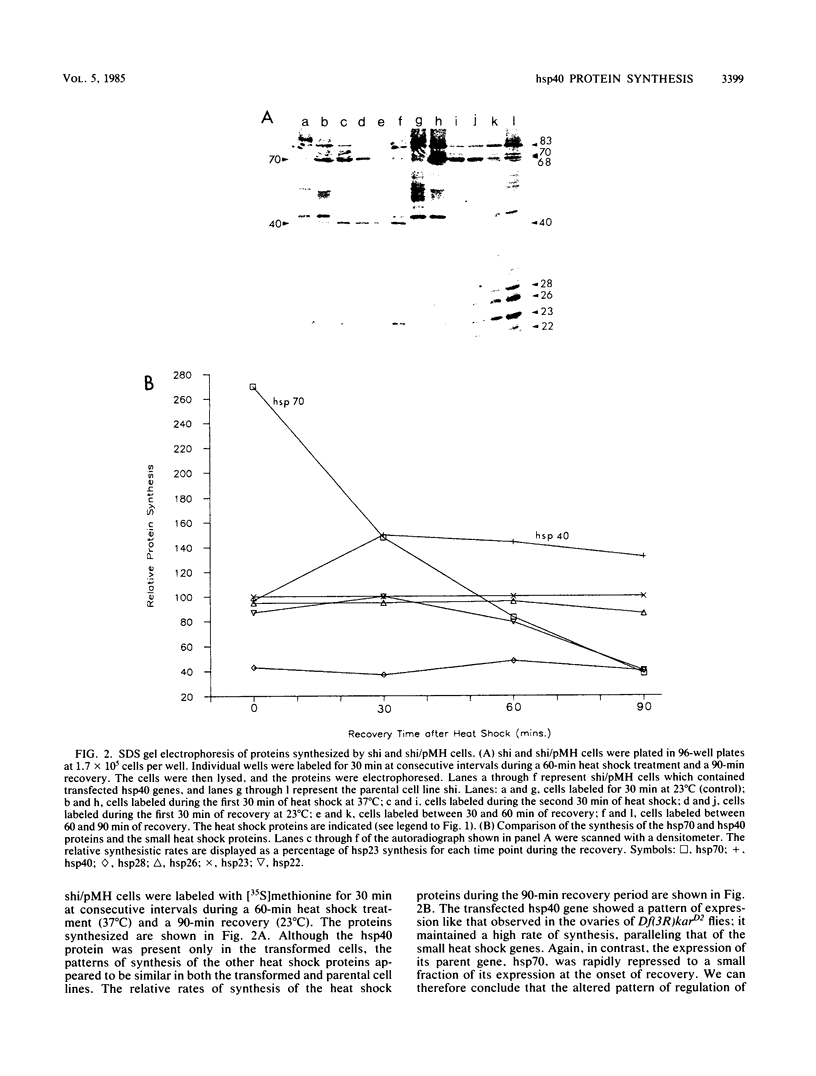
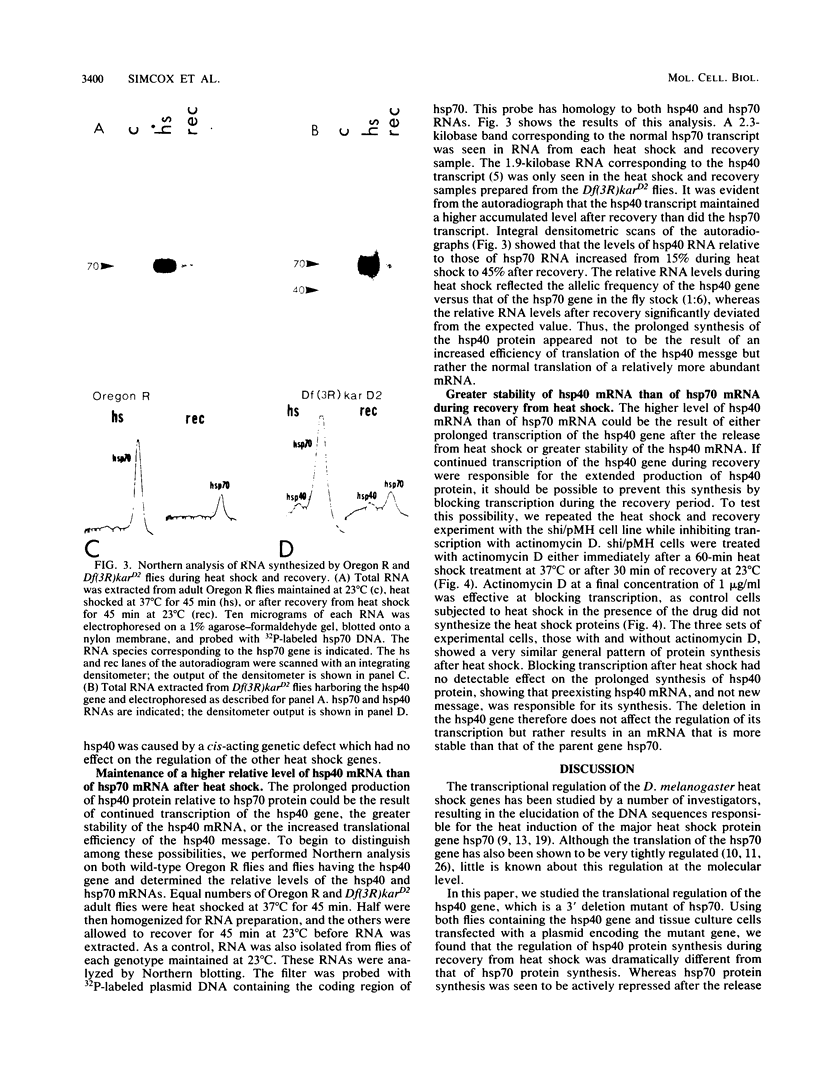
hsp40, an X-ray-induced deletion mutant of the major Drosophila melanogaster heat shock protein gene hsp70, was shown to be incorrectly regulated at the translational level. hsp40 protein synthesis persisted at a high level after the release from heat shock, whereas hsp70 protein production was rapidly repressed. This result was observed both in flies heterozygous for the hsp40 gene and in tissue culture cells transfected with the truncated gene. Analysis of the transcription of the hsp40 gene indicated that its mRNA, unlike hsp70 mRNA, was not actively destabilized after a return to control temperatures, permitting prolonged production of the mutant protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Ayme A., Southgate R., Tissières A. Nucleotide sequences responsible for the thermal inducibility of the Drosophila small heat-shock protein genes in monkey COS cells. J Mol Biol. 1985 Apr 20;182(4):469–475. doi: 10.1016/0022-2836(85)90233-5. [DOI] [PubMed] [Google Scholar]
- Benyajati C., Dray J. F. Cloned Drosophila alcohol dehydrogenase genes are correctly expressed after transfection into Drosophila cells in culture. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1701–1705. doi: 10.1073/pnas.81.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourouis M., Jarry B. Vectors containing a prokaryotic dihydrofolate reductase gene transform Drosophila cells to methotrexate-resistance. EMBO J. 1983;2(7):1099–1104. doi: 10.1002/j.1460-2075.1983.tb01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F. High-sensitivity S1 mapping with single-stranded [32P]DNA probes synthesized from bacteriophage M13mp templates. Gene. 1984 Oct;30(1-3):63–68. doi: 10.1016/0378-1119(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Caggese C., Caizzi R., Morea M., Scalenghe F., Ritossa F. Mutation generating a fragment of the major heat shock-inducible polypeptide in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 May;76(5):2385–2389. doi: 10.1073/pnas.76.5.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. M., Shearn A. Developmental regulation of Drosophila imaginal disc proteins: synthesis of a heat shock protein under non-heat-shock conditions. Dev Biol. 1983 Feb;95(2):325–330. doi: 10.1016/0012-1606(83)90033-7. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Inducible transcription and puffing in Drosophila melanogaster transformed with hsp70-phage lambda hybrid heat shock genes. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5509–5513. doi: 10.1073/pnas.81.17.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. Heat shock and recovery are mediated by different translational mechanisms. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6181–6185. doi: 10.1073/pnas.79.20.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiDomenico B. J., Bugaisky G. E., Lindquist S. The heat shock response is self-regulated at both the transcriptional and posttranscriptional levels. Cell. 1982 Dec;31(3 Pt 2):593–603. doi: 10.1016/0092-8674(82)90315-4. [DOI] [PubMed] [Google Scholar]
- Dudler R., Travers A. A. Upstream elements necessary for optimal function of the hsp 70 promoter in transformed flies. Cell. 1984 Sep;38(2):391–398. doi: 10.1016/0092-8674(84)90494-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Freund R., Schweber M., Wensink P. C., Meselson M. Sequence organization and transcription at two heat shock loci in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5613–5617. doi: 10.1073/pnas.75.11.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganelli C. M., Berger E. M. Transient expression of homologous genes in Drosophila cells. Science. 1984 Jun 1;224(4652):1004–1006. doi: 10.1126/science.224.4652.1004. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Sang J. H., Sinclair J. H., Burke J. F., Ish-Horowicz D. Rescue of a Drosophila temperature-sensitive mutant cell line by DNA transfection. Somat Cell Mol Genet. 1984 Nov;10(6):573–577. doi: 10.1007/BF01535222. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]