Abstract
A novel multi-component reaction for the synthesis of polyfunctionalized indoles and bis-indoles has been established. The reaction pathways were controlled by varying enamines with different substitution patterns to give polyfunctionalized indoles and bis-indoles selectively. The reaction proceeds at a fast speed within 15–30 min with water as the major byproduct, which makes work-up convenient.
Indoles are probably the most ubiquitous heterocycles in nature and have been referred to as “privileged structures” in drug discovery because of their capacity to bind many receptors with high affinity.1 Accordingly, many powerful methodologies for the synthesis of these heterocycles have been developed,2 the majority of these methods involve Fischer-type indole synthesis,3 reductive cyclization,4 metal-catalyzed coupling/condensation cascades5 and electrophilic activation of N-aryl amides.6 Recently, the utility of enamines in metal catalysis for the formation of valuable indoles has also been reported.7,8 The development of concise approaches to multi-functionalized indoles from readily available and inexpensive starting material is of great importance and challenging.
Meanwhile, the direct and selective functionalization of allylic C–H bonds into C–C and/or C–X bonds (X = O, N, halogen, etc.) has become an important topic because it provides a powerful tool for the synthesis of numerous complex molecules.9 In this regard, there has been enormous interest in developing sp3-C–H bond functionalization,10 especially those of metal-free couplings.11 Among the various known synthetic methodologies, the direct formations of C–C and/or C–X bonds from allylic C–H bonds have attracted great attention.12 These methodologies can provide a series of intrinsic advantages, such as higher atom economy, shorter synthetic routes, and less energy and manpower usage, which leads to “benign by design”.13 Therefore, the design of efficient allylic functionalization without the use of metal catalysts is a continuing challenge at the forefront of organic chemistry.
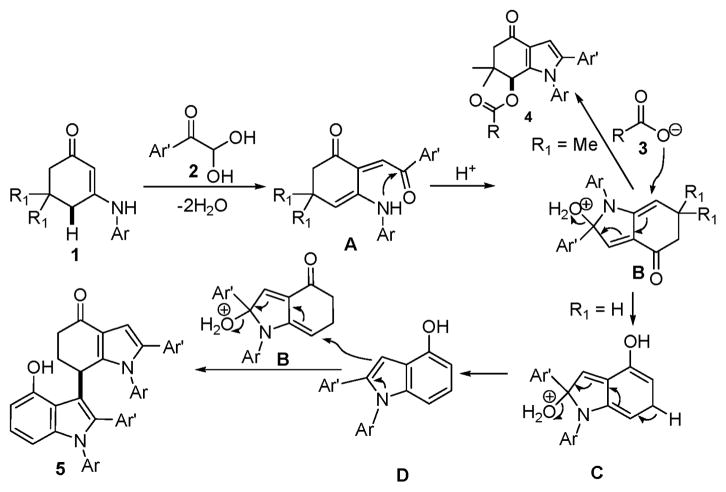
Over the past several years, our group has developed various multicomponent domino reactions (MDRs) that can offer easy access to useful multiple functionalized ring structures of chemical and pharmaceutical interest.14 During our continuous efforts on the development of useful multi-component domino reactions,15,16 herein, we report the challenging annulation of enamines with arylglyoxal monohydrate and the subsequent allylic activation with aliphatic carboxylic acids as nucleophilic reagents yielding multifunctionalized indoles (Scheme 1). The great aspect of the present domino reaction is shown by the fact that the formation of indole skeleton and its functionalization were readily achieved via metal-free allylic activation in an intermolecular manner and in a one-pot operation.
Scheme 1.
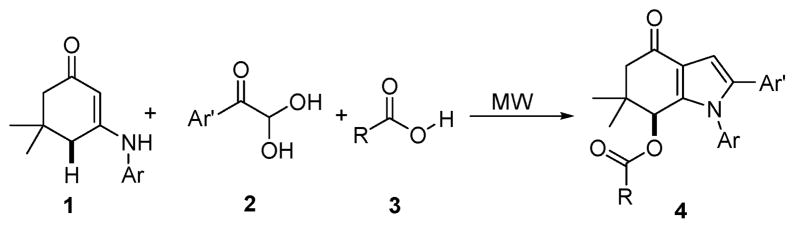
To begin this study, we chose 3-(p-tolylamino)-5,5-dimethyl-cyclohex-2-enone (1a), phenylglyoxal monohydrate (2a), and acetic acid (3a) as the standard substrates to search for suitable reaction conditions under microwave (MW) irradiation. Various solvents including DMF, benzene, CHCl3, EtOH, and HOAc were optimized. Non-protonic solvents, such as DMF, benzene, CHCl3, resulted in poor to moderate yields under MW irradiation for 10 min at 100 °C. In another case, when EtOH was selected as the solvent, the reaction occurred and product 4a was obtained in 54% isolated yield after purification is performed.‡ After systematic screening was made, we found that, in the presence of acetic acid, 1a was transformed into the desired product 4a in an excellent yield (84%). It is anticipated that acetic acid serves as nucleophile, reaction media and Bronsted acid promoter for the allylic functionalization simultaneously.
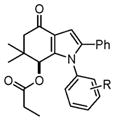
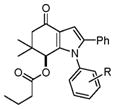
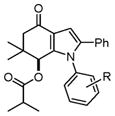
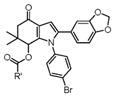
With these optimized conditions in hand, we examined the scope of this new multicomponent domino process by using various easily available starting materials. As revealed in Table 1, a range of invaluable polysubstituted indole derivatives can be synthesized in good to excellent yields. The reaction is easy to perform simply by subjecting a mixture of enamine 1 and arylglyoxal monohydrate 2 in various carboxylic acids to microwave heating. Subsequently, the enamine scope of this interesting transformation was investigated (Table 1). Several different N-substituents were compared and substituents bearing electron-withdrawing or electron-donating groups were found to be suitable for this domino reaction. Furthermore, using various carboxylic acids, such as propionic acid (3b), butyric acid (3c), and isobutyric acid (3d), together with different N-substituted enamines 1a–f resulted in the cyclization to corresponding substituted indoles 4e–4s smoothly. The benzo[d][1,3]dioxol-6-ylglyoxal monohydrate 2b was subjected to the reaction with various carboxylic acids, providing the corresponding benzo[d][1,3]dioxol-6-yl substituted indoles 4t–4v. The results exhibit the scope and generality of the novel allylic activation-based multicomponent domino reaction with respect to a range of enamine and carboxylic acid substrates. Indeed, the protocol provides a straightforward pathway to construct highly substituted indoles, which are generally prepared via metal-catalyzed coupling reactions.10
Table 1.
Domino synthesis of indoles 4 under MWa
| Entry | Product | R or R′ | Time/min | Yieldb/% |
|---|---|---|---|---|
| 1 |
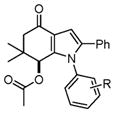 4a–4d |
4a, H (1a) | 18 | 85 |
| 2 | 4b, 4-Fluoro (1b) | 20 | 81 | |
| 3 | 4c, 4-Bromo (1d) | 22 | 82 | |
| 4 | 4d, 4-Methyl (1e) | 16 | 88 | |
| 5 |
 4c–4j |
4e, H (1a) | 18 | 86 |
| 6 | 4f, 4-Fluoro (1b) | 24 | 82 | |
| 7 | 4g, 4-Chloro (1c) | 20 | 84 | |
| 8 | 4h, 4-Bromo (1d) | 18 | 85 | |
| 9 | 4i, 4-Methyl (1e) | 16 | 87 | |
| 10 |
 4k–4n |
4j, 4-Methoxy (1f) | 15 | 89 |
| 11 | 4k, 4-Chloro (1b) | 22 | 79 | |
| 12 | 4l, 4-Bromo (1d) | 20 | 75 | |
| 13 | 4m, 4-Methyl (1e) | 18 | 81 | |
| 14 | 4n, 4-Ethoxy (1f) | 18 | 84 | |
| 15 |
 4o–4s |
4o, 4-Fluoro (1b) | 26 | 82 |
| 16 | 4p, 4-Chloro (1c) | 22 | 77 | |
| 17 | 4q, 4-Bromo (1d) | 22 | 79 | |
| 18 | 4r, 4-Methyl (1e) | 18 | 84 | |
| 19 | 4s, 4-Methoxy (1f) | 18 | 85 | |
| 20 |
 4t–4v |
4t, Methyl (3a) | 20 | 79 |
| 21 | 4u, Ethyl (3b) | 22 | 76 | |
| 22 | 4w, Propyl (3c) | 22 | 78 | |
| 23 | 4v, Isopropyl (3d) | 20 | 80 |
Reagents and conditions: 120 °C, microwave heating.
Isolated yield.
In view of these results, we turned our attention to investigate several differently substituted enamines. The reactions of arylglyoxal monohydrate (2a–2h) with N-substituted 3-amino-cyclohex-2-enones (1g–1j) in acetic acid were performed under the conditions described above for a short period (26–30 min). Interestingly, acetic acid was found to be the only efficient solvent and Bronsted acid catalyst, not as a nucleophile. Two molecules of arylglyoxal monohydrate and N-substituted 3-aminocyclohex-2-enones were introduced into the final poly-substituted bis-indoles (Scheme 2). The results are summarized in Table 2. Both electron-deficient and electron-rich aromatic groups on the N-substituted 3-aminocyclohex-2-enones gave very good chemical yields (5a–5l). Furthermore, functional groups like bromide and chloride were well tolerated. These functional groups provide ample opportunity for further functional group manipulations, for example, by modern cross-coupling reactions.
Scheme 2.
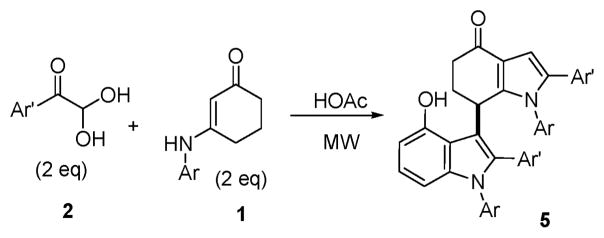
Table 2.
Domino synthesis of bis-indoles 5 under MWa
| Entry | 5 | Ar | Ar′ | Time/ min | Yieldb/ % |
|---|---|---|---|---|---|
| 1 | 5a | 4-Tolyl (1g) | Phenyl (2a) | 30 | 51 |
| 2 | 5b | 4-Tolyl (1g) | 4-Fluorophenyl (2c) | 28 | 54 |
| 3 | 5c | 4-Tolyl (1g) | 4-Chlorophenyl (2d) | 26 | 50 |
| 4 | 5d | 4-Tolyl (1g) | 4-Bromophenyl (2e) | 26 | 52 |
| 5 | 5e | 4-Tolyl (1g) | 4-Methoxyphenyl (2f) | 30 | 48 |
| 6 | 5f | 4-Tolyl (1g) | 3-Methoxyphenyl (2g) | 30 | 45 |
| 7 | 5g | 4-Chlorophenyl (1h) | 4-Fluorophenyl (2c) | 28 | 43 |
| 8 | 5h | 4-Chlorophenyl (1h) | 4-Tolyl (2h) | 30 | 40 |
| 9 | 5i | 4-Chlorophenyl (1h) | Benzo[d][1,3]dioxol-6-yl (2b) | 30 | 52 |
| 10 | 5j | 4-Fluorophenyl (1i) | Phenyl (2a) | 28 | 49 |
| 11 | 5k | 4-Fluorophenyl (1i) | Benzo[d][1,3]dioxol-6-yl (2b) | 30 | 47 |
| 12 | 5l | 4-Methoxyphenyl (1j) | Phenyl (2a) | 30 | 53 |
Reagents and conditions: HOAc (2.0 mL), 100 °C, microwave heating.
Isolated yield.
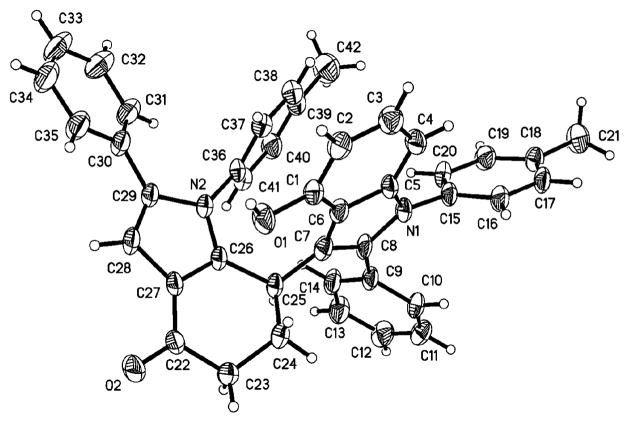
In all cases, the complexity of resulting products from this new reaction illustrates the remarkable chemo-, and regioselectivity of the sequence starting from very common and easily accessible inexpensive starting materials. The structural elucidation and the attribution of regioselectivity were unequivocally determined by NMR spectroscopic analysis and X-ray diffraction of single crystals that were obtained by slow evaporation of the solvent, as in the case of bis-indoles 5a (Fig. 1). During these domino processes, up to two indole rings and five sigma-bonds were formed accompanied by cleavage of two C=O and four C–O bonds of the aryl glyoxal, and direct indolation of hydroindoles B was simultaneously achieved via intermolecular coupling between sp2-C–H and sp3-C–H bonds (Scheme 3). This observation is truly rare, very interesting and important in organic chemistry. Only microwave irradiation can make the present multi-component domino reaction to occur rapidly and efficiently, while normal heating diminished both yield and speed.
Fig. 1.
X-Ray structure of 5a.
Scheme 3.
Complete regioselectivity and excellent yields (particularly for multicomponent reactions) were achieved for all cases that were examined. Furthermore, the reaction occurred at a very fast speed; in fact, all cases can be finished within 15–30 minutes. Water is nearly a sole by-product, which makes work-up convenient. In most cases, the products can precipitate out after cold water was poured into the reaction mixture. The continuing work on this reaction will be focused on the development of its asymmetric version in our lab.
The mechanism of this domino reaction is proposed in Scheme 3. An initial condensation generated imine-isomeric enamines A, which successively underwent intramolecular cyclization to give hydroindoles B. The key step of a divergence in reaction paths depends on the aromatization of hydroindoles B. With two methyl groups on the 5-position of cyclohex-2-enones, the 6-position of the hydroindoles B was activated to an electrophilic center and coupled with carboxylic acid, leading to multifunctionalized indoles 4. The hydroindoles B without methyl groups (R1 = H) were easily converted into the aromatic indoles D with a nucleophilic center at the 3-position, which through intermolecular coupling reaction with activated hydroindoles B resulted in the final polysubstituted bis-indoles.
In conclusion, a novel multicomponent domino reaction for the divergent synthesis of polyfunctionalized indoles and bis-indoles has been discovered. The reaction is easy to perform simply by mixing common reactants under microwave irradiation. The reaction is very fast and can be finished within 15–30 min with water as the major byproduct, making workup convenient.
Supplementary Material
Acknowledgments
We are grateful for financial support from the NSFC (No. 20928001, 21072163, 21002083, and 21102124), and Sci. Foundation in Interdisciplinary Major Res. Project of XZNU (No. 09XKXK01), PADA of Jiangsu Higher Education Institutions, Robert A. Welch Foundation (D-1361) and NIH (R21DA031860-01).
Footnotes
Electronic supplementary information (ESI) available: Experimental, full analytic and X-ray data. CCDC [846865, 846866]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/ c1cc15913e
Crystal data for 4a: C25H25NO3, Mr = 387.46, monoclinic, a = 8.6760(8) Å, b=12.8793(13) Å, c =9.4794(11) Å, U= 1045.73(19) Å3, T = 298(2) K, space group P21, Z = 2, 5349 reflections measured, 1936 unique (Rint = 0.0428) which were used in all calculations. The final wR(F2) was 0.0638 (all data). Crystal data for 5a: C42H34N2O2, Mr = 598.71, triclinic, a = 10.0878(13) Å, b = 11.1881(13) Å, c = 15.2089(16) Å, U = 1594.8(3) Å3, T = 298(2) K, space group P1̄, Z=2, 8283 reflections measured, 5520 unique (Rint=0.1165) which were used in all calculations. The final wR(F2) was 0.2184 (all data).
Contributor Information
Shu-Jiang Tu, Email: laotu@xznu.edu.cn.
Guigen Li, Email: guigen.li@ttu.edu.
Notes and references
- 1.Horton DA, Bourne GT, Smythe ML. Chem Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]
- 2.(a) Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873–2920. doi: 10.1021/cr040639b. [DOI] [PubMed] [Google Scholar]; (b) Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875–2911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]
- 3.Alex K, Tillack A, Schwarz N, Beller M. Angew Chem, Int Ed. 2008;47:2304–2307. doi: 10.1002/anie.200703823. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kuethe JT, Wong A, Davies IW. J Org Chem. 2004;69:7752–7754. doi: 10.1021/jo048887v. [DOI] [PubMed] [Google Scholar]; (b) Rutherford JL, Rainka MP, Buchwald SL. J Am Chem Soc. 2002;124:15168–15169. doi: 10.1021/ja0288993. [DOI] [PubMed] [Google Scholar]
- 5.(a) Coleman CM, O’Shea DF. J Am Chem Soc. 2003;125:4054–4055. doi: 10.1021/ja034283h. [DOI] [PubMed] [Google Scholar]; (b) Dunetz JR, Danheiser RL. J Am Chem Soc. 2005;127:5776–5777. doi: 10.1021/ja051180l. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Fayol A, Fang YQ, Lautens M. Org Lett. 2006;8:4203–4206. doi: 10.1021/ol061374l. [DOI] [PubMed] [Google Scholar]
- 6.Cui SL, Wang J, Wang YG. J Am Chem Soc. 2008;130:13526–13527. doi: 10.1021/ja805706r. [DOI] [PubMed] [Google Scholar]
- 7.Barluenga J, Trincado M, Rubio E, Gonzalez JM. Angew Chem, Int Ed. 2003;42:2406–2409. doi: 10.1002/anie.200351303. [DOI] [PubMed] [Google Scholar]
- 8.(a) Barluenga J, Rodriguez F, Fananas FJ. Chem–Asian J. 2009;4:1036–1048. doi: 10.1002/asia.200900018. [DOI] [PubMed] [Google Scholar]; (b) Bernini R, Fabrizi G, Sferrazza A, Cacchi S. Angew Chem, Int Ed. 2009;48:8078–8081. doi: 10.1002/anie.200902440. [DOI] [PubMed] [Google Scholar]
- 9.(a) Chen MS, Prabagaran N, Labenz NA, White MC. J Am Chem Soc. 2005;127:6970–6971. doi: 10.1021/ja0500198. [DOI] [PubMed] [Google Scholar]; (b) Delcamp JH, White MC. J Am Chem Soc. 2006;128:15076–15077. doi: 10.1021/ja066563d. [DOI] [PubMed] [Google Scholar]; (c) Fraunhoffer KJ, Bachovchin DA, White MC. Org Lett. 2005;7:223–226. doi: 10.1021/ol047800p. [DOI] [PubMed] [Google Scholar]; (d) Fraunhoffer KJ, Prabagaran N, Sirois LE, White MC. J Am Chem Soc. 2006;128:9032–9033. doi: 10.1021/ja063096r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For representative examples of sp3 C–H bonds functionalization, see: Dwight TA, Rue NR, Charyk D, Josselyn R, DeBoef B. Org Lett. 2007;9:3137–3139. doi: 10.1021/ol071308z.Li Z, Cao L, Li CJ. Angew Chem, Int Ed. 2007;46:6505–6507. doi: 10.1002/anie.200701782.Zhang YH, Li CJ. Angew Chem, Int Ed. 2006;45:1949–1952. doi: 10.1002/anie.200503255.
- 11.For organocatalysis, see: Berkessel A, Groger H, editors. Asymmetric Organocatalysis. Wiley-VCH; Weinheim, Germany: 2005. Acc Chem Res. 2004;37:8. special issue.List B. Synlett. 2001:1675–1686.List B. Tetrahedron. 2002;58:5573–5590.
- 12.(a) Arndtsen BA, Bergman RG, Mobley TA, Peterson TH. Acc Chem Res. 1995;28:154–162. [Google Scholar]; (b) Chatani N, Asaumi T, Yorimitsu S, Ikeda T, Kakiuchi F, Murai S. J Am Chem Soc. 2001;123:10935–10941. doi: 10.1021/ja011540e. [DOI] [PubMed] [Google Scholar]; (c) Chen HY, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. [DOI] [PubMed] [Google Scholar]; (d) Crabtree RH. J Organomet Chem. 2004;689:4083–4091. [Google Scholar]
- 13.(a) Winterton N. Green Chem. 2001;3:G73–G75. [Google Scholar]; (b) Zhang YH, Li CJ. J Am Chem Soc. 2006;128:4242–4243. doi: 10.1021/ja060050p. [DOI] [PubMed] [Google Scholar]; (c) Anastas PT, Warner JC. Green Chemistry Theory and Practice. Oxford University Press; New York: 1998. [Google Scholar]
- 14.(a) Jiang B, Li C, Shi F, Tu SJ, Kaur P, Wever W, Li G. J Org Chem. 2010;75:2962–2965. doi: 10.1021/jo1002278. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang B, Tu SJ, Kaur P, Wever W, Li G. J Am Chem Soc. 2009;131:11660–11661. doi: 10.1021/ja904011s. [DOI] [PubMed] [Google Scholar]; (c) Jiang B, Wang X, Shi F, Tu SJ, Ai T, Ballew A, Li G. J Org Chem. 2009;74:9486–9489. doi: 10.1021/jo902204s. [DOI] [PubMed] [Google Scholar]; (d) Li G, Wei HX, Kim SH, Carducci MD. Angew Chem, Int Ed. 2001;40:4277–4280. doi: 10.1002/1521-3773(20011119)40:22<4277::AID-ANIE4277>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (e) Ma N, Jiang B, Zhang G, Tu SJ, Wever W, Li G. Green Chem. 2010;12:1357–1361. [Google Scholar]
- 15.For domino reactions and atom economic synthesis, see ref. 15 and 16: Tietze LF, Brasche G, Gericke KM. Domino Reactions in Organic Synthesis. Wiley-VCH; Weinheim: 2006. Tietze LF, Brazel CC, Hoelsken S, Magull J, Ringe A. Angew Chem, Int Ed. 2008;47:5246–5249. doi: 10.1002/anie.200800626.Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206.Padwa A. Chem Soc Rev. 2009;38:3072–3081. doi: 10.1039/b816701j.Padwa A, Bur SK. Tetrahedron. 2007;63:5341–5378. doi: 10.1016/j.tet.2007.03.158.
- 16.(a) Huang Y, Walji AM, Larsen CH, MacMillan DWC. J Am Chem Soc. 2005;127:15051–15053. doi: 10.1021/ja055545d. [DOI] [PubMed] [Google Scholar]; (b) Lu M, Zhu D, Lu Y, Hou Y, Tan B, Zhong G. Angew Chem, Int Ed. 2008;47:10187–10191. doi: 10.1002/anie.200803731. [DOI] [PubMed] [Google Scholar]; (c) Snyder SA, Breazzano SP, Ross AG, Lin Y, Zografos AL. J Am Chem Soc. 2009;131:1753–1765. doi: 10.1021/ja806183r. [DOI] [PubMed] [Google Scholar]; (d) Yang JW, Fonseca MTH, List B. J Am Chem Soc. 2005;127:15036–15037. doi: 10.1021/ja055735o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.