Abstract
Previous microsatellite analyses of sympatric populations of Plasmodium vivax and P. falciparum in Brazil revealed higher diversity in the former species. However, it remains unclear whether regional species-specific differences in prevalence and transmission levels might account for these findings. Here, we examine sympatric populations of P. vivax (n = 87) and P. falciparum (n = 164) parasites from Pursat province, western Cambodia, where both species are similarly prevalent. Using 10 genome-wide microsatellites for P. falciparum and 13 for P. vivax, we found that the P. vivax population was more diverse than the sympatric P. falciparum population (average virtual heterozygosity [HE], 0.87 vs. 0.66, P = 0.003), with more multiple-clone infections (89.6% vs. 47.6%) and larger mean number of alleles per marker (16.2 vs. 11.1, P = 0.07). Both populations showed significant multi-locus linkage disequilibrium suggestive of a predominantly clonal mode of parasite reproduction. The higher microsatellite diversity found in P. vivax isolates, compared to sympatric P. falciparum isolates, does not necessarily result from local differences in transmission level and may reflect differences in population history between species or increased mutation rates in P. vivax.
Keywords: Plasmodium vivax, Plasmodium falciparum, population structure, genetic diversity, microsatellites
1. Introduction
Despite the continuous development of molecular typing methods and high-throughput platforms, microsatellites (tandem repeats of motifs of one to six nucleotides) remain among the most popular and informative genetic markers currently available for the study of eukaryotic organisms (Guichoux et al., 2011). The extensive variation found in microsatellites, which typically results in multiple alleles per locus, is mostly generated by strand-slippage events during DNA replication. Observed mutation rates (10−3 to 10−4 per locus per generation) result from the interplay between strand-slippage events and mismatch repair, which counteracts DNA slippage during replication (Schlötterer, 1998).
Microsatellites are abundant in the human malaria parasite Plasmodium falciparum; an average of one microsatellite locus is found every 2-3 kb of genome sequence (Su and Wellems, 1996). Microsatellites have been extensively used to compare the population structure of this species in different endemic settings, revealing striking geographic differences in the levels of genetic diversity and linkage disequilibrium (LD). Diversity was higher and LD lower in populations from holoendemic Africa (Anderson et al., 2000), whereas diversity was lower and LD higher in populations from hypoendemic South America (Anderson et al., 2000; Machado et al., 2004; Orjuela-Sánchez et al., 2009b), with intermediate patterns seen in Southeast Asia (Anderson et al., 2000; Anthony et al., 2005).
Microsatellite abundance correlates positively with genome AT content, which is extremely high in P. falciparum (the average AT content reaches 95% in repetitive domains) but is relatively low in the other major human malaria parasite, P. vivax (Tyagi et al., 2011). As a consequence, only 160 short repetitive sequences, many of them with features of classical microsatellites, have been characterized across the P. vivax genome (Carlton et al., 2008). Despite their relative paucity, microsatellites have provided valuable insights into the global genetic diversity of P. vivax, suggesting that a spectrum of population structures also exists for this species (Imwong et al., 2007; Ferreira et al., 2007; Karunaweera et al., 2008; Joy et al., 2008; Orjuela-Sánchez et al., 2009a; Van den Eede et al., 2010a, Van den Eede et al., 2010b; Gunawardena et al., 2010).
Two or more human malaria parasite species often occur in sympatry in endemic regions. Across most of the Amazon Basin and Southeast Asia, P. falciparum and P. vivax affect the same human populations and are transmitted essentially by the same vectors, but their population structure has been mostly examined separately. Two comparisons of microsatellite diversity in human malaria parasite species co-circulating in the same area in Brazil suggested that P. vivax infections are more diverse and comprise multiple clones more often than P. falciparum infections (Ferreira et al. 2007; Orjuela-Sánchez et al. 2009b). Moreover, a recent comparison of sequence diversity at the apical membrane protein-1 (ama-1) locus, which codes for a major malaria vaccine-candidate antigen, in sympatric parasites from Venezuela revealed much more polymorphism in P. vivax than in the P. falciparum orthologue (Ord et al., 2008). Since P. vivax currently predominates in South America, differences in species-specific transmission levels might translate into differences in genetic diversity observed in these studies. To investigate the potential confounding effect of transmission levels on population structure, here we sought to compare levels of microsatellite diversity in sympatric populations of P. vivax and P. falciparum from Pursat province, western Cambodia, an area where both species are similarly prevalent.
2. Materials and methods
2.1 Study area and population
The 87 P. vivax and 164 P. falciparum isolates completely typed in this study were obtained directly from patients enrolled into either of two studies of genetic resistance to malaria, conducted from June to December, 2008 (ClinicalTrials.gov identifiers, NCT00341003 and NCT00663546). During this time, we screened 1875 individuals with symptoms of malaria who presented to the Sampov Meas Referral Hospital in Pursat town (population, 57,000) (12°32′N, 103°55′E), located 174 km northwest of Phnom Penh, in western Cambodia (Figure 1). Of the 634 patients with detectable parasitemia, 278 (44%), 329 (52%) and 27 (4%) were infected with P. vivax, P. falciparum and both parasite species, confirming that P. vivax and P. falciparum are similarly prevalent in this region. Blood samples from 177 and 95 patients with single-species P. falciparum and P. vivax infections, respectively, were available for this study; no mixed-species infections were typed. While these patients presented from all six districts of Pursat province (where they reside permanently), they were exposed to infectious mosquito bites in the forested areas of Veal Veng district near the Cambodia-Thailand border. This is because they live close to these areas or travel to these areas for occupational activities such as hunting, mining and logging. In western Cambodia, both P. vivax and P. falciparum are transmitted by Anopheles dirus and An. minimus (Durnez et al., 2011).
Figure 1.
Map of Southeast Asia showing the location of Cambodia, its capital (Phnom Penh), and Pursat town (12°32′N, 103°55′E). The distance between Phnom Penh and Pursat, by road, is about 174 km.
Parasitized blood samples were collected under protocols approved by the Cambodian National Ethics Committee for Health Research and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, USA. All patients or their parents or legal guardians provided written informed consent.
2.2 Laboratory diagnosis of malaria
Giemsa-stained thick blood smears were examined by an expert microscopist. To estimate parasite density the total number of parasites per 200 white blood cells (WBCs) was multiplied by 40 to express the number of parasites per μL (assuming the patient has 8000 WBC per μL of whole blood). If no parasites were observed after counting 200 WBC, counting was continued up to 500 WBC and the total number of parasites per 500 WBC was used to calculate the parasite density. Blood samples were further examined for malaria parasites by nested PCR-based amplification of a species-specific segment of the 18S rRNA gene of human malaria parasites (Snounou et al., 1993). DNA templates for PCR amplification were isolated from 200 μL of whole blood, using a QIAamp DNA blood mini kit (Qiagen, Valencia, CA). To increase sample volume, 10 ng of genomic DNA were submitted to whole-genome amplification (WGA) with high-fidelity multiple displacement technology (Dean et al 2002) using the REPLI-g Mini kit (Qiagen, Valencia, United States).
2.3 Microsatellite typing of P. falciparum
Ten single-copy microsatellites with trinucleotide repeats, which map to 6 chromosomes of P. falciparum, were typed. Alleles were amplified by single-stage polymerase chain reaction (PCR) with the oligonucleotide primers listed in Table 1 (Anderson et al. 1999; Su et al. 1999). PCR mixtures (15 μl) contained 3 μl of DNA template (prepared as described above), 1 unit of Taq polymerase, 1.5 μl 10× buffer, 2 mM MgCl2, 0.1 mM of each dNTP, and 2 μM of each primer. All reagents were purchased from Fermentas (Vilnius, Lithuania), except for primers (both labeled with fluorescent dyes and unlabeled), which were supplied by Applied Biosystems (Foster City, CA). PCR cycling conditions were: 2 min, 94°C; (30 sec, 94 °C; 30 sec, 42°C; 30 sec, 40°C; 30 sec, 65°C) ×40 cycles; 5 min, 65 °C. After PCR amplification, products were pooled (TAA60+ARA2, PfG377+TA87, PfPK2+TA109, TA81+TA42, and Polyα+C2M3) according to their sizes and labels.
Table 1.
Sequences of oligonucleotide primers used to amplify 10 microsatellite loci of Plasmodium falciparum and virtual heterozygosity (HE) and number of alleles observed in 164 isolates from western Cambodia
| Namea | 5′ Label | Sequence 5′-3′ | Chromosome | Virtual heterozygosity (HE) |
No. of alleles |
|---|---|---|---|---|---|
| Polyα-F | AAAATATAGACGAACAGA | 4 | 0.882 | 17 | |
| Polyα-R | VIC | ATCAGATAATTGTTGGTA | c | ||
| TA60-F | VIC | CTCAAAGAAAAATAATTCA | 13 | 0.722 | 15 |
| TA60-R | AAAAAGGAGGATAAATACAT | ||||
| ARA2-F | NED | GTACATATGAATCACCAA | 11 | 0.698 | 10 |
| ARA2-R | GCIII GAGTATTATTAATA | ||||
| PfG377-F | GATCTCAACGGAAATTAT | 12 | 0.587 | 6 | |
| PfG377-R | NED | TTATGTTGGTACCGTGT | |||
| PfPK2-F | CTTTCATCGATACTACGA | 12 | 0.840 | 20 | |
| PfPK2-R | NED | CCTCAGACTGAAATGCAT | |||
| TA87-F | VIC | ATGGGTTAAATGAGGTACA | 6 | 0.747 | 11 |
| TA87-R | ACATGTTCATATTACTCAC | ||||
| TA109-F | FAM | TAGGGAACATCATAAGGAT | 6 | 0.345 | 9 |
| TA109-R | CCTATACCAAACATGCTAAA | ||||
| C2M3-F | NED | GGTTAATATGATCACAAAATG | 2 | 0.508 | 3 |
| C2M3-R | ATTGTTGATTCATGAAATGCA | c | |||
| TA81-F | FAM | GAAGAAATAAGGGAAGGT | 5 | 0.807 | 9 |
| TA81-R | TTTCACACAACACAGGATT | ||||
| TA42-F | VIC | ACAAAAGGGTGGTGATTCT | 5 | 0.466 | 12 |
| TA42-R | GTATTATTACTACTACTAAAG |
F = forward, R = reverse. The fluorescent dyes used to label primers were VIC (Applied Biosystems proprietary “green” fluorescent dye), NED (Applied Biosystems proprietary “yellow” fluorescent dye), and 6-FAM (6-carboxyfluorescein, “blue” fluorescent dye).
2.4 Microsatellite typing of P. vivax
Thirteen single-copy microsatellites with tri- or tetranucleotide repeats, which map to 10 chromosomes of P. vivax, were typed. Alleles were PCR-amplified with the oligonucleotide primers listed in Table 2 (Karunaweera et al., 2007). For each PCR reaction, 3 μl of genomic DNA (prepared as described above) were used with 2 mM MgCl2, 2 μM of each primer, 0.1 mM of each dNTP, 1 U of recombinant Taq polymerase, and 1.5 μl of 10× Taq polymerase buffer in a final volume of 15 μl. All reagents were purchased from Fermentas (Vilnius, Lithuania), except for primers (both labeled with fluorescent dyes and unlabeled), which were supplied by Applied Biosystems (Foster City, CA). PCR cycling conditions were: 2 min, 94°C; (30 s, 94°C; 40 s, 58°C; 50 s, 72°C) ×40 cycles; 5 min, 72°C. After PCR amplification, products were pooled as follows: MS1+MS3+MS9, MS2+MS5, MS4+MS6+MS10, MS7+MS15, and MS8+MS12+MS20.
Table 2.
Sequences of oligonucleotide primers used to amplify 13 microsatellite loci of Plasmodium vivax and virtual heterozygosity (HE) and number of alleles observed in 87 isolates from western Cambodia
| Namea | 5′ Label |
Sequence 5′-3′b | Chromosome | Virtual heterozygosity (HE) |
No.of alleles |
|---|---|---|---|---|---|
| MS1-F | FAM | TCAACTGTTGGAAGGGCAAT | 3 | 0.745 | 8 |
| MS1-R | ctgtcttTTGCTGCGTTTTTGTTTCTG | A | |||
| MS3-F | NED | GAAGATCCTGTGGAGGAGCA | 4 | 0.740 | 6 |
| MS3-R | ctgtcttCTCCTTCGCTCCTTTCCTTT | s | |||
| MS4-F | FAM | CGATTTACTGTTGACGCTGAA | 6 | 0.830 | 17 |
| MS4-R | ctgtcttCAAAGGAACATGCTCGATGA | ||||
| MS5-F | NED | CGTCCTCTATCGCGTACACA | 6 | 0.913 | 16 |
| MS5-R | ctgtcttAAAGGGAGAGGAGCGAAAAC | ||||
| MS6-F | VIC | GGTTCTTCGGTGATCTCTGC | 11 | 0.829 | 13 |
| MS6-R | ctgtcttGGAGGACATCAACGGGATT | ||||
| MS7-F | FAM | TTGCAGAAAATGCAGAGAGC | 12 | 0.884 | 14 |
| MS7-R | ctgtcttAGGGTCTTCAGCGTGTTGTT | A | |||
| MS8-F | NED | AGAGGAGGCAGAAATGCAGA | 12 | 0.939 | 26 |
| MS8-R | ctgtcttAGCCCCTTTGCGTTCTTTAT | ||||
| MS9-F | FAM | AGATGCCTACACGTTGACGA | 8 | 0.880 | 17 |
| MS9-R | ctgtcttGAAGCTGCCCATGTGGTAAT | ||||
| MS10-F | NED | TTATCCCTGCTGGATGTGAA | 13 | 0.947 | 22 |
| MS10-R | ctgtcttTCCTTCAGGTGGGACTTGTT | ||||
| MS12-F | FAM | AATGCGCATCCTATGTCTCC | 5 | 0.866 | 16 |
| MS12-R | ctgtcttCTGCTGTTGTTGTTGCTGCT | ||||
| MS15-F | FAM | TGTTTGCAAAGGAATCCACA | 5 | 0.891 | 16 |
| MS15-R | ctgtcttCGGCCAGATGAAAAGGATAA | ||||
| MS20-F | VIC | GCACAACAAATGCAAGATCC | 10 | 0.911 | 18 |
| MS20-R | ctgtcttGTGGCAGTGGCTCATCTTCT |
F = forward, R = reverse. The fluorescent dyes used to label primers were 6-FAM (6-carboxyfluorescein, “blue” fluorescent dye), VIC (Applied Biosystems proprietary “green” fluorescent dye), and NED (Applied Biosystems proprietary “yellow” fluorescent dye).
CTGTCTT tail (lowercase letters) was added to the 5′ end of the reverse primers to promote nontemplate-directed nucleotide addition to amplicons in a reproducible way (Raby et al., 2003).
2.5 Data analysis
Fragment size was measured on an ABI310 (Applied Biosystems) DNA sequencer. GeneScan 500-ROX (Applied Biosystems) was used as an internal size standard. The relative abundance of alleles (peak heights in electropherograms) was determined using STRand software (http://www.vgl.ucdavis.edu/informatics/strand.php). Major and minor microsatellite alleles coexisting in the same infection can theoretically be differentiated in this way, but minor alleles may be confused with artifacts such as stutter peaks or unspecific PCR amplification products. Stutter peaks are often produced by DNA strand slippage during PCR at intervals corresponding to nucleotide repeat sizes (de Valk et al., 2007). We scored two alleles at a locus when the minor peak was more than one-third the height of the predominant peak and its height exceeded 200 arbitrary fluorescence units (Havryliuk et al., 2008). Infections were considered to contain multiple clones if one or more loci showed more than one allele (Anderson et al. 2000). The proportions of multiple-clone infections were compared with standard χ2 tests with Yates correction. Multilocus haplotypes were defined as unique combinations of alleles at each locus analyzed; only the predominant alleles were considered for haplotype assignment in multiple-clone infections (Anderson et al. 1999).
We used the virtual heterozygosity estimate (HE) as a measure of overall genetic diversity: HE = [n/(n – 1)][1- Σpi2], where n is the number of isolates analyzed and pi is the frequency of the i-th allele in the population. HE gives the average probability that a pair of alleles randomly obtained from the population is different. Virtual heterozygosity ranges between 0 and 1. Haplotype diversity (Hd) was calculated in a similar way, giving the average probability (ranging between 0 and 1) that a pair of haplotypes randomly obtained from the population is different. The number of alleles per locus and virtual heterozygosity estimates were compared between species using standard Mann-Whitney U tests.
The standardized index of association (ISA) was used to test for evidence of overall multilocus LD in parasite populations. This test compares the variance (VD) of the number of alleles shared between all pairs of haplotypes observed in the population (D) with the variance expected under random association of alleles (VE) as follows: ISA = (VD/VE -1) (r – 1), where r is the number of loci analyzed. VE is derived from 10,000 simulated data sets in which alleles were randomly reshuffled among haplotypes. Significant LD is detected if VD is greater than 95% of the values derived from the reshuffled data sets. Data were analyzed with LIAN 3.5 software (Haubold and Hudson, 2000) available at: http://pubmlst.org/perl/mlstanalyse/mlstanalyse.pl?site=pubmlst&page=lia n&referer=pubmlst.org. Unique haplotypes were analyzed separately to distinguish between clonal and “epidemic” population structures of parasites (Smith et al. 1993).
Finally, we compared the allele frequency distribution of P. vivax and P. falciparum microsatellites as a test for recent population bottlenecks (Luikart et al., 1988). Alleles at low frequencies (e.g., below 0.1) tend to become less abundant after a major population bottleneck. The typically L-shaped distribution observed in populations at mutation-drift equilibrium, indicating the predominance of rare alleles, tends to become flattened as rare alleles are lost as a result of the bottleneck. Frequency distributions were compared with a standard χ2 test.
3. Results and discussion
3.1 Genetic diversity and linkage disequilibrium
We were able to genotype completely the vast majority of available parasite samples available (87 of 95 [91.5%] of P. vivax samples and 164 of 177 [92.6%] of P. falciparum samples). The P. vivax population in Pursat province was more diverse than the sympatric P. falciparum population, with more multiple-clone infections (χ2 = 41.05, 1 degree of freedom, P < 0.00001) and greater number of alleles per locus (P = 0.07, Mann-Whitney U test; Table 3). HE estimates ranged between 0.882 and 0.345 for P. falciparum microsatellites (Table 1) and between 0.947 and 0.740 for P. vivax microsatellites (Table 2). The difference in average HE estimates between these species is statistically significant (P = 0.003, Mann-Whitney U test).
Table 3.
Proportions of mixed-clone infections, mean number of microsatellite alleles per locus (and standard error of the mean, SE), genetic diversity (average virtual heterozygosity [HE] and SE) and linkage disequilibrium (standardized index of association [IAS]) in malaria parasite populations from Pursat, western Cambodia
|
IAS by infection typea |
|||||
|---|---|---|---|---|---|
| Parasite species | Multiple-clone infections, % |
Alleles per locus, mean ± SE (range) |
HE, mean ± SE (range) |
all | Those with unique haplotypes |
| P. falciparum | 47.6 | 21.2 ± 1.6 | 0.66 ± 0.06 | 0.051 | 0.036 |
| (3-20) | (0.34-0.88) | (n = 164) | (n = 151) | ||
| P. vivax | 89.6 | 16.1 ± 1.5 | 0.87 ± 0.02 | 0.010 | 0.010 |
| (6-26) | (0.74-0.95) | (n = 87) | (n = 87)b | ||
All ISA values were significantly larger than zero (P = 0.001), denoting significant multilocus linkage disequilibrium. For P. falciparum, we recalculated ISA considering only the 86 single-clone infections and obtained an even greater value of 0.068 (P =0.001).
All Plasmodium vivax isolates had unique haplotypes.
Both parasite populations showed highly significant multilocus LD. We found 151 unique 10-locus haplotypes in 164 P. falciparum isolates (one haplotype was shared by 2 parasites, two haplotypes were shared by 4 parasites and one haplotype was shared by 5 parasites), giving an Hd estimate of 0.998). The LD for P. falciparum remained significant when each haplotype was counted once (Table 3), indicating that recent epidemic expansions of particular lineages (Smith et al. 1993) do not account for the linkage observed. Given that wrong assignment of predominant haplotypes in multiple-clone infections may generate falsely recombinant haplotypes, we repeated the LD analysis considering only the 86 single-clone P. falciparum infections, obtaining an even greater IAS estimate (0.068, P = 0.001). Each of the 87 P. vivax isolates displayed a unique 13-locus haplotype (Hd = 1). The significant LD suggests that local populations of parasites of both species have relatively low rates of effective meiotic recombination, consistent with a predominantly clonal mode of reproduction that has also been found in other P. falciparum and P. vivax populations from Southeast Asia (Anderson et al., 2000; Anthony et al., 2005; Imwong et al., 2007; Van den Eede et al., 2010a) and South America (Machado et al., 2004; Ferreira et al., 2007; Orjuela-Sánchez et al., 2009a; Orjuela-Sánchez et al., 2009b; Van Den Eede et al., 2010b). Alternatively, natural selection on some of these microsatellite loci or neighboring sites may have originated nonrandom allele associations.
3.2 Allele frequency distribution and recent population history
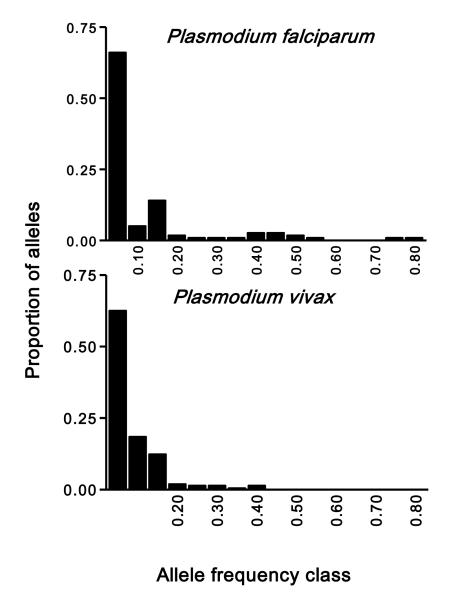
Major demographic events, such as a severe reduction in population size in the recent past (e.g. as a consequence of drug pressure) provide a possible explanation for the reduced diversity in P. falciparum from Cambodia. Population genetics theory predicts that a major population bottleneck within the past few generations would have resulted in a loss of rare alleles, distorting the overall distribution of microsatellite allele frequencies (Luikart et al., 1998). In fact, we found a significant difference in the frequency distributions of alleles from each species (χ2 = 25.6, 8 degrees of freedom, P =0.001), although both distributions were clearly L-shaped (Figure 2). Rare alleles (those found at frequencies below 0.1) predominated in both populations, but were less abundant in P. falciparum (71.4% of all alleles) than in P. vivax (81.0% of all alleles). The overall P. falciparum allele frequency distribution is slightly shifted to the right; 8 (7.1%) P. falciparum alleles, but none of P. vivax, were found at frequencies above 0.4. In conclusion, although we found no support for a major recent bottleneck in the P. falciparum population from Pursat, we did find a trend towards some loss of rare alleles in this population. The observed between-species difference in allele frequency distributions may result from demographic events, but natural selection on some of our microsatellite loci may also play a role. In fact, more P. vivax than P. falciparum microsatellites (9 vs. 4) map to protein-coding sequences, although none of them is located in genes encoding major surface antigens or drug-resistance traits that are expected to be under strong selective pressure (Ferreira et al., 2007).
Figure 2.
Distribution of microsatellite allele frequencies in P. falciparum isolates (n = 164) and Plasmodium vivax (n = 87) collected in Pursat province, western Cambodia. Data are presented for all loci combined (13 P. vivax microsatellites with a total of 211 alleles and 10 P. falciparum microsatellites with a total of 112 alleles). An L-shaped frequency distribution is expected for non-bottlenecked populations at mutation-drift equilibrium (Luikart et al., 1998).
3.3 Multiplicity of infection
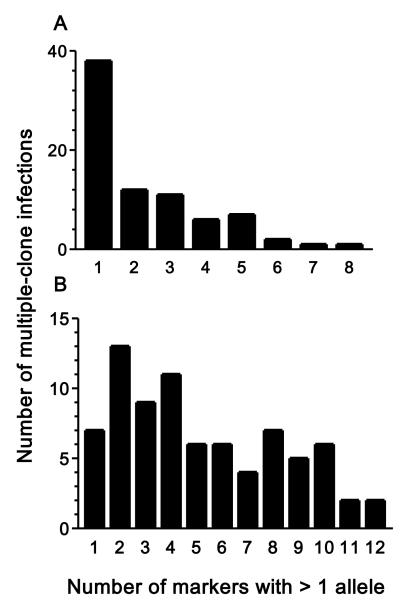
Multiple clones present in the same parasite isolate (i. e., multiple-clone infections) were detected when more than one allele was found in one or more single-copy microsatellites analyzed for each species (Table 3). However, the number of markers with more than one allele detected in multiple-clone infections varied widely (between 1 and 8 for P. falciparum and between 1 and 12 for P. vivax). Note that most (38 of 78, 61.5%) multiple-clone P. falciparum infections had a single microsatellite (out of 10 typed) with more than one allele, suggesting that a fairly number of markers are required to find multiple-clone P. falciparum infections in western Cambodia. In contrast, 74.4% (58 of 78) of all multiple-clone P. vivax infections had three or more (out of 13) markers with more than one allele (Figure 3), suggesting that a reduced set of markers would be able to diagnose all or nearly all multiple-clone P. vivax infections in the study site. A more conservative approach to define multiple-clone infections would be to consider only those with more than one allele at two or more microsatellite loci. Applying this criterion would result in an even greater disparity in the proportion of multiple-clone infections between P. vivax (81.6%) and P. falciparum (24.3%), which is clearly statistically significant (χ2 = 73.15, 1 degree of freedom, P < 0.00001).
Figure 3.
Frequency distribution of Plasmodium falciparum (A) and P. vivax (B) isolates collected in Pursat province, western Cambodia, according to the number of single-copy microsatellite loci with more than one allele. Data for 78 multiple-clone infections detected for each species. The maximum number of markers with >1 allele was eight for P. falciparum and twelve for P. vivax.
Increasing the number of markers examined theoretically improves our ability to detect multiple-clone infections (i. e., to find at least one marker with more than one allele) (Havryliuk and Ferreira, 2009). Differences in the number of microsatellites examined might account, at least in part, for the between-species differences in multiplicity of infection in Pursat, since more markers were used for P. vivax typing. To examine the minimum number of markers required to estimate the prevalence of multiple-clone infections for different species, we first ranked microsatellite loci according to their HE estimates (shown in Tables 1 and 2). When only the marker with the highest HE value was used (Poly-∝ for P. falciparum and MS10 for P. vivax), it detected 23 out of 78 P. falciparum multiple-clone infections diagnosed with the complete 10-marker set (29.5% of the total) and 30 out of 78 P. vivax multiple-clone infections diagnosed with the complete 13-marker set (38.5% of the total). By combining this marker with the one with the second largest HE (PfPk2 for P. falciparum and MS8 for P. vivax), we detected 43 P. falciparum and 47 P. vivax multiple-clone infections.
The remaining microsatellites were then added one at time to the panel of markers, in order of decreasing HE (Orjuela-Sánchez et al., 2009b). Sixty-four P. falciparum and 74 P. vivax multiple-clone infections would have been diagnosed with a restricted panel comprising only the six microsatellite loci with the highest HE values, resulting in estimates of prevalence of multiple-clone infections of 85.1% for P. vivax and 42.1% for P. falciparum.
Microsatellite panels with equal number of markers for each species would always detect a higher prevalence of multiple-clone infections among P. vivax isolates, compared to P. falciparum isolates from the same region of Cambodia (Figure 4). Ten microsatellites would suffice for diagnosing all 78 multiple-clone P. vivax infections. We conclude that our ability to detect multiple-clone infections in P. vivax is influenced by the greater diversity in microsatellite markers in this species, but not by the fact that a larger number of markers were typed.
Figure 4.
Relationship between the number of microsatellite markers typed and the prevalence of multiple-clone infections detected in 164 Plasmodium falciparum and 87 P. vivax isolates collected in Pursat province, western Cambodia. The markers were added one by one in decreasing order of virtual heterozygosity (HE; see Tables 1 and 2). By combining the nine microsatellite loci with the highest HE values, all P. vivax multiple-clone infection could be detected. Note that when the same number of markers for P. falciparum and P. vivax is used, a greater prevalence of multiple-clone infections is always diagnosed among P. vivax isolates.
The high prevalence of multiple-clone P. vivax infections in Cambodia is far from surprising. In fact, most populations of this species so far examined show high clonal diversity, which would allow for frequent mating between genetically unrelated gametocytes found in the same mosquito blood meal, favoring outbreeding during the sexual reproduction of parasites in the vector. Hypnozoites, the dormant liver stages, provide a biologic basis for increased multiplicity of infection in P. vivax, compared to non-relapsing species such as P. falciparum. If super-infection with a different clone occurs weeks or months after the primary infection, genetically diverse strains are likely to accumulate in the same patient. If the dormant stages awaken at the time the second brood of parasites concludes its schizogony in the liver, genetically diverse strains are likely to be found in the bloodstream and their gametocytes may be taken up by the same vector (Havryliuk and Ferreira, 2009). Alternatively, several populations of dormant stages may be reactivated at once by an external stimulus, such as an unrelated systemic febrile illness (White, 2011).
Malaria parasites display sexual reproduction during their life cycles in the mosquito vector, but effective recombination rates may be quite variable among populations. In areas where most human hosts harbor a single parasite clone, selfing is common, since only genetically identical male and female gametes are taken up by mosquitoes during a single blood meal. This situation would maintain linkage disequilibrium among physically unlinked loci during several generations. Where multiple-clone infections are common in humans, gametes from different clones are often available in the same bloodmeal; they may fuse and originate detectable recombination events (outbreeding), which break down preexisting associations between physically unlinked loci and generate new multilocus haplotypes. In human P. vivax infections, virtually all patients have mature gametocytes at the time of diagnosis (Lima et al., 2012), suggesting that all circulating clones are potentially able to produce gametocytes early during the course of infection.
Paradoxically, however, frequent multiple-clone infections do not disrupt the clonal structure observed in Cambodia and other P. vivax populations from hypo- to mesoendemic regions (Imwong et al., 2007; Ferreira et al., 2007; Orjuela-Sánchez et al., 2009a; Gunawardena et al., 2010; Van den Eede et al., 2010b). A possible explanation is that the proportions of mature gametocytes produced by each genetically distinct clone in multiple-clone infections may be so different (say, 95% vs. 5%) that selfing remains the most likely outcome when two gametes taken at random fuse in the mosquito vector. Furthermore, the maintenance of high inbreeding rates, leading to strong LD, does not translate into reduced levels of genetic diversity in P. vivax. If there is so much inbreeding, how so many microsatellite haplotypes are generated? Our results suggest that microsatellite repeats do not require frequent meiotic recombination for their diversification, creating novel alleles and haplotypes; mitotic recombination events may play a major role in generating these new alleles. Alternatively, LD may not necessarily imply a predominantly clonal mode of parasite propagation, but might result, at least in part, from strong natural selection on some of the microsatellite markers used. However, none of them map to either coding sequences or genome regions that are expected to be under such an intense selective pressure.
3.4 Number of genetic markers and haplotype diversity
Increasing the number of markers examined may also improve our ability to score more multilocus haplotypes and inflate our estimates of haplotype diversity. Therefore, it is essential to define the minimal number of markers that would accurately estimate the overall genetic diversity when comparing malaria parasite populations and species. Figure 5 shows that the highest possible number of P. vivax multilocus haplotypes (87 haplotypes in 87 isolates, corresponding to Hd = 1) would have been scored with a rather restricted panel consisting of only four microsatellite loci with the highest HE values (MS2, MS5, MS8, and MS10). In contrast, nine of the ten microsatellites would be required to score all P. falciparum haplotypes (151 haplotypes in 164 isolates). We thus conclude that the greater haplotype diversity found in the P. vivax population from Cambodia, when compared with sympatric P. falciparum isolates, does not result from the use of a larger number of genetic markers to characterize the former species; in fact, we would have obtained identical results with only four informative markers.
Figure 5.
Relationship between the number of microsatellite markers typed and the number of genetically distinct haplotypes (unique combinations of alleles) detected in 164 Plasmodium falciparum and 87 P. vivax isolates collected in Pursat province, western Cambodia. The markers were added one by one in decreasing order of virtual heterozygosity (HE; see Tables 1 and 2). By combining the four microsatellite loci with the highest HE values, we were able to distinguish all P. vivax haplotypes, indicating that the greater haplotype diversity in P. vivax than P. falciparum (87 different haplotypes in 87 isolates vs. 151 different haplotypes in 164 isolates) is not merely a consequence of the use of more microsatellite markers (13 vs. 10) to characterize P. vivax isolates.
3.5 Conclusions
This is the first study comparing the population structure of sympatric P. vivax and P. falciparum isolates in Southeast Asia. We found higher microsatellite diversity in P. vivax, supported by a greater average virtual heterozygosity and a larger mean number of alleles per marker. Moreover, we identified more multiple-clone infections among P. vivax than P. falciparum isolates. These between-species differences seem to result neither from differences in the prevalence of each species in the study site nor from the use of a greater number of microsatellite markers to characterize the P. vivax population (since similar differences would have been observed by using the same number of markers for each species).
Most studies have shown extensive diversity in di-, tri- and tetranucleotide microsatellites in P. vivax populations from areas of low (Sri Lanka, Mexico and Brazil), intermediate (Myanmar, Vietnam and Cambodia) and high (Papua New Guinea) levels of malaria transmission (Imwong et al., 2006; Imwong et al., 2007; Ferreira et al., 2007; Karunaweera et al., 2008; Joy et al., 2008; Orjuela-Sánchez et al., 2009a; Van den Eede et al., 2010a, Van den Eede et al., 2010b; Gunawardena et al., 2010; Koepfli et al., 2011; but see also Leclerc et al., 2004 and Bruce et al., 2006). Therefore, although microsatellite repeats are relatively rare across the genome of P. vivax, the vast majority of those so far characterized are highly polymorphic.
It remains unclear whether (and, if so, why) microsatellites are intrinsically more diverse in P. vivax than in sympatric populations of P. falciparum. Our results might have originated from a bias in the selection of microsatellite-type repeats used for typing parasites of each species, which may not reflect a genome-wide pattern. However, a recent analysis of six whole-genome sequences of each species has consistently revealed significantly more diversity not only in microsatellites but also in single-nucleotide polymorphisms of P. vivax compared to P. falciparum (Neafsey et al., 2012).
Several factors might explain the disparity in microsatellite diversity observed in the two major human malaria parasite species. Historical demography is one of them. Cambodia is close to the putative region of origin of P. vivax (Cornejo and Escalante, 2006), where genetic diversity is expected to be highest for this species. In contrast, part of the P. falciparum diversity originally seen in Africa may have been lost as parasite populations migrated to Southeast Asia (Tanabe et al., 2010). Nevertheless, a recent comparison of whole-genome sequences of isolates of both species from different continents suggests that the greater diversity in P. vivax is not a geographically restricted phenomenon (Neafsey et al., 2012). Alternatively, more recent demographic events, such as drastic changes in the population size of P. falciparum, might have reduced the overall diversity of parasites from Cambodia. Partial support to this hypothesis is provided by the analysis of microsatellite allele frequency distributions (Figure 2), which is consistent with some loss of rare alleles in the P. falciparum population from Pursat.
Finally, repetitive sequences in P. vivax might show greater plasticity, perhaps as a consequence of a partially defective DNA mismatch repair. Little is currently known about DNA mismatch repair pathways in malaria parasites; however, DNA mismatch repair defects induced by mutations in MSH-2 (a homologue of a protein involved in the well-characterized methyl-directed MutHLS mismatch repair system of Escherichia coli) favor microsatellite instability in the rodent malaria parasite P. berghei (Bethke et al., 2007). A better understanding of DNA mismatch repair pathways in different malaria parasites may be required to interpret microsatellite-based comparisons between species.
Highlights.
Plasmodium vivax was hypothesized to be genetically more diverse than P. falciparum.
We found more microsatellite diversity in P. vivax than in P. falciparum from Cambodia.
These findings may reflect differences in population biology or genome plasticity between species.
Acknowledgments
This research was supported by funds from the National Institutes of Health (NIH) grant RO1 AI 075416 to M.U.F., the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant 470570/2006-7 to M.U.F. and the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grant 07/51199-0 to M.U.F. This study was supported in part by funds from the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH, provided to R.M.F. P.O.S. and M.U.F. received scholarships from CNPq, and P.T.R. received a scholarship from FAPESP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the patients from Pursat province for their cooperation, and Seila Suon and Sokunthea Sreng for their collaborative efforts in Pursat. We also thank Carmen S. A. Takata, Márcio M. Yamamoto, Erika S. Phelps and Michael A. Krause for laboratory and analytical support.
Abbreviations
- AMA-1
apical membrane protein-1
- CAPES
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil
- CNPq
Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FAPESP
Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil
- Hd
haplotype diversity
- HE
virtual heterozygosity
- ISA
standardized index of association
- LD
linkage disequilibrium
- NIAID
National Institute of Allergy and Infectious Diseases
- NIH
National Institutes of Health, United States
- PCR
polymerase chain reaction
- WBC
white blood cells
- WGA
whole genome amplification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson TJC, Su XZ, Bockarie M, Lagog M, Day KP. Twelve microsatellite markers for characterization of Plasmodium falciparum from finger-prick blood samples. Parasitology. 1999;119:113–125. doi: 10.1017/s0031182099004552. [DOI] [PubMed] [Google Scholar]
- Anderson TJC, Haubold B, Williams JT, Estrada-Franco JG, Richardson L, Mollinedo R, Bockarie M, Mokili J, Mharakurwa S, French N, Whitworth J, Velez ID, Brockman AH, Nosten F, Ferreira MU, Day KP. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Molecular Biology and Evolution. 2000;17:1467–1482. doi: 10.1093/oxfordjournals.molbev.a026247. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Conway DJ, Cox-Singh J, Matusop A, Ratnam S, Shamsul S, Singh B. Fragmented population structure of Plasmodium falciparum in a region of declining endemicity. Journal of Infectious Diseases. 2005;191:1558–1564. doi: 10.1086/429338. [DOI] [PubMed] [Google Scholar]
- Bethke L, Thomas S, Walker K, Lakhia R, Rangarajan R, Wirth D. The role of DNA mismatch repair in generating genetic diversity and drug resistance in malaria parasites. Molecular and Biochemical Parasitology. 2007;155:18–25. doi: 10.1016/j.molbiopara.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton JM, Adams JH, Silva JC, Bidwell SL, Lorenzi H, Caler E, Crabtree J, Angiuoli SV, Merino EF, Amedeo P, Cheng Q, Coulson RM, Crabb BS, del Portillo HA, Essien K, Feldblyum TV, Fernandez-Becerra C, Gilson PR, Gueye AH, Guo X, Kang’a S, Kooij TW, Korsinczky M, Meyer EV, Nene V, Paulsen I, White O, Ralph SA, Ren Q, Sargeant TJ, Salzberg SL, Stoeckert CJ, Sullivan SA, Yamamoto MM, Hoffman SL, Wortman JR, Gardner MJ, Galinski MR, Barnwell JW, Fraser-Liggett CM. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornejo OE, Escalante AA. The origin and age of Plasmodium vivax. Trends in Parasitology. 2006;22:558–563. doi: 10.1016/j.pt.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean FB, Hosono S, Fang L, Wu X, Farugi AF, Bray-Ward P, Sun Z, Zong Q, Du Y, Du J, Driscoll M, Song W, Kingsmore SF, Egholm M, Lasken RS. Comprehensive human genome amplification using multiple displacement amplification. Proceedings of National Academy of Sciences of USA. 2002;99:5261–5266. doi: 10.1073/pnas.082089499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Valk HA, Meis JFGM, Klaasen CHW. Microsatellite based typing of Aspergillus fumigatus: Strengths, pitfalls and solutions. Journal of Microbiological Methods. 2007;69:268–272. doi: 10.1016/j.mimet.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Durnez L, Van Bortel W, Denis L, Roelants P, Veracx A, Trung HD, Sochantha T, Coosemans M. False positive circumsporozoite protein ELISA: a challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malaria Journal. 2011;10:195. doi: 10.1186/1475-2875-10-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MU, Karunaweera ND, da Silva-Nunes M, Silva NS, Hartl DL, Wirth DF. Population structure and transmission dynamics of Plasmodium vivax in rural Amazonia. Journal of Infectious Diseases. 2007;195:1218–1226. doi: 10.1086/512685. [DOI] [PubMed] [Google Scholar]
- Guichoux E, Lagache L, Wagner S, Chumeil P, Léger P, Lepais O, Lepoittevin C, Malausa T, Revardel E, Salin F, Petit RJ. Current trends in microsatellite genotyping. Molecular Ecology Resources. 2011;11:591–611. doi: 10.1111/j.1755-0998.2011.03014.x. [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Karunaweera ND, Ferreira MU, Phone-Kyaw M, Pollack RJ, Alifrangis M, Rajakaruna RS, Konradsen F, Amerasinghe PH, Schousboe ML, Galappaththy GN, Abeyasinghe RR, Hartl DL, Wirth DF. Geographic structure of Plasmodium vivax: microsatellite analysis of parasite populations from Sri Lanka, Myanmar, and Ethiopia. American Journal of Tropical Medicine and Hygiene. 2010;82:235–242. doi: 10.4269/ajtmh.2010.09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubold B, Hudson RR. LIAN 3.0: detecting linkage disequilibrium in multilocus data. Linkage Analysis. Bioinformatics. 2000;16:847–848. doi: 10.1093/bioinformatics/16.9.847. [DOI] [PubMed] [Google Scholar]
- Havryliuk T, Ferreira MU. A closer look at multiple-clone Plasmodium vivax infections: detection methods, prevalence and consequences. Memórias do Instituto Oswaldo Cruz. 2009;104:67–73. doi: 10.1590/s0074-02762009000100011. [DOI] [PubMed] [Google Scholar]
- Havryliuk T, Orjuela-Sánchez. P, Ferreira MU. Plasmodium vivax: microsatellite analysis of multiple-clone infections. Experimental Parasitology. 2008;120:330–336. doi: 10.1016/j.exppara.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Imwong M, Nair S, Pukrittayakamee S, Sudimack D, Williams JT, Mayxay M, Newton PN, Kim JR, Nandy A, Osorio L, Carlton JM, White NJ, Day NPJ, Anderson TJC. Contrasting genetic structure in Plasmodium vivax populations from Asia and South America. International Journal for Parasitology. 2007;37:1013–1022. doi: 10.1016/j.ijpara.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Imwong M, Sudimack D, Pukrittayakamee S, Osório L, Carlton JM, Day NP, White NJ, Anderson TJ. Microsatellite variation, repeat array length, and population history of Plasmodium vivax. Molecular Biology and Evolution. 2006;23:1016–1018. doi: 10.1093/molbev/msj116. [DOI] [PubMed] [Google Scholar]
- Joy DA, Gonzalez-Ceron L, Carlton JM, Gueye A, Fay M, McCutchan TF, Su XZ. Local adaptation and vector-mediated population structure in Plasmodium vivax malaria. Molecular Biology and Evolution. 2008;25:1245–1252. doi: 10.1093/molbev/msn073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunaweera ND, Ferreira MU, Munasinghe A, Barnwell JW, Collins WE, King CL, Kawamoto F, Hartl DL, Wirth DF. Extensive microsatellite diversity in the human malaria parasite Plasmodium vivax. Gene. 2008;410:105–112. doi: 10.1016/j.gene.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Karunaweera ND, Ferreira MU, Hartl DL, Wirth DF. Fourteen polymorphic microsatellite DNA markers for the human malaria parasite Plasmodium vivax. Molecular Ecology Notes. 2007;7:172–175. [Google Scholar]
- Koepfli C, Ross A, Kiniboro B, Smith TA, Zimmerman PA, Siba P, Mueller I, Felger I. Multiplicity and diversity of Plasmodium vivax infections in a highly endemic region in Papua New Guinea. PLoS Neglected Tropical Diseases. 2011;5:e1424. doi: 10.1371/journal.pntd.0001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima NF, Bastos MS, Ferreira MU. Plasmodium vivax: reverse transcriptase real-time PCR for gametocyte detection and quantitation in clinical samples. Experimental Parasitology. 2012;132:348–354. doi: 10.1016/j.exppara.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc MC, Durand P, Gauthier C, Patot S, Billotte N, Menegon M, Severini C, Ayala FJ, Renaud F. Meager genetic variability of the human malaria agent Plasmodium vivax. Proceedings of the National Academy of Sciences of the USA. 2004;101:14455–14460. doi: 10.1073/pnas.0405186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luikart G, Allendorf FW, Cornuet JM, Sherwin WB. Distortion of allele frequency distributions provides a test for recent population bottlenecks. Journal of Heredity. 1998;89:238–247. doi: 10.1093/jhered/89.3.238. [DOI] [PubMed] [Google Scholar]
- Machado RLD, Póvoa MM, Calvosa VSP, Ferreira MU, Rossit ARB, dos Santos EJM, Conway DJ. Genetic structure of Plasmodium falciparum populations in the Brazilian Amazon region. Journal of Infectious Diseases. 2004;190:1547–1555. doi: 10.1086/424601. [DOI] [PubMed] [Google Scholar]
- Neafsey DE, Galinsky K, Jiang RH, Young L, Sykes SM, Saif S, Gujja S, Goldberg JM, Young S, Zeng Q, Chapman SB, Dash AP, Anvikar AR, Sutton PL, Birren BW, Escalante AA, Barnwell JW, Carlton JM. The malaria parasite Plasmodium vivax exhibits greater genetic diversity than Plasmodium falciparum. Nature Genetics. 2012;44:1046–1050. doi: 10.1038/ng.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord RL, Tami A, Sutherland CJ. ama1 genes of sympatric Plasmodium vivax and P. falciparum from Venezuela differ significantly in genetic diversity and recombination frequency. PLoS One. 2008;3:e3366. doi: 10.1371/journal.pone.0003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Sánchez P, da Silva-Nunes M, da Silva NS, Scopel KK, Gonçalves RM, Malafronte RS, Ferreira MU. Population dynamics of genetically diverse Plasmodium falciparum lineages: community-based prospective study in rural Amazonia. Parasitology. 2009a;136:1097–1105. doi: 10.1017/S0031182009990539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjuela-Sánchez P, da Silva NS, da Silva-Nunes M, Ferreira MU. Recurrent parasitemias and population dynamics of Plasmodium vivax polymorphisms in rural Amazonia. American Journal of Tropical Medicine and Hygiene. 2009b;81:961–968. doi: 10.4269/ajtmh.2009.09-0337. [DOI] [PubMed] [Google Scholar]
- Raby BA, Silverman EK, Lazarus R, Lange C, Kwiatkowiski DJ, Weiss ST. Chromosome 12q harbors multiple genetic loci related to asthma and asthma-related phenotypes. Human Molecular Genetics. 2003;12:1973–1979. doi: 10.1093/hmg/ddg208. [DOI] [PubMed] [Google Scholar]
- Russell B, Suwanarusk R, Lek-Uthai U. Plasmodium vivax genetic diversity: microsatellite length matters. Trends in Parasitology. 2006;22:399–401. doi: 10.1016/j.pt.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Schlötterer C. Genome evolution: Are microsatellites really simple sequences? Current Biology. 1998;8:R132–134. doi: 10.1016/s0960-9822(98)70989-3. [DOI] [PubMed] [Google Scholar]
- Smith JM, Smith NH, O’Rourke M, Spratt BG. How clonal are bacteria? Proceedings of the National Academy of Sciences of the USA. 1993;90:4384–4388. doi: 10.1073/pnas.90.10.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of four human malaria parasite species in field samples by polymerase chain reaction and detection of a high prevalence of mixed infections. Molecular and Biochemical Parasitology. 1993;58:283–292. doi: 10.1016/0166-6851(93)90050-8. [DOI] [PubMed] [Google Scholar]
- Su X, Ferdig MT, Huang Y, Huynh CQ, Liu A, You J, Wootton JC, Wellems TE. A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science. 1999;286:1351–1353. doi: 10.1126/science.286.5443.1351. [DOI] [PubMed] [Google Scholar]
- Su X, Wellems TE. Toward a high-resolution Plasmodium falciparum linkage map: polymorphic markers from hundreds of simple sequence repeats. Genomics. 1996;33:430–444. doi: 10.1006/geno.1996.0218. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Mita T, Jombart T, Eriksson A, Horibe S, Palacpac N, Ranford-Cartwright L, Sawai H, Sakihama N, Ohmae H, Nakamura M, Ferreira MU, Escalante AA, Prugnolle F, Björkman A, Färnert A, Kaneko A, Horii T, Manica A, Kishino H, Balloux F. Plasmodium falciparum accompanied the human expansion out of Africa. Current Biology. 2010;20:1283–1289. doi: 10.1016/j.cub.2010.05.053. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Sharma M, Das A. Comparative genomic analysis of simple sequence repeats in three Plasmodium species. Parasitology Research. 2011;108:451–458. doi: 10.1007/s00436-010-2086-5. [DOI] [PubMed] [Google Scholar]
- Van den Eede P, Erhart A, van der Auwera G, van Overmeir C, Thang ND, Hung le X, Anné J, D’Alessandro U. High complexity of Plasmodium vivax infections in symptomatic patients from a rural community in central Vietnam detected by microsatellite genotyping. American Journal of Tropical Medicine and Hygiene. 2010a;82:223–227. doi: 10.4269/ajtmh.2010.09-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eede P, van der Auwera G, Delgado C, Huyse T, Soto-Calle VE, Gamboa D, Grande T, Rodríguez H, Llanos A, Anné J, Erhart A, D’Alessandro U. Multilocus genotyping reveals high heterogeneity and strong local population structure of the Plasmodium vivax population in the Peruvian Amazon. Malaria Journal. 2010b;9:151. doi: 10.1186/1475-2875-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malaria Journal. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]