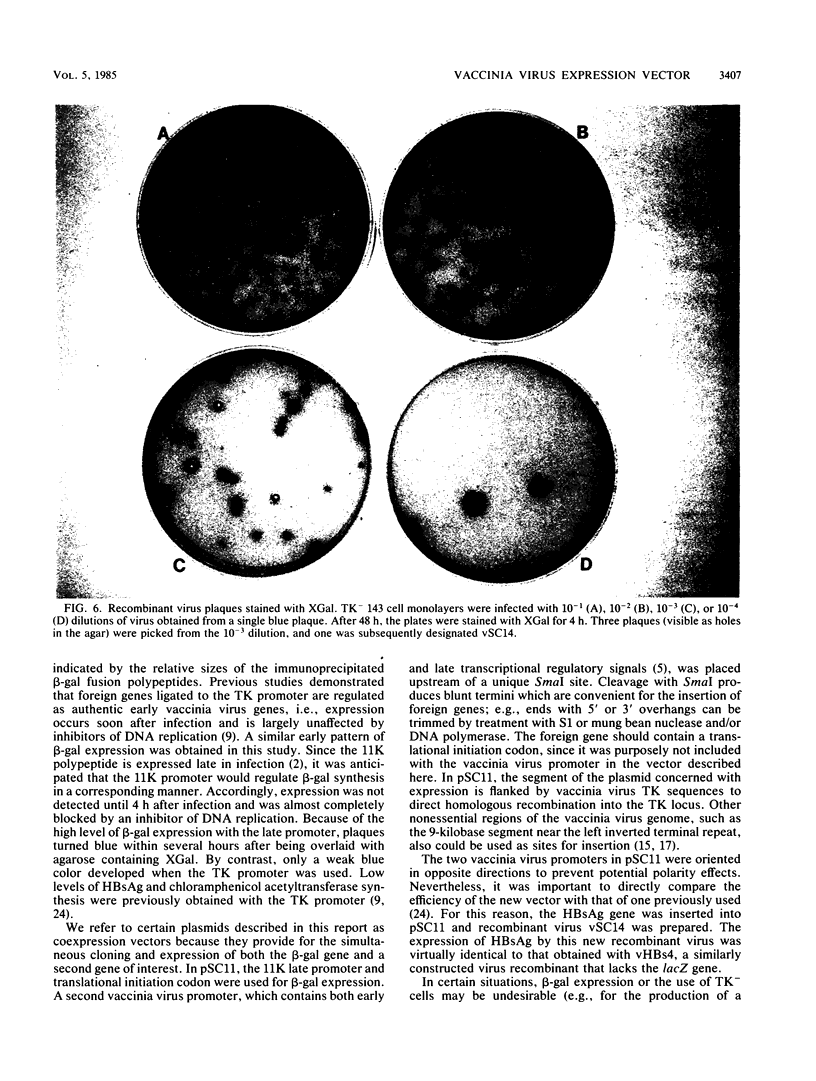
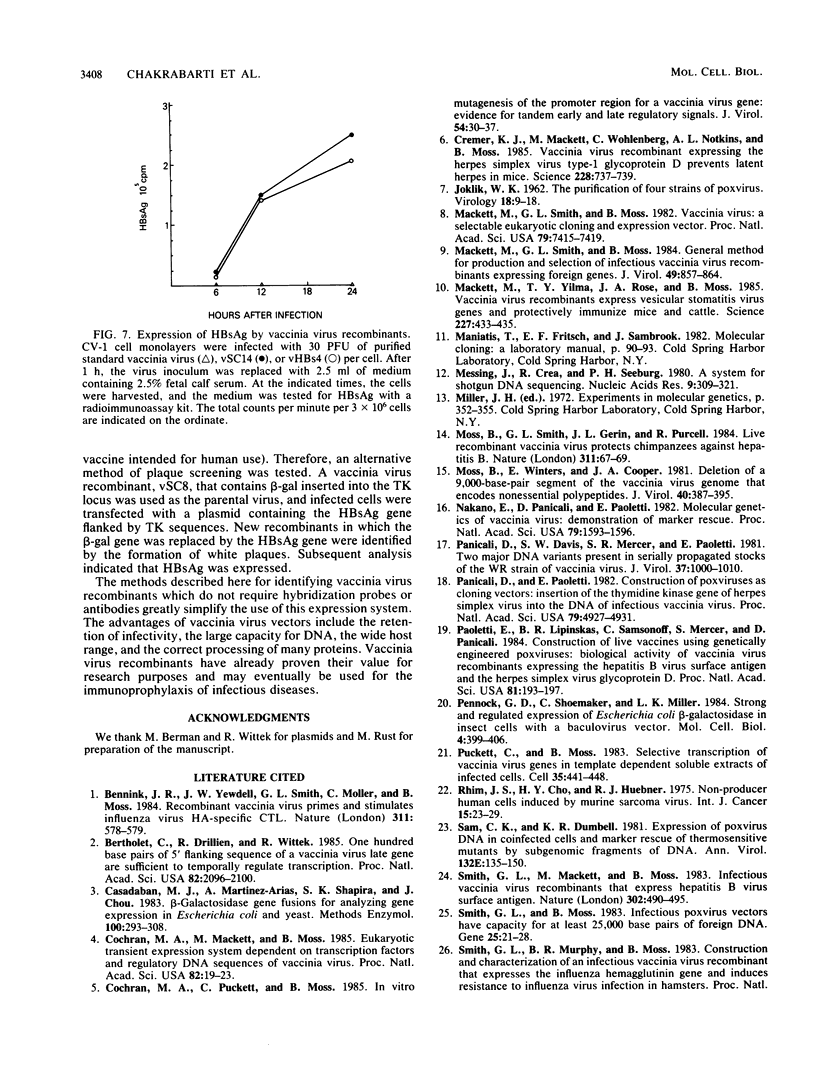
Abstract
We constructed a plasmid coexpression vector that directs the insertion of a foreign gene of interest together with the Escherichia coli beta-galactosidase (beta gal) gene into the thymidine kinase (TK) locus of the vaccinia virus genome. Tissue culture cells that had been infected with vaccinia virus were transfected with a plasmid vector containing a foreign gene. TK- recombinants could be selected by a plaque assay on TK- cells in the presence of 5-bromodeoxyuridine and distinguished from spontaneous TK- mutants by the addition of a beta-gal indicator to the agarose overlay. Plaques that expressed beta-gal stained dark blue within several hours at 37 degrees C. Alternatively, TK- selection could be eliminated, and recombinant plaques could be readily identified solely by their blue color. The reverse procedure, in which the starting virus expresses beta-gal (i.e., forms blue plaques) and the desired recombinant has deleted the entire beta-gal gene (i.e., forms white plaques), is another alternative. Each protocol was tested by constructing vaccinia virus recombinants that express hepatitis B virus surface antigen.
Full text
PDF
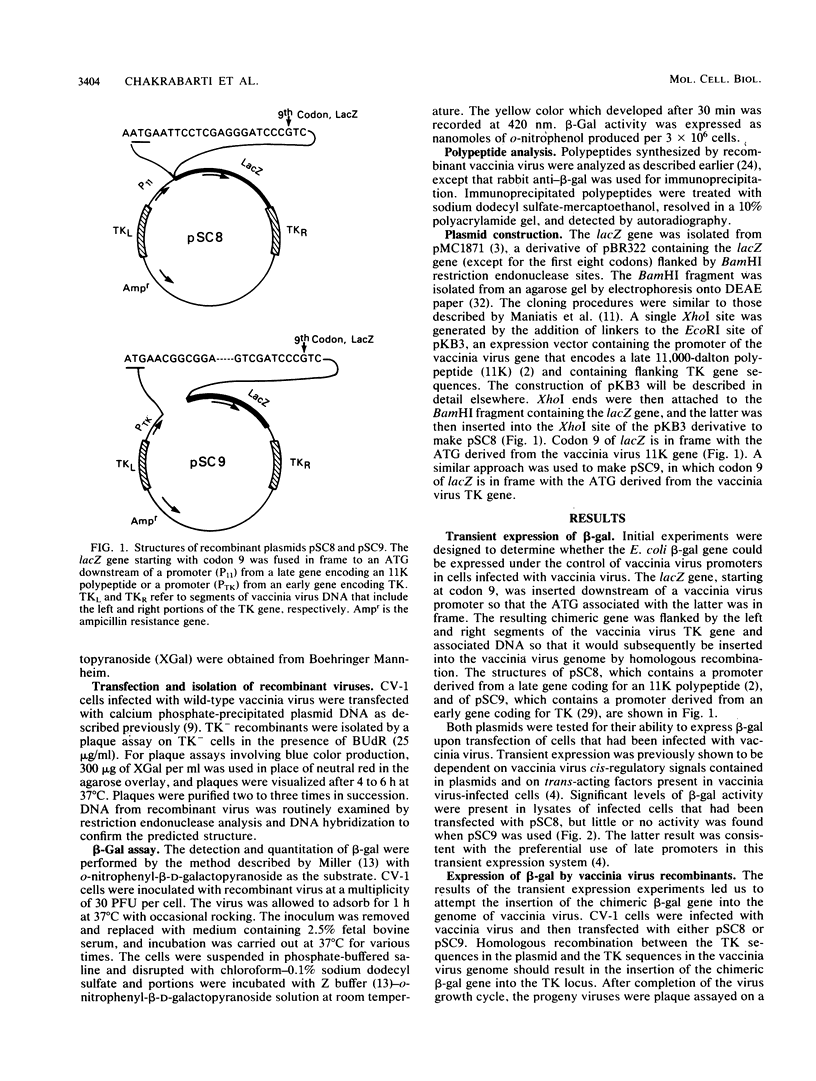
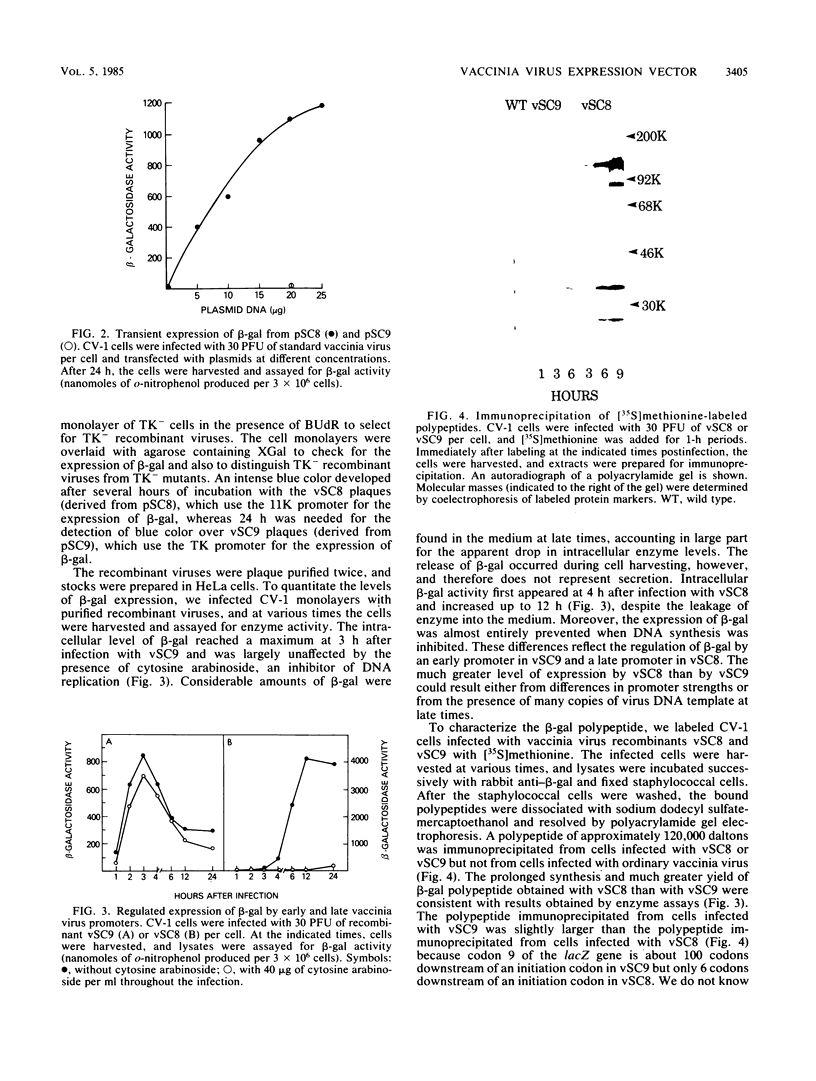
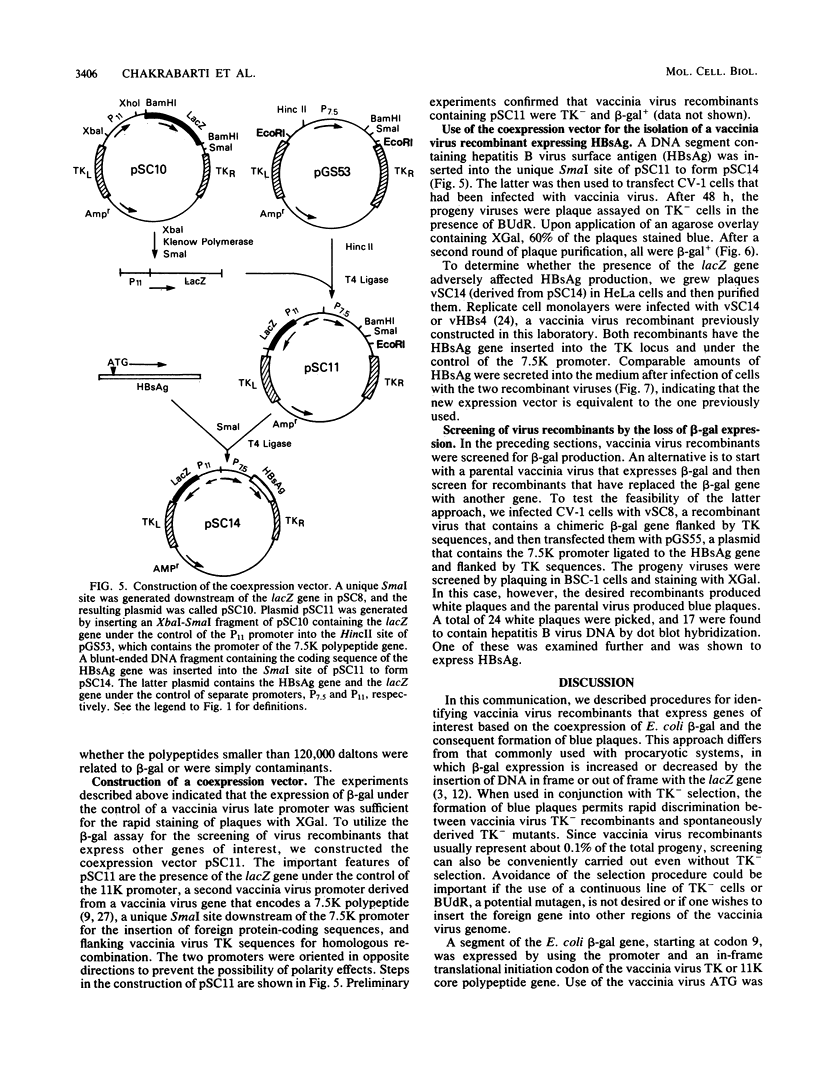

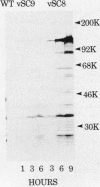
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennink J. R., Yewdell J. W., Smith G. L., Moller C., Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1984 Oct 11;311(5986):578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer K. J., Mackett M., Wohlenberg C., Notkins A. L., Moss B. Vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D prevents latent herpes in mice. Science. 1985 May 10;228(4700):737–740. doi: 10.1126/science.2986288. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Smith G. L., Gerin J. L., Purcell R. H. Live recombinant vaccinia virus protects chimpanzees against hepatitis B. Nature. 1984 Sep 6;311(5981):67–69. doi: 10.1038/311067a0. [DOI] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper J. A. Deletion of a 9,000-base-pair segment of the vaccinia virus genome that encodes nonessential polypeptides. J Virol. 1981 Nov;40(2):387–395. doi: 10.1128/jvi.40.2.387-395.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano E., Panicali D., Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Mercer S. R., Paoletti E. Two major DNA variants present in serially propagated stocks of the WR strain of vaccinia virus. J Virol. 1981 Mar;37(3):1000–1010. doi: 10.1128/jvi.37.3.1000-1010.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc Natl Acad Sci U S A. 1984 Jan;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock G. D., Shoemaker C., Miller L. K. Strong and regulated expression of Escherichia coli beta-galactosidase in insect cells with a baculovirus vector. Mol Cell Biol. 1984 Mar;4(3):399–406. doi: 10.1128/mcb.4.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puckett C., Moss B. Selective transcription of vaccinia virus genes in template dependent soluble extracts of infected cells. Cell. 1983 Dec;35(2 Pt 1):441–448. doi: 10.1016/0092-8674(83)90177-0. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Mackett M., Moss B. Infectious vaccinia virus recombinants that express hepatitis B virus surface antigen. Nature. 1983 Apr 7;302(5908):490–495. doi: 10.1038/302490a0. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Moss B. Infectious poxvirus vectors have capacity for at least 25 000 base pairs of foreign DNA. Gene. 1983 Nov;25(1):21–28. doi: 10.1016/0378-1119(83)90163-4. [DOI] [PubMed] [Google Scholar]
- Smith G. L., Murphy B. R., Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Regulation of expression and nucleotide sequence of a late vaccinia virus gene. J Virol. 1984 Sep;51(3):662–669. doi: 10.1128/jvi.51.3.662-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor T. J., Macfarlan R. I., Reagan K. J., Dietzschold B., Curtis P. J., Wunner W. H., Kieny M. P., Lathe R., Lecocq J. P., Mackett M. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Smith G. L., Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]