Abstract
BACKGROUND
We investigated whether thyroid transcription factor–1 (TTF-1) expression correlates with the IASLC/ATS/ERS classification and whether it stratifies patients with stage I lung adenocarcinoma with respect to recurrence.
METHODS
Patients with stage I lung adenocarcinoma were classified according to the IASLC/ATS/ERS classification. We constructed tissue microarrays and performed immunostaining for TTF-1; 452 cases were available for analysis. Tumors were dichotomized by intensity of nuclear TTF-1 expression: negative (score 0) or positive (score 1–3). Cumulative incidence of recurrence (CIR) was used to estimate recurrence probabilities.
RESULTS
TTF-1 expression was identified in 92% of all patients, including 100% of patients with minimally invasive or lepidic-predominant adenocarcinoma, 94% of acinar-predominant, 98% of papillary-predominant, 93% of micropapillary-predominant, 86% of solid-predominant, 67% of colloid-predominant, and 47% of invasive-mucinous. The CIR for patients with negative TTF-1 expression (n = 34; 5-year CIR, 40%) was significantly higher than that for patients with positive TTF-1 (n = 418; 5-year CIR, 15%; p < 0.001). Among the intermediate-grade tumors, the CIR for patients with negative TTF-1 expression (n = 16; 5-year CIR, 45%) was significantly higher than that for patients with positive TTF-1 (n = 313, 5-year CIR, 14%; p < 0.001). In multivariate analysis, negative TTF-1 expression significantly correlated with increased risk of recurrence (hazard ratio, 2.55; p = 0.009).
CONCLUSIONS
TTF-1 expression is an independent predictor of recurrence, stratifying intermediate-grade tumors into 2 prognostic subsets, and it correlates with the IASLC/ATS/ERS classification.
Keywords: lung adenocarcinoma, thyroid transcription factor–1, histologic subtype, recurrence
INTRODUCTION
Lung cancer is the leading cause of death from cancer.1 At present, tumor-nodal-metastasis (TNM) stage is the most important prognostic factor for lung cancer.2 For patients with stage I lung cancer, however, survival outcomes remain variable.3 There is a need to refine the prognostic factors for early-stage lung cancer. The International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) has proposed a new lung adenocarcinoma classification.4 Histologic subtyping according to this classification has powerful prognostic value.5–7
Thyroid transcription factor–1 (TTF-1), a homeodomain-containing nuclear transcriptional protein of the Nkx2 gene family, is expressed in epithelial cells of the fetal through the adult lung.8 TTF-1 is also expressed in lung carcinoma, and previous studies have reported a prognostic association with TTF-1 expression in non-small cell lung cancer (NSCLC).9–27 Most of them have shown that lack of TTF-1 expression correlates with worse prognosis. In these studies, however, most cohorts were heterogeneous in histology (adenocarcinoma and squamous cell carcinoma) and/or in TNM stage (early and advanced). No study has specifically investigated the prognostic utility of TTF-1 expression using a uniform cohort of early-stage lung adenocarcinomas, and the association between TTF-1 expression and the IASLC/ATS/ERS classification.
In this study, for stage I lung adenocarcinoma, we determined whether TTF-1 expression correlates with the IASLC/ATS/ERS classification; and whether TTF-1 expression stratifies patients with respect to recurrence.
MATERIALS AND METHODS
Patients
The current retrospective study was approved by the institutional review board (WA0269-08). We reviewed all patients with pathologic stage I solitary lung adenocarcinoma who underwent surgical resection at our institution between 1995 and 2005. A total of 514 cases had tumor slides available for histologic evaluation. Among them, 471 cases had tumor blocks available for construction of tissue microarrays. Clinical data were collected from the prospectively maintained database. Disease stage was based on the seventh edition of the American Joint Committee on Cancer TNM Staging Manual.2
Histologic Evaluation
All available hematoxylin and eosin (H&E)–stained tumor slides (mean, 5 slides/case [range, 1–12 slides/case]) were reviewed by 2 pathologists (K.K. and W.D.T.) separately, who were blinded to the patients’ clinical outcomes, by use of an Olympus BX51 microscope (Olympus Co., Tokyo, Japan) with a standard, 22-mm-diameter eyepiece. Discrepancies between the two pathologists in assignment of the predominant subtype were later resolved by consensus at a multiple-headed microscope. The percentage of each histologic pattern was recorded in 5% increments. Tumors were classified, according to the IASLC/ATS/ERS classification, as adenocarcinoma in situ (AIS); minimally invasive adenocarcinoma (MIA); and invasive adenocarcinoma, which was subdivided into lepidic-predominant, acinar-predominant, papillary-predominant, micropapillary-predominant, solid-predominant, colloid-predominant and invasive-mucinous adenocarcinoma.4 Invasive-mucinous adenocarcinoma was divided into 2 groups: pure mucinous (when having more than 90% invasive-mucinous pattern) and mixed mucinous/nonmucinous (when having at least 10% of each component).4 Tumors were grouped by architectural grading, as low (AIS, MIA, or lepidic-predominant), intermediate-grade (papillary-predominant or acinar-predominant), and high-grade (micropapillary-predominant, solid-predominant, colloid-predominant, or invasive-mucinous).5, 28
Nuclear features were examined with a high-power field (HPF) of ×400 magnification (0.237 mm2). Nuclear atypia was graded as previously reported: mild, moderate, and severe.29 Tumors were classified by mitotic count per 10 HPF as low (0–1); intermediate (2–4); and high (≥5).29 The following factors were also investigated: visceral pleural invasion;2 lymphatic and vascular invasion; and presence of necrosis.Lymphatic invasion was defined by the presence of tumor cells within an endothelium-lined space with lymphocytes. Vascular invasion was defined by the presence of tumor cells within blood vessels.
Tissue Microarray
Formalin-fixed, paraffin-embedded tumor specimens were used for construction of tissue microarrays. In brief, 4 representative tumor areas, 2 from the most predominant histologic pattern and 2 from the second predominant pattern, were marked on H&E-stained slides, and cylindrical 0.6-mm tissue cores were arrayed from the corresponding paraffin blocks into a recipient block by an automated tissue arrayer (ATA-27; Beecher Instruments, Sun Prairie, WI), resulting in 7 tissue microarray blocks. In all, 452 patients had adequate cores available for immunohistochemical analysis.
Immunohistochemical Analysis and Scoring of TTF-1
In brief, 4-μm-thick sections from the blocks were deparaffinized. Antigen retrieval was conducted using citrate buffer (pH 6.0). The standard avidin-biotin-complex peroxidase technique was used for immunostaining of anti–TTF-1 antibody (SPT24, NovoCastra; diluted at 1:50). Sections were stained using a Ventana Discovery XT automated immunohistochemical stainer (Ventana, Tucson, AZ), in accordance with the manufacturer’s guidelines. Normal lung tissues were stained as positive control in parallel with the study tissues.
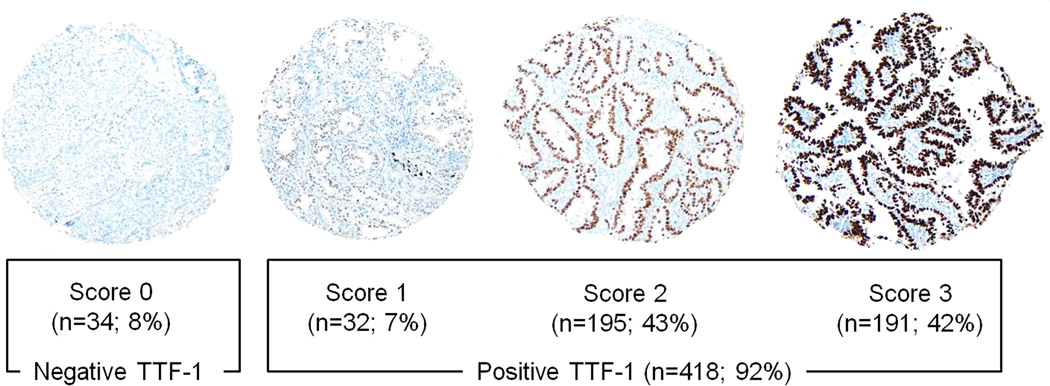
As nuclear TTF-1 expression showed diffuse pattern, expressed at least 50% of the tumor area of each core, in most (>90%) cases, TTF-1 expression was evaluated based on the intensity. The intensity of immunostaining was scored as 0 (no expression), 1 (mild), 2 (intermediate), or 3 (strong) in each tumor core, as shown in Figure 1. The average intensity score for the tumor cores was considered to be the TTF-1 expression for each patient. On average, 3.2 tumor cores per patient were available for analysis.
Figure 1.
Thyroid transcription factor–1 (TTF-1) immunohistochemical analysis using tissue microarray (original magnification, ×200)
With regard to the TTF-1 expression score, 34 tumors had a score 0, 32 had a score 1, 195 had a score 2, and 191 had a score 3.
Statistical Analysis
Associations between clinicopathologic factors and TTF-1 expression were analyzed using Fisher’s exact test, for categorical variables, and the Wilcoxon test, for continuous variables.
Time-to-recurrence analyses were performed using competing-risks methodology, which is the appropriate technique when a large proportion of patients die before experiencing recurrence. The Kaplan-Meier method estimates the probability of recurrence with the assumption that no deaths occur, which is unrealistic in this early-stage population, in which a large number of deaths without documented recurrence are observed. Instead, cumulative incidence of recurrence (CIR) estimates the risk of recurrence by accounting for death as a competing event.30, 31 Patients were followed up from the time of surgery, and censored if they were alive without documented recurrence at the time of the last follow-up.
We investigated the effect of clinicopathological factors and TTF-1 on CIR. Differences in CIR were assessed using the methods of Gray (in univariate nonparametric analyses) and Fine-Gray (in multivariate analyses).30, 31 We first examined the univariate association between clinicopathologic factors and CIR to determine candidate variables for inclusion in a multivariate model.
All significance tests were 2-sided and used a 5% level of significance. Statistical analyses were conducted using SAS statistical software (version 9.2; SAS Institute, Cary, NC) and R (R Development Core Team, 2010), including the “survival” and “cmprsk” packages.
RESULTS
Association between Patient Clinicopathologic Factors and Recurrence
Of all 452 patients, the median age was 69 years (range, 33–89 years). Most patients were women (63%), and had stage IA disease (68%). Of all, 84% underwent lobectomy (Table 1).
Table 1.
Association between Clinicopathologic Factors and Recurrence
| Variable | n (%) | 5-year CIR | P |
|---|---|---|---|
| All patients | 452 (100) | ||
| Age | 0.63 | ||
| ≤65 | 161 (36) | 18% | |
| >65 | 291 (64) | 16% | |
| Sex | 0.010 | ||
| Female | 285 (63) | 13% | |
| Male | 167 (37) | 23% | |
| Smoking | 0.17 | ||
| Never | 72 (16) | 11% | |
| Former/current | 380 (84) | 18% | |
| Surgery | 0.008 | ||
| Lobectomy | 379 (84) | 15% | |
| Sublobar resection | 73 (16) | 27% | |
| Pathologic stage | <0.001 | ||
| IA | 307 (68) | 12% | |
| IB | 145 (32) | 26% | |
| Architectural grade | 0.001 | ||
| Low | 35 (8) | 7% | |
| Intermediate | 329 (73) | 15% | |
| High | 88 (19) | 26% | |
| Pleural invasion | 0.050 | ||
| Absence | 364 (80) | 15% | |
| Presence | 88 (20) | 24% | |
| Lymphatic invasion | 0.005 | ||
| Absence | 330 (73) | 14% | |
| Presence | 122 (27) | 24% | |
| Vascular invasion | 0.003 | ||
| Absence | 325 (72) | 13% | |
| Presence | 127 (28) | 25% | |
| Necrosis | <0.001 | ||
| Absence | 371 (82) | 11% | |
| Presence | 81 (18) | 41% | |
| Nuclear atypia | 0.010 | ||
| Mild | 226 (50) | 12% | |
| Moderate | 120 (26) | 19% | |
| Severe | 106 (24) | 24% | |
| Mitotic count | <0.001 | ||
| Low | 196 (43) | 9% | |
| Intermediate | 97 (22) | 17% | |
| High | 159 (35) | 27% |
Significant p values (< 0.05) are shown in bold.
CIR, cumulative incidence of recurrence
According to the histologic subtyping, 9 tumors were MIA (8 nonmucinous and 1 mixed mucinous/nonmucinous), 26 were lepidic-predominant, 203 were acinar-predominant, 126 were papillary-predominant, 14 were micropapillary-predominant, 56 were solid-predominant, 3 were colloid-predominant, and 15 were invasive-mucinous (6 pure mucinous and 9 mixed mucinous/nonmucinous).
Of all the patients, 73 had a recurrence, and 102 died of any cause without a documented recurrence. Twenty-two tumors recurred in lung, 13 in lymph nodes, and 38 in distant organs. The median follow-up for patients who did not have a recurrence was 57.3 months (range, 0.3–160.1 months). In univariate analysis, male sex (p = 0.010), sublobar resection (p = 0.008), higher stage (stage IB; p < 0.001), higher architectural grade (p = 0.001), lymphatic invasion (p = 0.005), vascular invasion (p = 0.003), presence of necrosis (p < 0.001), greater nuclear atypia (p = 0.010), and higher mitotic count (p < 0.001) were associated with increased risk of recurrence (Table 1).
Association between TTF-1 and Histologic Subtype or Clinicopathologic Factors
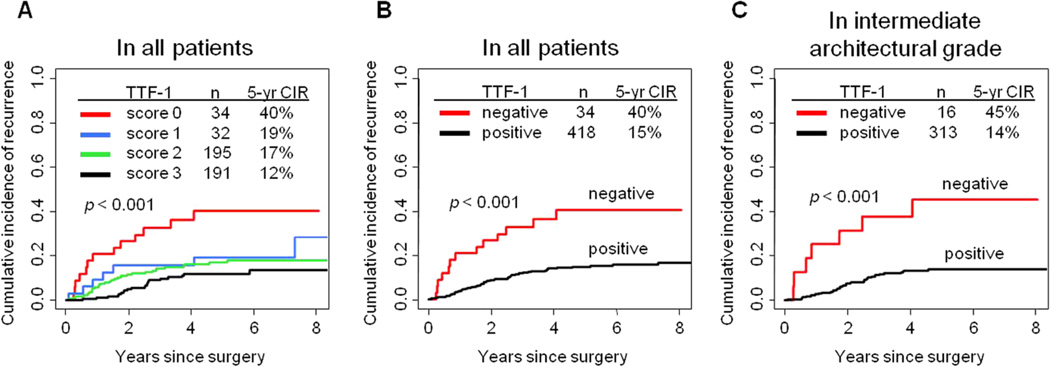
With regard to the TTF-1 expression score, 34 tumors had a score 0, 32 had a score 1, 195 had a score 2, and 191 had a score 3 (Figure 1). When stratifying tumors by TTF-1 score, the CIR for patients with a score 0 was significantly higher (5-year CIR, 40%) than that for patients with a score 1 (19%), 2 (17%), or 3 (12%; p < 0.001) (Figure 2A). On the basis of the 5-year CIR for each score, we decided to dichotomize TTF-1 expression into negative (score 0) versus positive (score 1–3).
Figure 2.
Association between thyroid transcription factor-1 (TTF-1) expression and recurrence
(A) When we stratified tumors into 4 groups by TTF-1 expression score, the cumulative incidence of recurrence (CIR) for patients with a score of 0 was significantly higher (5-year CIR, 40%) than that for patients with a score of 1 (19%), 2 (17%), or 3 (12%). (B) The CIR for patients with negative TTF-1 expression (5-year CIR, 40%) was significantly higher than that for patients with positive TTF-1 expression (5-year CIR, 15%). (C) Among patients with intermediate architectural grade, the CIR for patients with negative TTF-1 expression (5-year CIR, 45%) was significantly higher than that for patients with positive TTF-1 expression (5-year CIR, 14%).
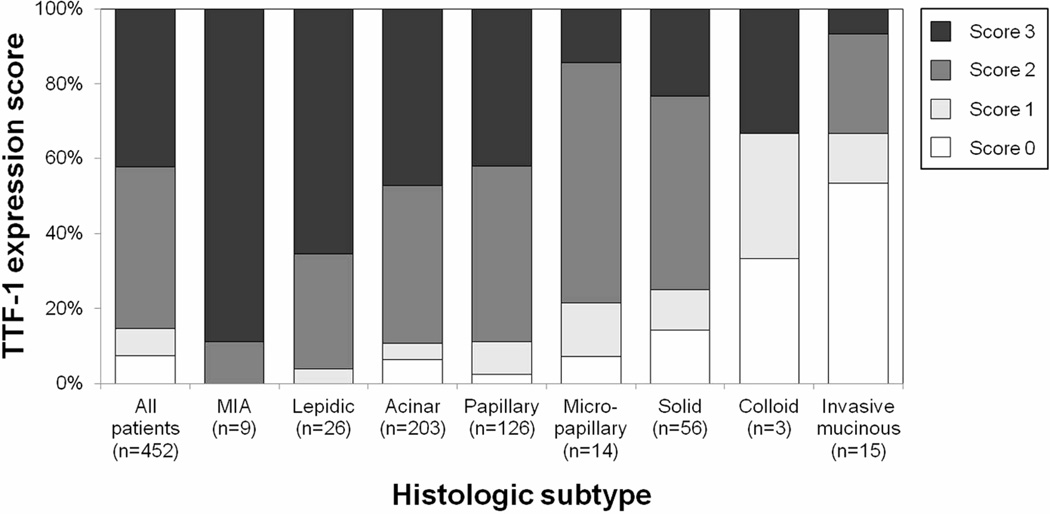
All MIA and lepidic-predominant tumors showed TTF-1 expression. TTF-1 expression was present in 94% (190/203) of acinar-predominant tumors, in 98% (123/126) of papillary-predominant, in 93% (13/14) of micropapillary-predominant, in 86% (48/56) of solid-predominant, in 67% (2/3) of colloid-predominant, and in 47% (7/15) of invasive-mucinous (Figure 3). Of the MIA tumors, 100% (7/7) of nonmucinous MIA tumors showed strong TTF-1 expression, and 1 mixed mucinous/nonmucinous MIA tumor showed intermediate expression. Strong TTF-1 expression was identified in 65% (17/26) of lepidic-predominant tumors, in 47% (96/203) of acinar-predominant, in 42% (53/126) of papillary-predominant, in 14% (2/14) of micropapillary-predominant, and in 23% (13/56) of solid-predominant. Of the invasive-mucinous tumors, positive TTF-1 expression was identified in 50% (3/6) of pure mucinous tumors and in 44% (4/9) of mixed mucinous/nonmucinous. Strong TTF-1 expression was not identified in pure mucinous tumors, but it was identified in 11% (1/9) of mucinous/nonmucinous and in 33% (1/3) of colloid-predominant. TTF-1 expression was the most frequently identified in low-grade tumors (100%), followed by intermediate-grade (95%) and high-grade (80%; p < 0.001) (Table 2).
Figure 3.
Association between thyroid transcription factor–1 (TTF-1) expression and histologic subtype
All minimally invasive adenocarcinoma (MIA) and lepidic-predominant tumors showed TTF-1 expression. TTF-1 expression was present in 94% of acinar-predominant tumors, in 98% of papillary-predominant tumors, in 93% of micropapillary-predominant tumors, in 86% of solid-predominant tumors, in 67% of colloid-predominant tumors, and in 47% of invasive-mucinous tumors.
Table 2.
Association between Thyroid Transcription Factor–1 (TTF-1) Expression and Clinicopathologic Factors
| Variable | TTF-1 expression, n (%) | P | |
|---|---|---|---|
| Negative | Positive | ||
| All patients | 34 (8) | 418 (92) | |
| Age | 0.47 | ||
| Median | 68 | 69 | |
| (Range) | (45–84) | (33–89) | |
| Sex | 0.85 | ||
| Female | 22 (8) | 263 (92) | |
| Male | 12 (7) | 155 (93) | |
| Smoking | 0.84 | ||
| Never | 5 (7) | 67 (93) | |
| Former/current | 29 (8) | 351 (92) | |
| Surgery | 0.46 | ||
| Lobectomy | 27 (7) | 352 (93) | |
| Sublobar resection | 7 (10) | 66 (90) | |
| Tumor size | 0.003 | ||
| Median | 2.9 | 2.0 | |
| (Range) | (0.6–4.5) | (0.3–5.0) | |
| Pathologic stage | 0.020 | ||
| IA | 17 (6) | 290 (94) | |
| IB | 17 (12) | 128 (88) | |
| Architectural grade | <0.001 | ||
| Low | 0 (0) | 35(100) | |
| Intermediate | 16 (5) | 313 (95) | |
| High | 18 (21) | 70 (80) | |
| Pleural invasion | 0.46 | ||
| Absence | 29 (8) | 335 (92) | |
| Presence | 5 (6) | 83 (94) | |
| Lymphatic invasion | 0.20 | ||
| Absence | 28 (9) | 302 (91) | |
| Presence | 6 (5) | 116 (95) | |
| Vascular invasion | 0.86 | ||
| Absence | 24 (7) | 301 (93) | |
| Presence | 10 (8) | 117 (92) | |
| Necrosis | <0.001 | ||
| Absence | 20 (5) | 351 (95) | |
| Presence | 14 (17) | 67 (83) | |
| Nuclear atypia | 0.72 | ||
| Mild | 16 (7) | 210 (93) | |
| Moderate | 11 (9) | 109 (91) | |
| Severe | 7 (7) | 99 (93) | |
Significant p values (p < 0.05) are shown in bold.
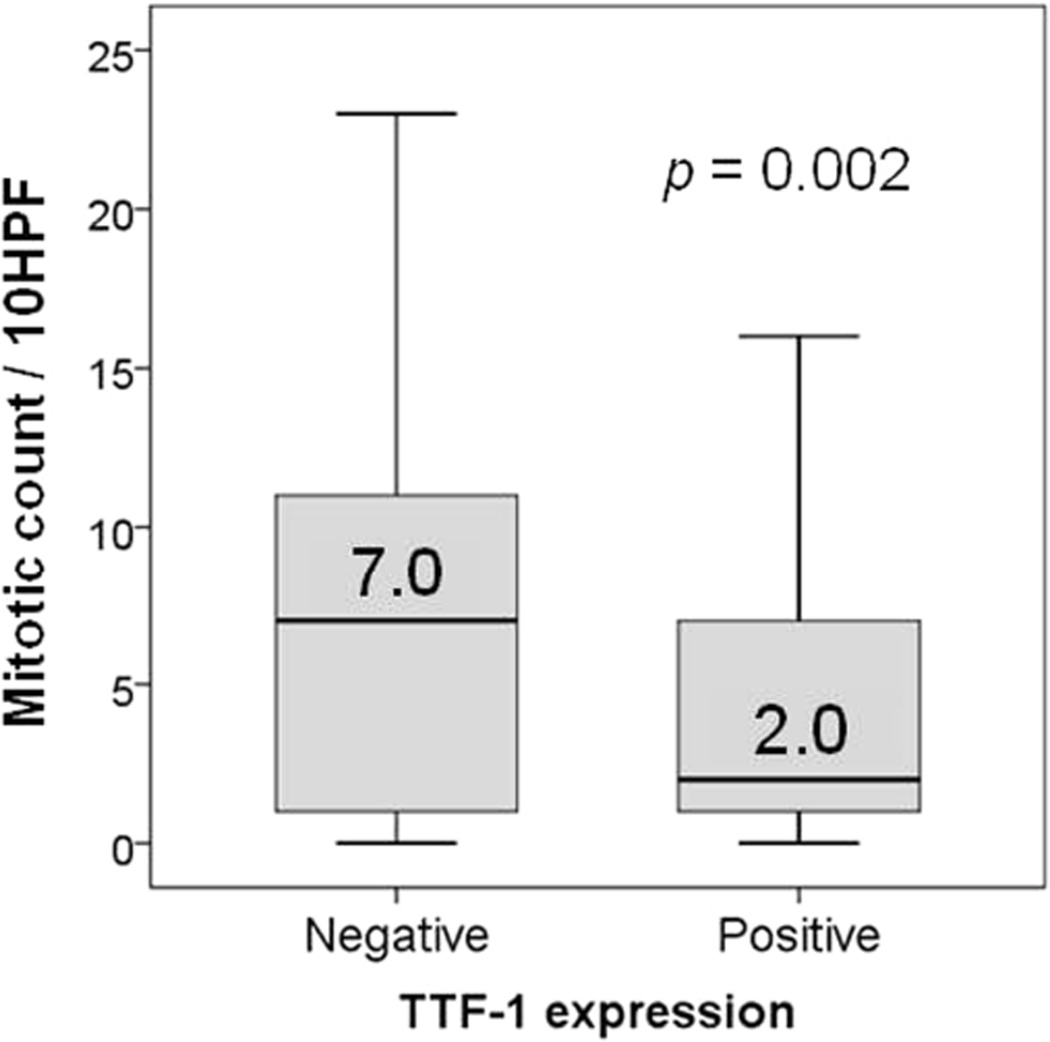
Negative TTF-1 expression significantly correlated with higher mitotic count (median, 7 [range, 0–39]), compared with positive expression (median, 2 [range, 0–43]; p = 0.002) (Figure 4). Negative TTF-1 expression was also associated with larger tumor size (p = 0.003), higher stage (stage IB; p = 0.020), and presence of necrosis (p < 0.001) (Table 2).
Figure 4.
Association between thyroid transcription factor–1 (TTF-1) expression and mitotic count/high-power field (HPF)
Negative TTF-1 expression was significantly associated with higher mitotic count (median, 7/10 HPF), compared with positive TTF-1 expression (median, 2/10 HPF).
Association between TTF-1 Expression and CIR
The CIR for patients with negative TTF-1 expression (n = 34, 5-year CIR, 40%) was significantly higher than patients with positive TTF-1 expression (n = 418, 5-year CIR, 15%; p < 0.001) (Figure 2B). This result was confirmed in a subgroup analysis limited to the 379 patients who underwent lobectomy: the CIR for patients with negative TTF-1 expression (n = 27; 5-year CIR, 39%) was significantly higher than that for patients with positive TTF-1 expression (n = 352; 5-year CIR, 13%; p < 0.001).
Among intermediate-grade tumors, the CIR for patients with negative TTF-1 expression (n = 16; 5-year CIR, 45%) was significantly higher than that for patients with positive TTF-1 expression (n = 313, 5-year CIR, 14%; p < 0.001) (Figure 2C). Among high-grade tumors, the CIR for patients with negative TTF-1 expression (n = 18, 5-year CIR, 35%) was higher than that for patients with positive TTF-1 expression (n = 70; 5-year CIR, 23%), although the difference was not statistically significant (p = 0.44).
Among acinar-predominant tumors, the CIR for patients with negative TTF-1 expression (n = 13, 5-year CIR, 38%) was higher than that for patients with positive TTF-1 expression (n = 190; 5-year CIR, 14%; p = 0.008). As for the other histologic subtypes, however, the small sample sizes prevented comparisons.
In multivariate analysis, of all the patients, negative TTF-1 expression remained at significantly increased risk of recurrence (hazard ratio [HR], 2.55; p = 0.009) (Table 3). Among intermediate-grade tumors, TTF-1 expression was an independent predictor of recurrence (HR, 3.84; p = 0.002). Among high-grade tumors, however, TTF-1 expression did not influence the risk of recurrence (HR, 1.60; p = 0.36).
Table 3.
Results of Multivariate Cox Proportional Hazards Model
| Variable | Hazard ratio | 95% CI | P |
|---|---|---|---|
| TTF-1 expression | |||
| negative vs. positive | 2.55 | 1.27–5.15 | 0.009 |
| Architectural grade | |||
| high vs. intermediate | 1.67 | 0.98–2.86 | 0.060 |
| high vs. low | 3.20 | 0.76–13.5 | 0.11 |
| Surgical procedure | |||
| sublobar vs. lobar | 2.38 | 1.38–4.13 | 0.002 |
| Pathologic TNM stage | |||
| IB vs. IA | 2.15 | 1.31–2.53 | 0.002 |
| Lymphatic invasion | |||
| presence vs. absence | 1.65 | 1.00–2.74 | 0.050 |
Note. Significant p values (p < 0.05) are shown in bold.
CI, confidence interval; TTF-1, thyroid transcriptional factor–1
DISCUSSION
We have demonstrated that lack of TTF-1 expression is more frequently identified in high-grade tumors and an independent predictor of recurrence in patients with stage I lung adenocarcinoma, especially in intermediate-grade tumors.
Several studies have reported no association between TTF-1 expression and lung adenocarcinomas differentiation.17, 20 However, we found an inverse association between TTF-1 expression and architectural grade based on the predominant subtype. To our knowledge, the association between TTF-1 expression and the IASLC/ATS/ERS classification has not been previously investigated in stage I lung adenocarcinomas, although studies using small cohorts of patients have suggested an association between higher TTF-1 expression and the lepidic pattern.14, 16, 21 In addition, TTF-1 expression correlates with a lower Ki-67 proliferation index in NSCLC.10, 12 In our study, TTF-1 positive tumors significantly correlated with lower mitotic count and smaller tumor size.
Tumors formerly classified as mucinous bronchioloalveolar carcinoma—which is called invasive-mucinous adenocarcinoma according to the IASLC/ATS/ERS classification—have no or less-frequent TTF-1 expression.14, 32–35 Pure mucinous tumors may have no or very low TTF-1 expression, and mixed mucinous/nonmucinous tumors more frequently express TTF-1 than pure mucinous.34 Similarly, in our study, strong TTF-1 expression was not identified in pure mucinous tumors.
To our knowledge, 19 studies investigating the association between TTF-1 expression and survival have been published.9–27 TTF-1 positive tumors are associated with better survival in 12 studies,11, 13, 16, 17, 19–22, 24–27 are associated with worse survival in 1 study,9 and have no association with survival in 6 studies.10, 12, 14, 15, 18, 23 Most of these studies used heterogeneous cohorts in histology and/or TNM stage. One study investigated the prognostic significance of TTF-1 expression in a more-uniform cohort that comprised patients with early-stage adenocarcinomas, although the study cohort was very selectively collected, including 50 patients with bronchioloalveolar carcinoma and 50 with conventional invasive adenocarcinoma.16 In a meta-analysis of 10 eligible studies published until 2005, TTF-1 combined HR for adenocarcinoma was 0.53 (95% confidence interval = 0.29–0.95).36 Our study, which comprises a uniform, large cohort of stage I lung adenocarcinomas, confirmed the prognostic significance of TTF-1 expression.
Previous studies have defined positive TTF-1 expression, for survival analysis, by various methods. Several studies used percentage of positive tumor cells, with a cutoff value of 1%–75%9–13, 15, 17–19, 23, 26, 27; others used staining intensity alone21 or a combination of positive percentage and intensity.16, 24, 25 Because of the high percentage of tumors positive for TTF-1, we used staining intensity only to classify the degree of TTF-1 expression, and we demonstrated the CIR differences by 4 groups of TTF-1 expression intensity, even though we finally dichotomized tumors into positive and negative. One limitation of the present study, which used tissue microarray, is that TTF-1–negative tumors might be focally positive if stained with whole-tissue blocks. As we have previously reported, however, when whole-tissue blocks were used, TTF-1 positivity was predominantly bimodal, either diffusely positive (84%) or completely negative (11%). Furthermore, the total positive rate in the whole-tissue block study (89%) was similar to that in the present, tissue microarray study (92%).37 Therefore, we believe that our conclusion would not be dramatically changed even if TTF-1 negativity were confirmed using whole-tissue block.
Several studies that performed multivariate analysis have shown that lack of TTF-1 expression is an independent predictor of worse prognosis.17, 20–22, 25, 27 However, it has been unclear whether lack of TTF-1 expression remains an independent prognostic predictor, even after the IASLC/ATS/ERS classification is adjusted for. In the current study, negative TTF-1 expression was an independent predictor of recurrence, after this classification was adjusted for.
One limitation of using the IASLC/ATS/ERS classification is that the majority of patients (73% in our study) are classified as intermediate-grade. Therefore, it is necessary to recognize poor prognostic factors for this group. In our study, TTF-1 expression stratifies intermediate-grade tumors into 2 groups with respect to recurrence.
In conclusion, in lung adenocarcinoma, the morphologic feature (histologic subtype) correlates with a specific molecular expression (TTF-1). For patients with stage I lung adenocarcinoma, TTF-1 expression is an independent predictor of recurrence. Since the morphologic assessment of H&E-stained slides and immunohistochemical analysis have become routine clinical practice, prognostic stratification using the IASLC/ATS/ERS classification and TTF-1 immunohistochemistry can be readily implemented in the treatment of patients with lung adenocarcinoma.
ACKNOWLEDGMENTS
We thank Joe Dycoco, for his help with the lung adenocarcinoma database at the Division of Thoracic Service, Department of Surgery; Avani Giri and Louie Lopez, for their help making the tissue microarray; Irina Linkov, for her technical assistance with the immunohistochemical analysis; and David Sewell, for his editorial assistance.
FUNDING SOURCES
This work was supported, in part, by the International Association for the Study of Lung Cancer Young Investigator Award; National Lung Cancer Partnership/LUNGevity Foundation Research Grant; American Association for Thoracic Surgery Third Edward D. Churchill Research Scholarship; Mesothelioma Applied Research Foundation grant in memory of Lance S. Ruble; William H. Goodwin and Alice Goodwin, the Commonwealth Foundation for Cancer Research and the Experimental Therapeutics Center; New York State Empire Clinical Research Investigator Program; the National Cancer Institute (grants R21CA164568, R21CA164585, U54CA137788 and U54CA132378); and the U.S. Department of Defense (grant LC110202 and PR101053).
Footnotes
CONFLICT INTEREST DISCLOSURES
The authors made no disclosure.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Cancer Staging Manual. 7th ed. New York, NY: Springer; 2009. pp. 253–270. [Google Scholar]
- 3.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol. 2007;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(2):244–285. doi: 10.1097/JTO.0b013e318206a221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24(5):653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 6.Kadota K, Suzuki K, D’Angelo SP, et al. Validation of the proposed IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6(Supplement 2):S286. [Google Scholar]
- 7.Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30(13):1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 8.Ikeda K, Clark JC, Shaw-White JR, et al. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem. 1995;270(14):8108–8114. doi: 10.1074/jbc.270.14.8108. [DOI] [PubMed] [Google Scholar]
- 9.Puglisi F, Barbone F, Damante G, et al. Prognostic value of thyroid transcription factor-1 in primary, resected, non-small cell lung carcinoma. Mod Pathol. 1999;12(3):318–324. [PubMed] [Google Scholar]
- 10.Pelosi G, Fraggetta F, Pasini F, et al. Immunoreactivity for thyroid transcription factor-1 in stage I non-small cell carcinomas of the lung. Am J Surg Pathol. 2001;25(3):363–372. doi: 10.1097/00000478-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Haque AK, Syed S, Lele SM, Freeman DH, Adegboyega PA. Immunohistochemical study of thyroid transcription factor-1 and HER2/neu in non-small cell lung cancer: strong thyroid transcription factor-1 expression predicts better survival. Appl Immunohistochem Mol Morphol. 2002;10(2):103–109. doi: 10.1097/00129039-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Myong NH. Thyroid transcription factor-1 (TTF-1) expression in human lung carcinomas: its prognostic implication and relationship with expressions of p53 and Ki-67 proteins. J Korean Med Sci. 2003;18(4):494–500. doi: 10.3346/jkms.2003.18.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan D, Li Q, Deeb G, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34(6):597–604. doi: 10.1016/s0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 14.Stenhouse G, Fyfe N, King G, Chapman A, Kerr KM. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol. 2004;57(4):383–387. doi: 10.1136/jcp.2003.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au NH, Cheang M, Huntsman DG, et al. Evaluation of immunohistochemical markers in non-small cell lung cancer by unsupervised hierarchical clustering analysis: a tissue microarray study of 284 cases and 18 markers. J Pathol. 2004;204(1):101–109. doi: 10.1002/path.1612. [DOI] [PubMed] [Google Scholar]
- 16.Saad RS, Liu YL, Han H, Landreneau RJ, Silverman JF. Prognostic significance of thyroid transcription factor-1 expression in both early-stage conventional adenocarcinoma and bronchioloalveolar carcinoma of the lung. Hum Pathol. 2004;35(1):3–7. doi: 10.1016/j.humpath.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 17.Barlesi F, Pinot D, Legoffic A, et al. Positive thyroid transcription factor 1 staining strongly correlates with survival of patients with adenocarcinoma of the lung. Br J Cancer. 2005;93(4):450–452. doi: 10.1038/sj.bjc.6602717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berghmans T, Mascaux C, Martin B, Ninane V, Sculier JP. Prognostic role of thyroid transcription factor-1 in stage III non-small cell lung cancer. Lung Cancer. 2006;52(2):219–224. doi: 10.1016/j.lungcan.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Martins SJ, Takagaki TY, Silva AG, et al. Prognostic relevance of TTF-1 and MMP-9 expression in advanced lung adenocarcinoma. Lung Cancer. 2009;64(1):105–109. doi: 10.1016/j.lungcan.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostou VK, Syrigos KN, Bepler G, Homer RJ, Rimm DL. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol. 2009;27(2):271–278. doi: 10.1200/JCO.2008.17.0043. [DOI] [PubMed] [Google Scholar]
- 21.Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med. 2009;13(8B):1977–1986. doi: 10.1111/j.1582-4934.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perner S, Wagner PL, Soltermann A, et al. TTF1 expression in non-small cell lung carcinoma: association with TTF1 gene amplification and improved survival. J Pathol. 2009;217(1):65–72. doi: 10.1002/path.2443. [DOI] [PubMed] [Google Scholar]
- 23.Anami Y, Iijima T, Suzuki K, et al. Bronchioloalveolar carcinoma (lepidic growth) component is a more useful prognostic factor than lymph node metastasis. J Thorac Oncol. 2009;4(8):951–958. doi: 10.1097/JTO.0b013e3181ad8631. [DOI] [PubMed] [Google Scholar]
- 24.Tang X, Kadara H, Behrens C, et al. Abnormalities of the TITF-1 lineage-specific oncogene in NSCLC: implications in lung cancer pathogenesis and prognosis. Clin Cancer Res. 2011;17(8):2434–2443. doi: 10.1158/1078-0432.CCR-10-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solis LM, Behrens C, Raso MG, et al. Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer. 2011 doi: 10.1002/cncr.26584. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Wan L, Shen H, et al. Thyroid transcription factor-1 amplification and expressions in lung adenocarcinoma tissues and pleural effusions predict patient survival and prognosis. J Thorac Oncol. 2012;7(1):76–84. doi: 10.1097/JTO.0b013e318232b98a. [DOI] [PubMed] [Google Scholar]
- 27.Chung KP, Huang YT, Chang YL, et al. Clinical significance of thyroid transcription factor-1 in advanced lung adenocarcinoma under epidermal growth factor receptor tyrosine kinase inhibitor treatment. Chest. 2012;141(2):420–428. doi: 10.1378/chest.10-3149. [DOI] [PubMed] [Google Scholar]
- 28.Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34(8):1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 29.Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol. 2012;25(8):1117–1127. doi: 10.1038/modpathol.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chappell R. Competing Risk Analyses: How Are They Different and Why Should You Care? Clin Cancer Res. 2012;18(8):2127–2129. doi: 10.1158/1078-0432.CCR-12-0455. [DOI] [PubMed] [Google Scholar]
- 31.Dignam JJ, Zhang Q, Kocherginsky M. The Use and Interpretation of Competing Risks Regression Models. Clin Cancer Res. 2012;18(8):2301–2308. doi: 10.1158/1078-0432.CCR-11-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaufmann O, Dietel M. Thyroid transcription factor-1 is the superior immunohistochemical marker for pulmonary adenocarcinomas and large cell carcinomas compared to surfactant proteins A and B. Histopathology. 2000;36(1):8–16. doi: 10.1046/j.1365-2559.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 33.Saad RS, Cho P, Silverman JF, Liu Y. Usefulness of Cdx2 in separating mucinous bronchioloalveolar adenocarcinoma of the lung from metastatic mucinous colorectal adenocarcinoma. Am J Clin Pathol. 2004;122(3):421–427. doi: 10.1309/UMF7-15KR-G2V1-98YD. [DOI] [PubMed] [Google Scholar]
- 34.Lau SK, Desrochers MJ, Luthringer DJ. Expression of thyroid transcription factor-1, cytokeratin 7, and cytokeratin 20 in bronchioloalveolar carcinomas: an immunohistochemical evaluation of 67 cases. Mod Pathol. 2002;15(5):538–542. doi: 10.1038/modpathol.3880560. [DOI] [PubMed] [Google Scholar]
- 35.Hurbin A, Wislez M, Busser B, et al. Insulin-like growth factor-1 receptor inhibition overcomes gefitinib resistance in mucinous lung adenocarcinoma. J Pathol. 2011;225(1):83–95. doi: 10.1002/path.2897. [DOI] [PubMed] [Google Scholar]
- 36.Berghmans T, Paesmans M, Mascaux C, et al. Thyroid transcription factor 1—a new prognostic factor in lung cancer: a meta-analysis. Ann Oncol. 2006;17(11):1673–1676. doi: 10.1093/annonc/mdl287. [DOI] [PubMed] [Google Scholar]
- 37.Rekhtman N, Ang DC, Sima CS, Travis WD, Moreira AL. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol. 2011;24(10):1348–1359. doi: 10.1038/modpathol.2011.92. [DOI] [PubMed] [Google Scholar]