Abstract
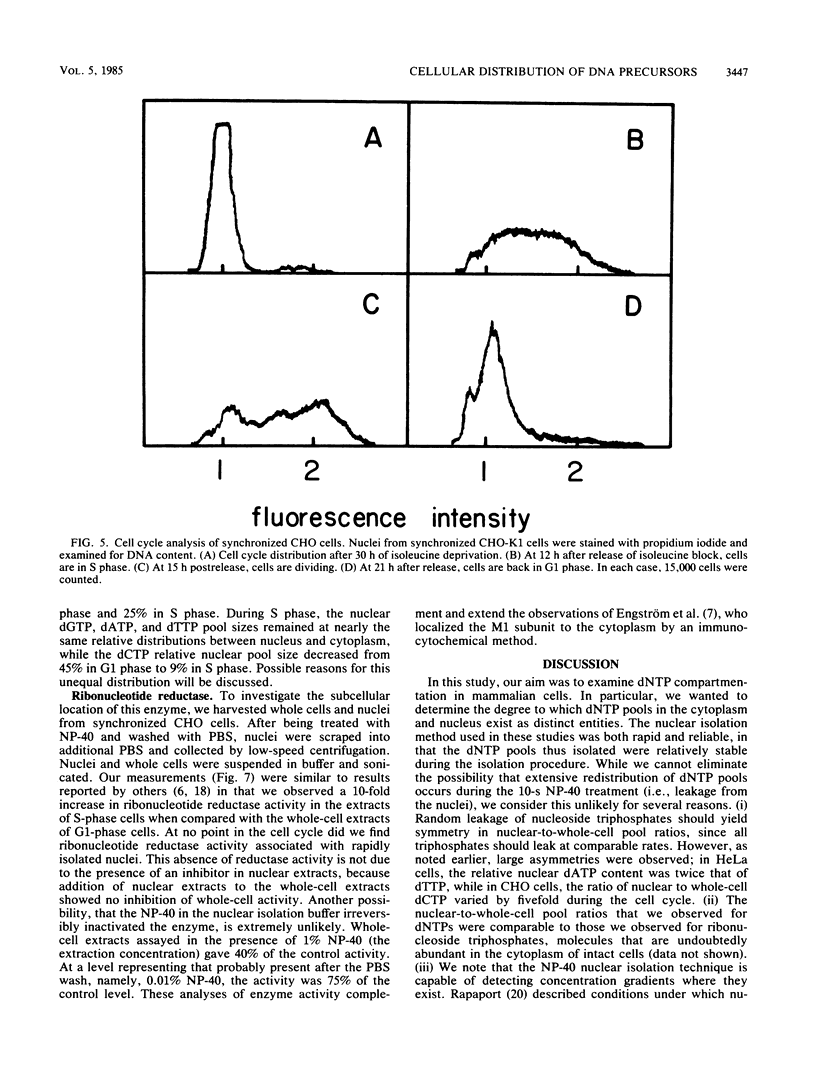
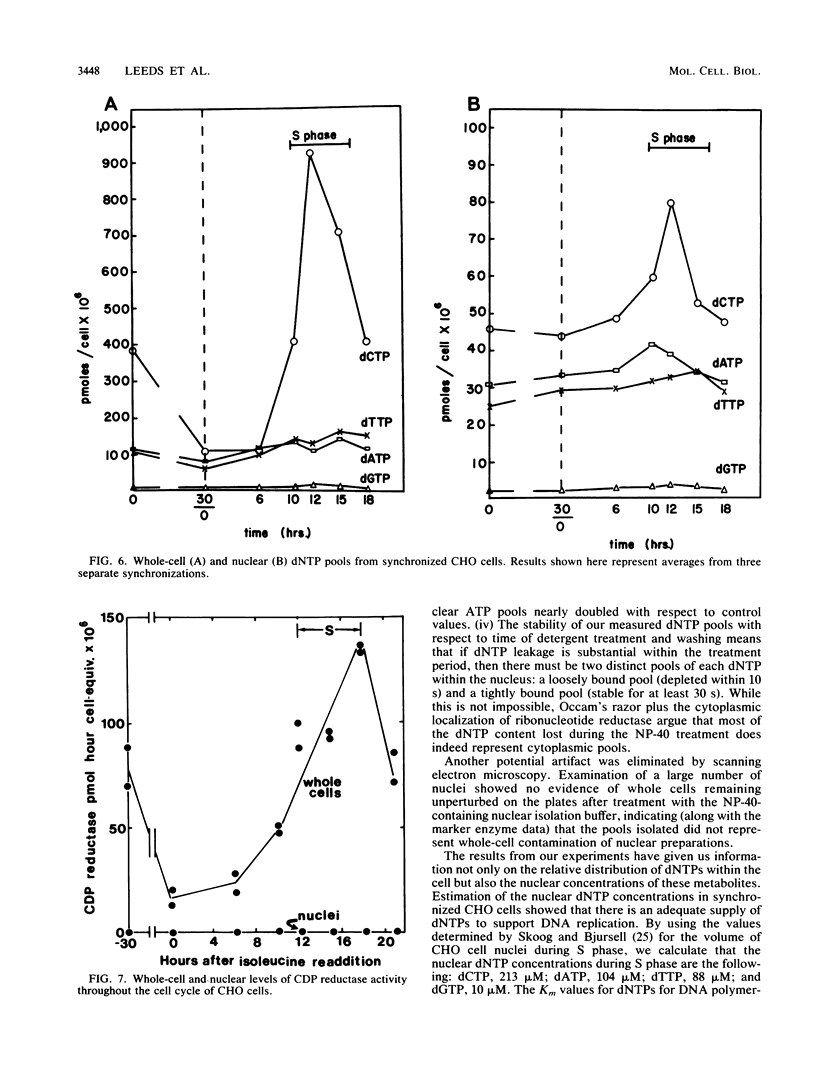
Nuclear and whole-cell deoxynucleoside triphosphate (dNTP) pools were measured in HeLa cells at different densities and throughout the cell cycle of synchronized CHO cells. Nuclei were prepared by brief detergent (Nonidet P-40) treatment of subconfluent monolayers, a procedure that solubilizes plasma membranes but leaves nuclei intact and attached to the plastic substratum. Electron microscopic examination of monolayers treated with Nonidet P-40 revealed protruding nuclei surrounded by cytoskeletal remnants. Control experiments showed that nuclear dNTP pool sizes were stable during the time required for isolation, suggesting that redistribution of nucleotides during the isolation procedure was minimal. Examination of HeLa whole-cell and nuclear dNTP levels revealed that the nuclear proportion of each dNTP was distinct and remained constant as cell density increased. In synchronized CHO cells, all four dNTP whole-cell pools increased during S phase, with the dCTP pool size increasing most dramatically. The nuclear dCTP pool did not increase as much as the whole-cell dCTP pool during S phase, lowering the relative nuclear dCTP pool. Although the whole-cell dNTP pools decreased after 30 h of isoleucine deprivation, nuclear pools did not decrease proportionately. In summary, nuclear dNTP pools in synchronized CHO cells maintained a relatively constant concentration throughout the cell cycle in the face of larger fluctuations in whole-cell dNTP pools. Ribonucleotide reductase activity was measured in CHO cells throughout the cell cycle, and although there was a 10-fold increase in whole-cell activity during S phase, we detected no reductase in nuclear preparations at any point in the cell cycle.
Full text
PDF
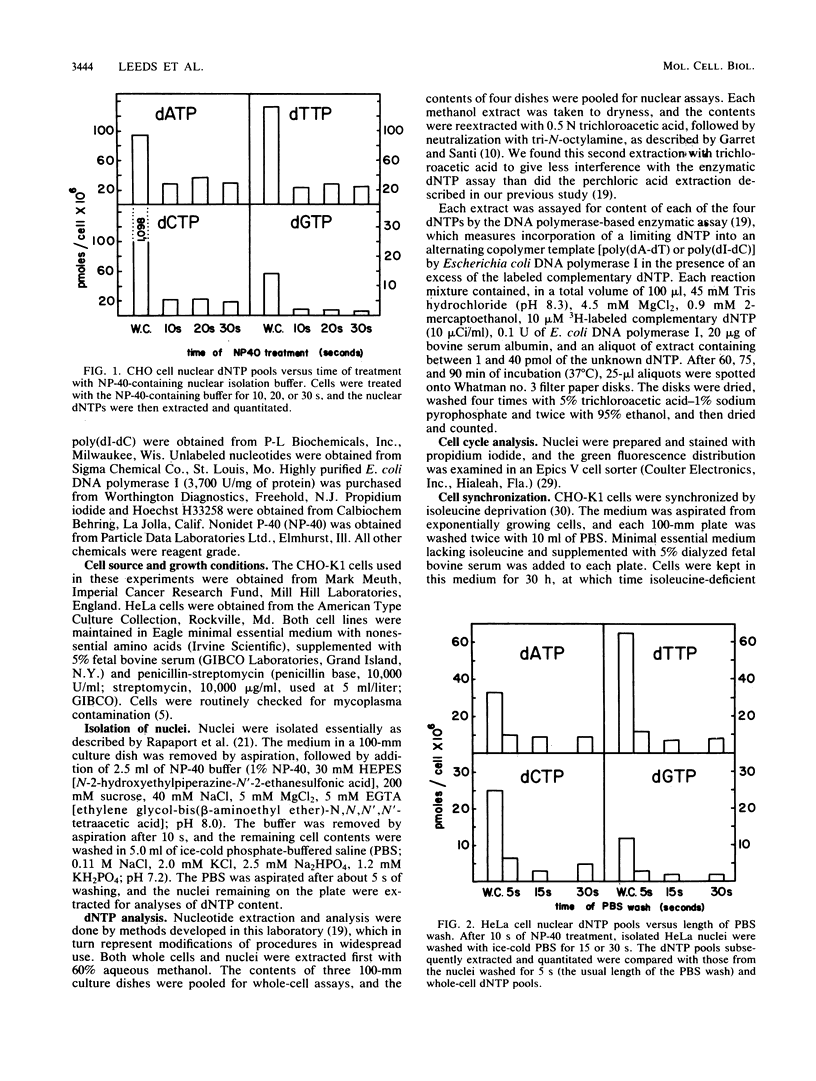
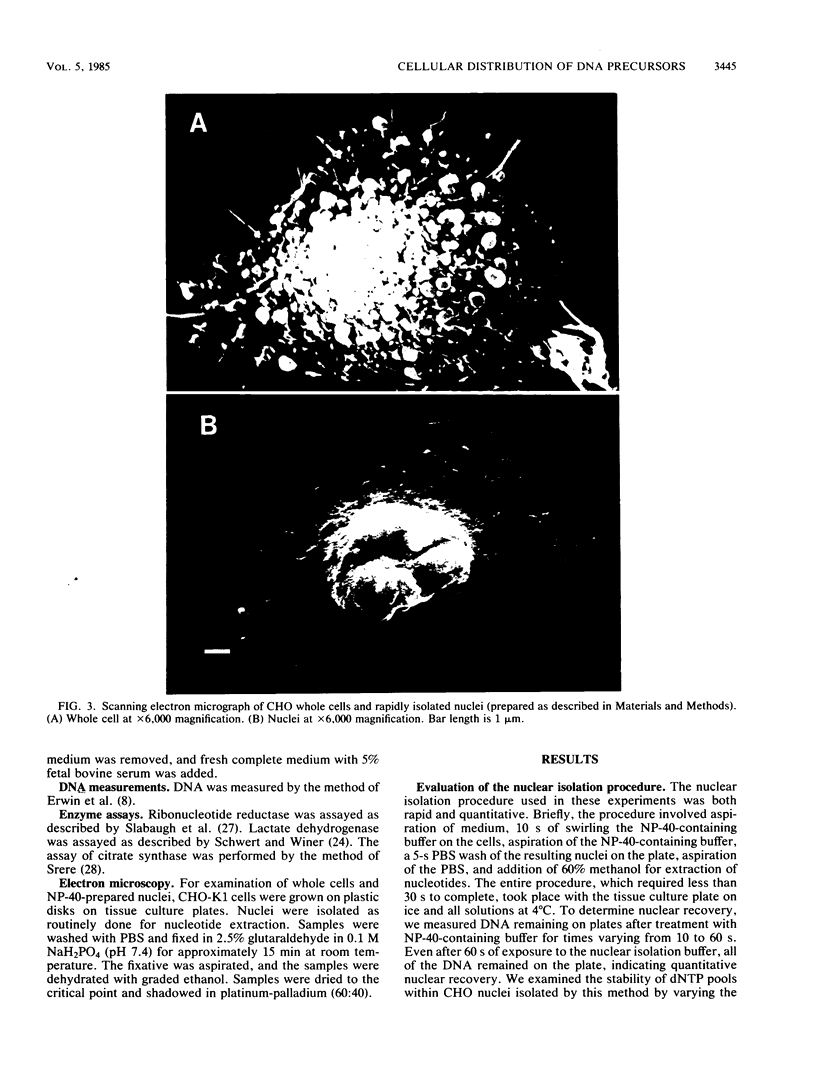
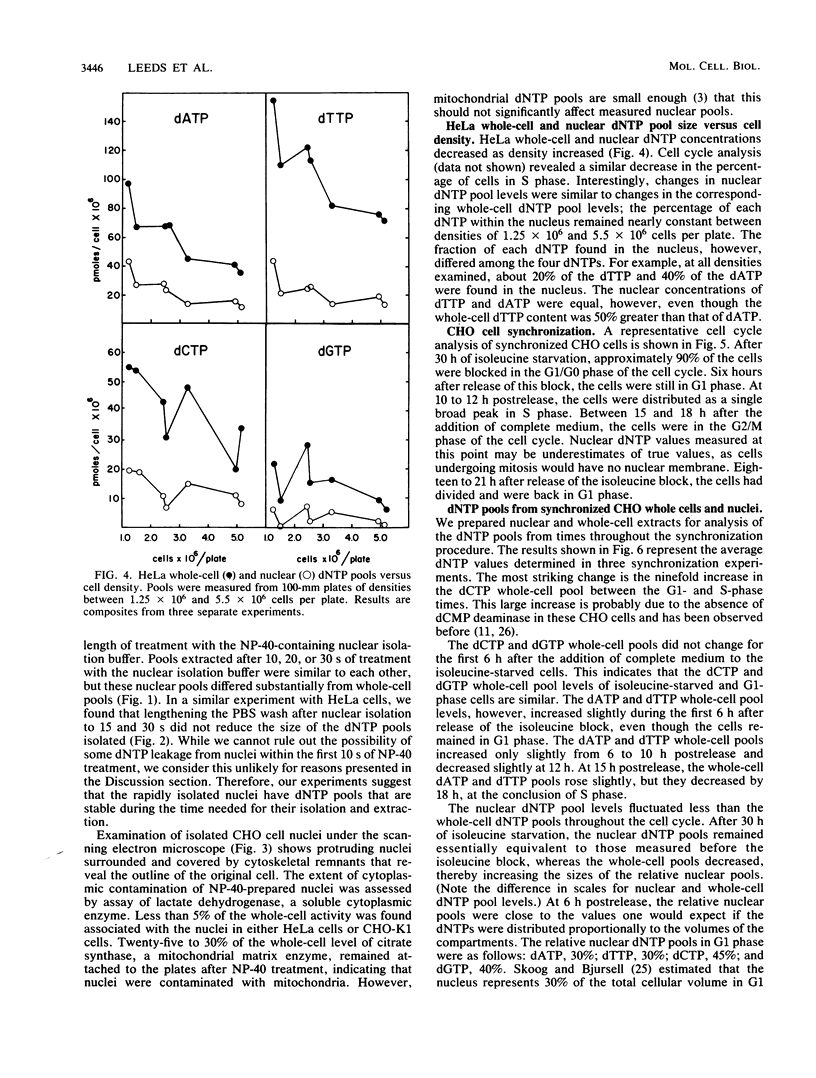


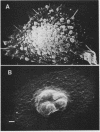
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensch K. G., Tanaka S., Hu S. Z., Wang T. S., Korn D. Intracellular localization of human DNA polymerase alpha with monoclonal antibodies. J Biol Chem. 1982 Jul 25;257(14):8391–8396. [PubMed] [Google Scholar]
- Bestwick R. K., Mathews C. K. Unusual compartmentation of precursors for nuclear and mitochondrial DNA in mouse L cells. J Biol Chem. 1982 Aug 25;257(16):9305–9308. [PubMed] [Google Scholar]
- Bestwick R. K., Moffett G. L., Mathews C. K. Selective expansion of mitochondrial nucleoside triphosphate pools in antimetabolite-treated HeLa cells. J Biol Chem. 1982 Aug 25;257(16):9300–9304. [PubMed] [Google Scholar]
- Caradonna S. J., Adamkiewicz D. M. Purification and properties of the deoxyuridine triphosphate nucleotidohydrolase enzyme derived from HeLa S3 cells. Comparison to a distinct dUTP nucleotidohydrolase induced in herpes simplex virus-infected HeLa S3 cells. J Biol Chem. 1984 May 10;259(9):5459–5464. [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Engström Y., Rozell B., Hansson H. A., Stemme S., Thelander L. Localization of ribonucleotide reductase in mammalian cells. EMBO J. 1984 Apr;3(4):863–867. doi: 10.1002/j.1460-2075.1984.tb01897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin B. G., Stoscheck C. M., Florini J. R. A rapid fluorometric method for the estimation of DNA in cultured cells. Anal Biochem. 1981 Jan 15;110(2):291–294. doi: 10.1016/0003-2697(81)90194-9. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Wang T. S., Korn D. Enzymological characterization of DNA polymerase alpha. Basic catalytic properties processivity, and gap utilization of the homogeneous enzyme from human KB cells. J Biol Chem. 1979 Jul 10;254(13):6128–6137. [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Hordern J., Henderson J. F. Comparison of purine and pyrimidine metabolism in G1 and S phases of HeLa and Chinese hamster ovary cells. Can J Biochem. 1982 Apr;60(4):422–433. doi: 10.1139/o82-050. [DOI] [PubMed] [Google Scholar]
- Jackson R. C. The regulation of thymidylate biosynthesis in Novikoff hepatoma cells and the effects of amethopterin, 5-fluorodeoxyuridine, and 3-deazauridine. J Biol Chem. 1978 Oct 25;253(20):7440–7446. [PubMed] [Google Scholar]
- Mathews C. K. Enzymatic channeling of DNA precursors. Basic Life Sci. 1985;31:47–66. doi: 10.1007/978-1-4613-2449-2_4. [DOI] [PubMed] [Google Scholar]
- McCormick P. J., Danhauser L. L., Rustum Y. M., Bertram J. S. Changes in ribo- and deoxyribonucleoside triphosphate pools within the cell cycle of a synchronized mouse fibroblast cell line. Biochim Biophys Acta. 1983 Mar 15;756(1):36–40. doi: 10.1016/0304-4165(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Newman C. N., Miller J. H. Mechanism of UV-induced deoxynucleoside triphosphate pool imbalance in CHO-K1 cells. Mutat Res. 1985 Jan-Mar;145(1-2):95–101. doi: 10.1016/0167-8817(85)90046-x. [DOI] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Dynamics of pyrimidine deoxynucleoside triphosphate pools in relationship to DNA synthesis in 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1347–1351. doi: 10.1073/pnas.80.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Evidence for the involvement of substrate cycles in the regulation of deoxyribonucleoside triphosphate pools in 3T6 cells. J Biol Chem. 1985 Aug 5;260(16):9216–9222. [PubMed] [Google Scholar]
- Nordenskjöld B. A., Skoog L., Brown N. C., Reichard P. Deoxyribonucleotide pools and deoxyribonucleic acid synthesis in cultured mouse embryo cells. J Biol Chem. 1970 Oct 25;245(20):5360–5368. [PubMed] [Google Scholar]
- North T. W., Bestwick R. K., Mathews C. K. Detection of activities that interfere with the enzymatic assay of deoxyribonucleoside 5'-triphosphates. J Biol Chem. 1980 Jul 25;255(14):6640–6645. [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport E. Compartmentalized ATP pools produced from adenosine are nuclear pools. J Cell Physiol. 1980 Nov;105(2):267–274. doi: 10.1002/jcp.1041050210. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Garcia-Blanco M. A., Zamecnik P. C. Regulation of DNA replication in S phase nuclei by ATP and ADP pools. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1643–1647. doi: 10.1073/pnas.76.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog K. L., Nordenskjöld B. A., Bjursell K. G. Deoxyribonucleoside-triphosphate pools and DNA synthesis in synchronized hamster cells. Eur J Biochem. 1973 Mar 15;33(3):428–432. doi: 10.1111/j.1432-1033.1973.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Slabaugh M. B., Johnson T. L., Mathews C. K. Vaccinia virus induces ribonucleotide reductase in primate cells. J Virol. 1984 Nov;52(2):507–514. doi: 10.1128/jvi.52.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornthwaite J. T., Sugarbaker E. V., Temple W. J. Preparation of tissues for DNA flow cytometric analysis. Cytometry. 1980 Nov;1(3):229–237. doi: 10.1002/cyto.990010309. [DOI] [PubMed] [Google Scholar]
- Tobey R. A. Production and characterization of mammalian cells reversibly arrested in G1 by growth in isoleucine-deficient medium. Methods Cell Biol. 1973;6:67–112. doi: 10.1016/s0091-679x(08)60048-5. [DOI] [PubMed] [Google Scholar]
- veer Reddy G. P., Pardee A. B. Coupled ribonucleoside diphosphate reduction, channeling, and incorporation into DNA of mammalian cells. J Biol Chem. 1982 Nov 10;257(21):12526–12531. [PubMed] [Google Scholar]