Abstract
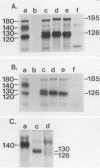
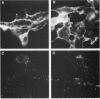
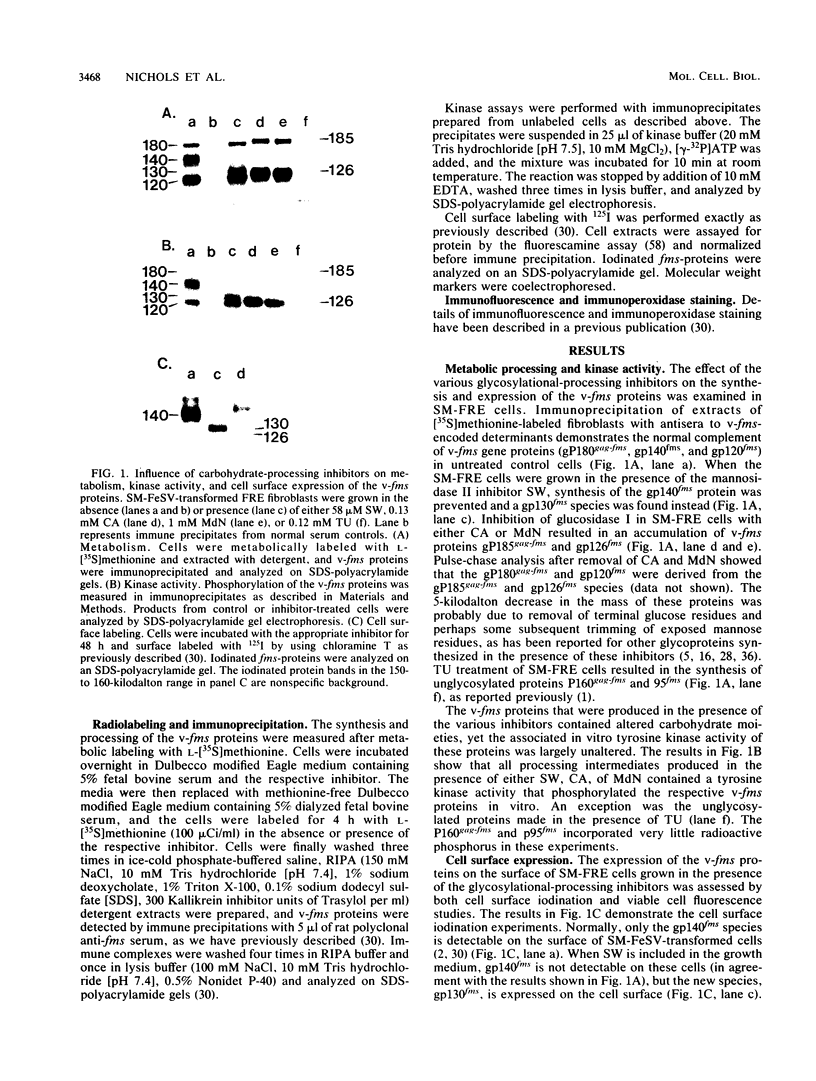
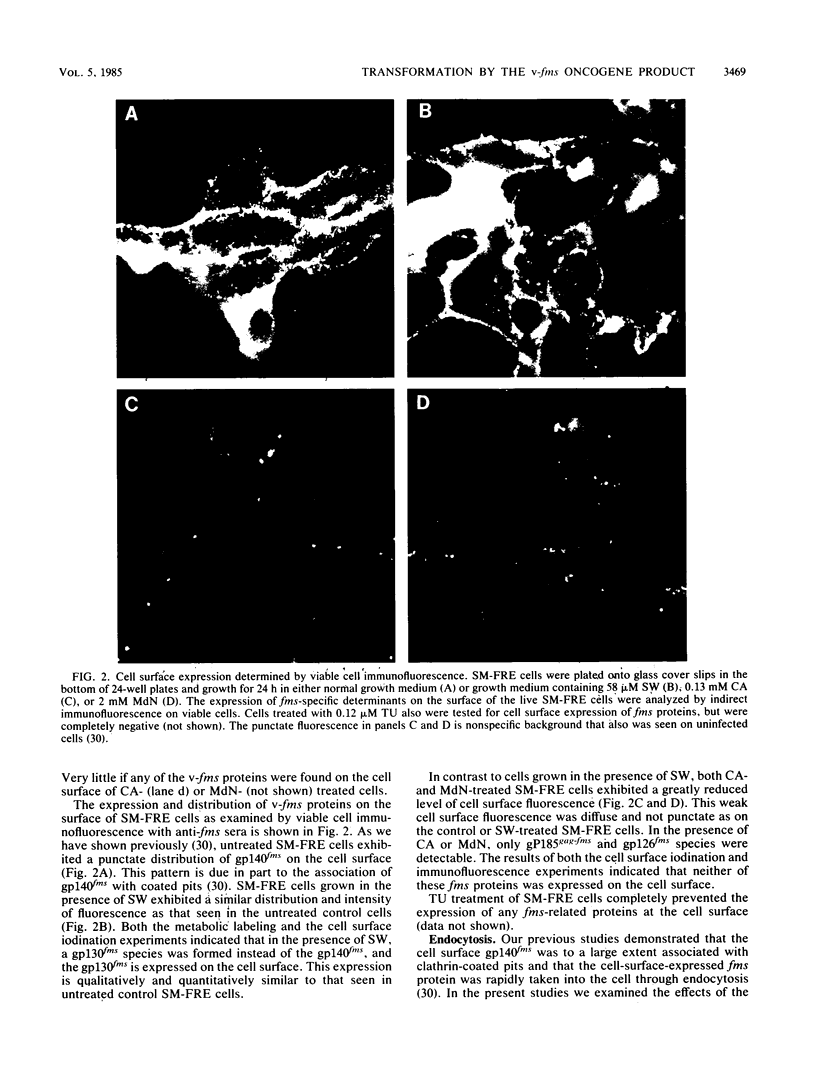
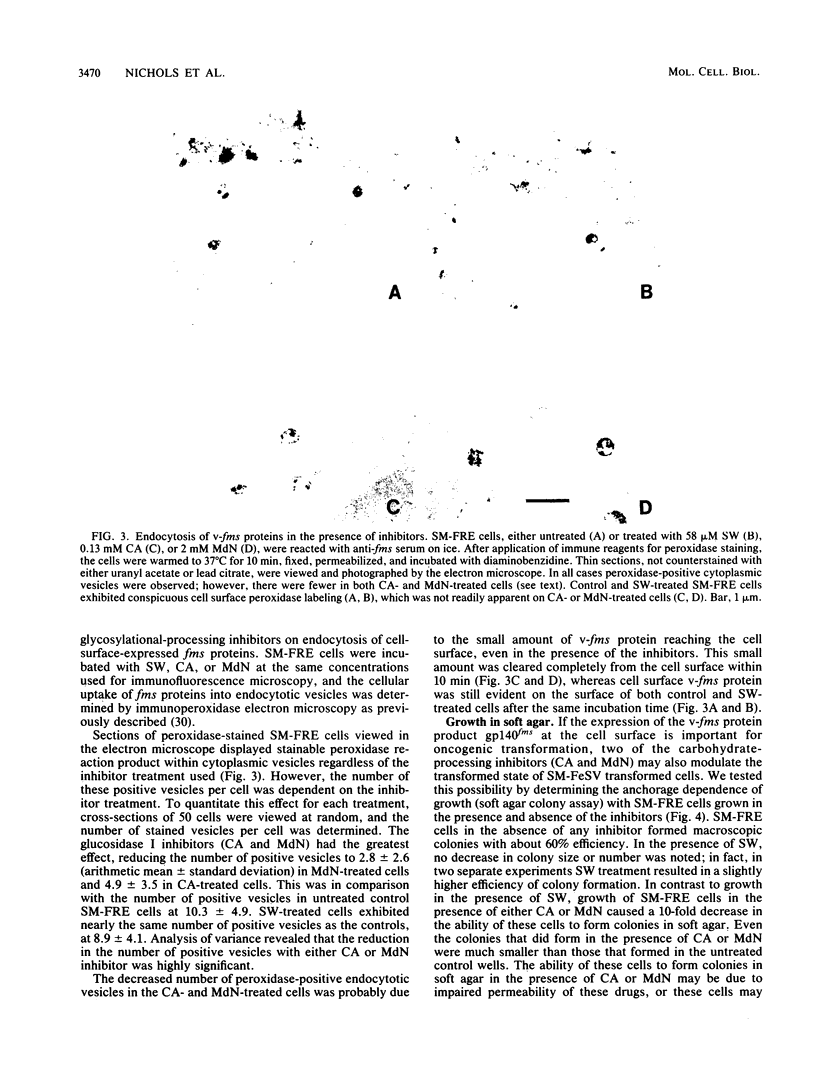
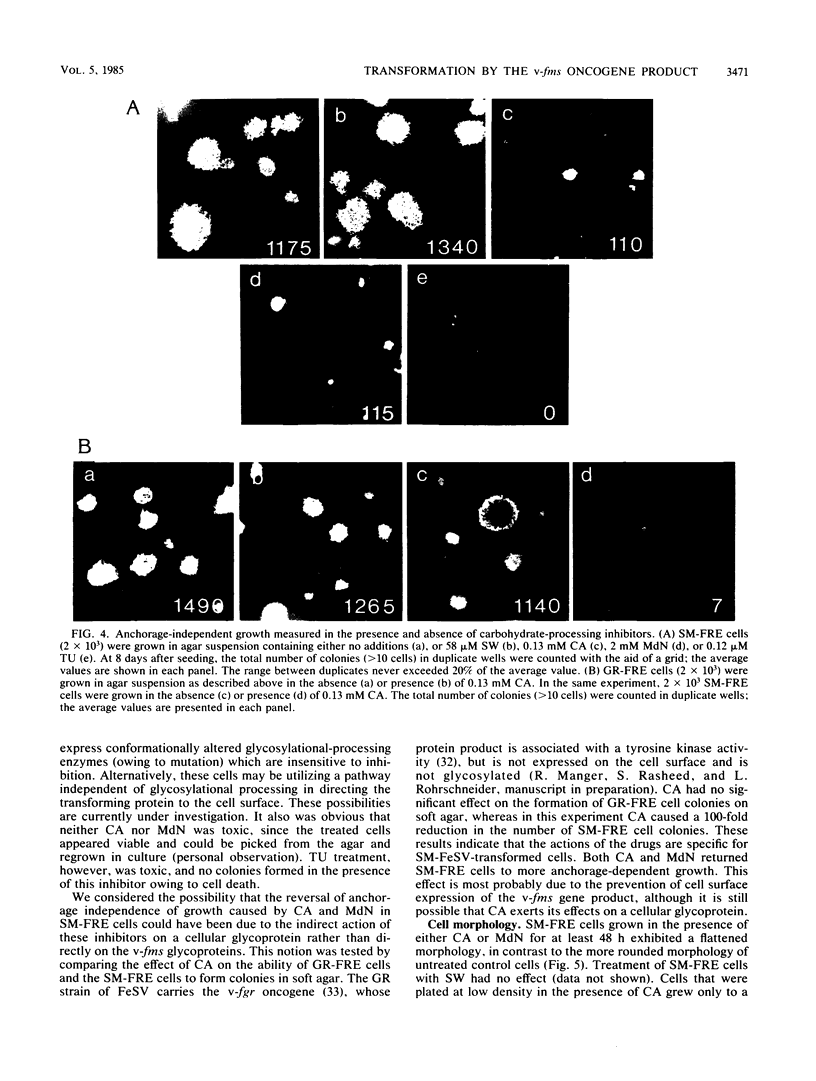
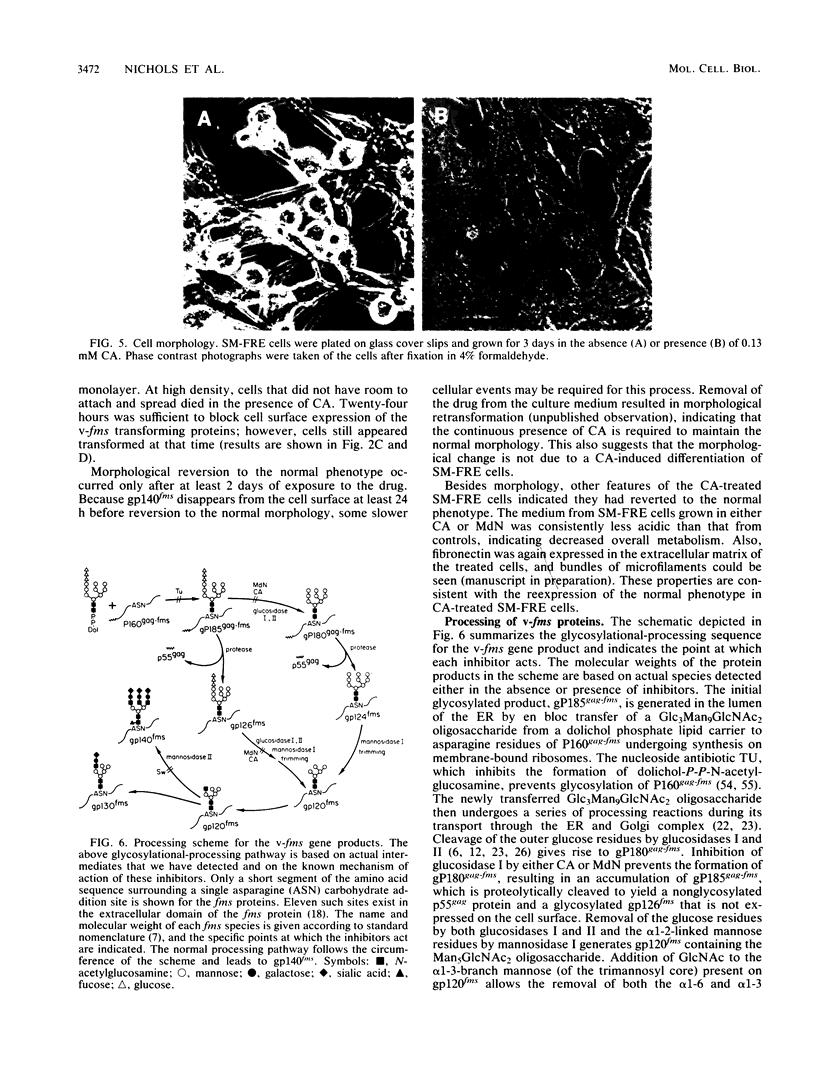
The effect of glycosylational-processing inhibitors on the synthesis, cell surface expression, endocytosis, and transforming function of the v-fms oncogene protein (gp140fms) was examined in McDonough feline sarcoma virus-transformed Fischer rat embryo (SM-FRE) cells. Swainsonine (SW), a mannosidase II inhibitor, blocked complete processing, but an abnormal v-fms protein containing hybrid carbohydrate structures was expressed on the cell surface. SW-treated SM-FRE cells retained the transformed phenotype. In contrast, two glucosidase I inhibitors (castanospermine [CA] and N-methyl-1-deoxynojirimycin [MdN]) blocked carbohydrate remodeling at an early stage within the endoplasmic reticulum and prevented cell surface expression of v-fms proteins. CA-treated SM-FRE cells reverted to the normal phenotype. Neither SW, CA, nor MdN affected either endocytosis or the tyrosine kinase activity associated with the v-fms gene product in vitro. These results demonstrate the necessity of carbohydrate processing for cell surface expression of the v-fms gene product and illustrate the unique ability to modulate the transformed state of SM-FRE cells with the glycosylational-processing inhibitors CA and MdN.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Furth M., Wolff L., Ruscetti S. K., Sherr C. J. Monoclonal antibodies to the transformation-specific glycoprotein encoded by the feline retroviral oncogene v-fms. J Virol. 1982 Nov;44(2):696–702. doi: 10.1128/jvi.44.2.696-702.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. J., Gonda M. A., Rettenmier C. W., Sherr C. J. Subcellular localization of glycoproteins encoded by the viral oncogene v-fms. J Virol. 1984 Sep;51(3):730–741. doi: 10.1128/jvi.51.3.730-741.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugham R. G., Tanzer M. L. Abnormal glycosylation of human cellular fibronectin in the presence of swainsonine. J Biol Chem. 1983 Oct 10;258(19):11883–11889. [PubMed] [Google Scholar]
- Bishop J. M. Cellular oncogenes and retroviruses. Annu Rev Biochem. 1983;52:301–354. doi: 10.1146/annurev.bi.52.070183.001505. [DOI] [PubMed] [Google Scholar]
- Bosch J. V., Schwarz R. T. Processing of gPr92env, the precursor to the glycoproteins of Rous sarcoma virus: use of inhibitors of oligosaccharide trimming and glycoprotein transport. Virology. 1984 Jan 15;132(1):95–109. doi: 10.1016/0042-6822(84)90094-1. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Touster O. Purification and characterization of glucosidase II, an endoplasmic reticulum hydrolase involved in glycoprotein biosynthesis. J Biol Chem. 1982 Sep 10;257(17):9990–10000. [PubMed] [Google Scholar]
- Coffin J. M., Varmus H. E., Bishop J. M., Essex M., Hardy W. D., Jr, Martin G. S., Rosenberg N. E., Scolnick E. M., Weinberg R. A., Vogt P. K. Proposal for naming host cell-derived inserts in retrovirus genomes. J Virol. 1981 Dec;40(3):953–957. doi: 10.1128/jvi.40.3.953-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of glycoprotein synthesis. Methods Enzymol. 1983;98:135–154. doi: 10.1016/0076-6879(83)98144-2. [DOI] [PubMed] [Google Scholar]
- Elting J. J., Chen W. W., Lennarz W. J. Characterization of a glucosidase involved in an initial step in the processing of oligosaccharide chains. J Biol Chem. 1980 Mar 25;255(6):2325–2331. [PubMed] [Google Scholar]
- Fischer H. D., Gonzalez-Noriega A., Sly W. S., Morré D. J. Phosphomannosyl-enzyme receptors in rat liver. Subcellular distribution and role in intracellular transport of lysosomal enzymes. J Biol Chem. 1980 Oct 25;255(20):9608–9615. [PubMed] [Google Scholar]
- Fitting T., Kabat D. Evidence for a glycoprotein "signal" involved in transport between subcellular organelles. Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J Biol Chem. 1982 Dec 10;257(23):14011–14017. [PubMed] [Google Scholar]
- Frankel A. E., Gilbert J. H., Porzig K. J., Scolnick E. M., Aaronson S. A. Nature and distribution of feline sarcoma virus nucleotide sequences. J Virol. 1979 Jun;30(3):821–827. doi: 10.1128/jvi.30.3.821-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Gross V., Tran-Thi T. A., Vosbeck K., Heinrich P. C. Effect of swainsonine on the processing of the asparagine-linked carbohydrate chains of alpha 1-antitrypsin in rat hepatocytes. Evidence for the formation of hybrid oligosaccharides. J Biol Chem. 1983 Mar 25;258(6):4032–4036. [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Requirement for a signal sequence in biological expression of the v-sis oncogene. Science. 1984 Dec 7;226(4679):1197–1199. doi: 10.1126/science.6095451. [DOI] [PubMed] [Google Scholar]
- Harpaz N., Schachter H. Control of glycoprotein synthesis. Processing of asparagine-linked oligosaccharides by one or more rat liver Golgi alpha-D-mannosidases dependent on the prior action of UDP-N-acetylglucosamine: alpha-D-mannoside beta 2-N-acetylglucosaminyltransferase I. J Biol Chem. 1980 May 25;255(10):4894–4902. [PubMed] [Google Scholar]
- Hettkamp H., Bause E., Legler G. Inhibition by nojirimycin and 1-deoxynojirimycin of microsomal glucosidases from calf liver acting on the glycoprotein oligosaccharides Glc1-3Man9GlcNAc2. Biosci Rep. 1982 Nov;2(11):899–906. doi: 10.1007/BF01114896. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Ivatt R. J. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Hubbard S. C., Robbins P. W. Synthesis and processing of protein-linked oligosaccharides in vivo. J Biol Chem. 1979 Jun 10;254(11):4568–4576. [PubMed] [Google Scholar]
- Kang M. S., Elbein A. D. Alterations in the structure of the oligosaccharide of vesicular stomatitis virus G protein by swainsonine. J Virol. 1983 Apr;46(1):60–69. doi: 10.1128/jvi.46.1.60-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S., Li E., Tabas I. The synthesis of complex-type oligosaccharides. II. Characterization of the processing intermediates in the synthesis of the complex oligosaccharide units of the vesicular stomatitis virus G protein. J Biol Chem. 1978 Nov 10;253(21):7771–7778. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Lemansky P., Gieselmann V., Hasilik A., von Figura K. Cathepsin D and beta-hexosaminidase synthesized in the presence of 1-deoxynojirimycin accumulate in the endoplasmic reticulum. J Biol Chem. 1984 Aug 25;259(16):10129–10135. [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Glucose removal from N-linked oligosaccharides is required for efficient maturation of certain secretory glycoproteins from the rough endoplasmic reticulum to the Golgi complex. J Cell Biol. 1984 May;98(5):1720–1729. doi: 10.1083/jcb.98.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger R., Najita L., Nichols E. J., Hakomori S., Rohrschneider L. Cell surface expression of the McDonough strain of feline sarcoma virus fms gene product (gp 140fms). Cell. 1984 Dec;39(2 Pt 1):327–337. doi: 10.1016/0092-8674(84)90011-4. [DOI] [PubMed] [Google Scholar]
- McDonough S. K., Larsen S., Brodey R. S., Stock N. D., Hardy W. D., Jr A transmissible feline fibrosarcoma of viral origin. Cancer Res. 1971 Jul;31(7):953–956. [PubMed] [Google Scholar]
- Naharro G., Dunn C. Y., Robbins K. C. Analysis of the primary translational product and integrated DNA of a new feline sarcoma virus, GR-FeSV. Virology. 1983 Mar;125(2):502–507. doi: 10.1016/0042-6822(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrate in biological function of the adhesive glycoprotein fibronectin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3343–3347. doi: 10.1073/pnas.76.7.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrieras N., Bause E., Legler G., Vasilov R., Claesson L., Peterson P., Ploegh H. Effects of the glucosidase inhibitors nojirimycin and deoxynojirimycin on the biosynthesis of membrane and secretory glycoproteins. EMBO J. 1983;2(6):823–832. doi: 10.1002/j.1460-2075.1983.tb01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Quinn C. O., Kitchingman G. R., Look A. T., Sherr C. J. Transmembrane orientation of glycoproteins encoded by the v-fms oncogene. Cell. 1985 Apr;40(4):971–981. doi: 10.1016/0092-8674(85)90357-5. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Datema R., Schwarz R. T. N-methyl-1-deoxynojirimycin, a novel inhibitor of glycoprotein processing, and its effect on fowl plague virus maturation. Virology. 1983 Oct 15;130(1):238–242. doi: 10.1016/0042-6822(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Romero P. A., Saunier B., Herscovics A. Comparison between 1-deoxynojirimycin and N-methyl-1-deoxynojirimycin as inhibitors of oligosaccharide processing in intestinal epithelial cells. Biochem J. 1985 Mar 15;226(3):733–740. doi: 10.1042/bj2260733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Look A. T., Sherr C. J. Cell surface expression of v-fms-coded glycoproteins is required for transformation. Mol Cell Biol. 1984 Oct;4(10):1999–2009. doi: 10.1128/mcb.4.10.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Sherr C. J., Barker P. E., Ruddle F. H. Molecular cloning of the c-fms locus and its assignment to human chromosome 5. J Virol. 1983 Dec;48(3):770–773. doi: 10.1128/jvi.48.3.770-773.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Sharar A. L., McDonough S. The SM strain of feline sarcoma virus. Biologic and antigenic characterization of virus. Proc Soc Exp Biol Med. 1972 Sep;140(4):1365–1368. doi: 10.3181/00379727-140-36675. [DOI] [PubMed] [Google Scholar]
- Saul R., Ghidoni J. J., Molyneux R. J., Elbein A. D. Castanospermine inhibits alpha-glucosidase activities and alters glycogen distribution in animals. Proc Natl Acad Sci U S A. 1985 Jan;82(1):93–97. doi: 10.1073/pnas.82.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunier B., Kilker R. D., Jr, Tkacz J. S., Quaroni A., Herscovics A. Inhibition of N-linked complex oligosaccharide formation by 1-deoxynojirimycin, an inhibitor of processing glucosidases. J Biol Chem. 1982 Dec 10;257(23):14155–14161. [PubMed] [Google Scholar]
- Schechter A. L., Stern D. F., Vaidyanathan L., Decker S. J., Drebin J. A., Greene M. I., Weinberg R. A. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984 Dec 6;312(5994):513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Beug H., Hayman M. J. Effects of inhibitors of glycoprotein processing on the synthesis and biological activity of the erb B oncogene. EMBO J. 1985 Jan;4(1):105–112. doi: 10.1002/j.1460-2075.1985.tb02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979 Nov 25;254(22):11655–11663. [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. Swainsonine causes the production of hybrid glycoproteins by human skin fibroblasts and rat liver Golgi preparations. J Biol Chem. 1983 Jun 25;258(12):7578–7585. [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Woolford J., Rothwell V., Rohrschneider L. Characterization of the human c-fms gene product and its expression in cells of the monocyte-macrophage lineage. Mol Cell Biol. 1985 Dec;5(12):3458–3466. doi: 10.1128/mcb.5.12.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]