Abstract
Results from the Viking biology experiments indicate the presence of reactive oxidants in martian soils that have previously been attributed to peroxide and superoxide. Instruments on the Mars Phoenix Lander and the Mars Science Laboratory detected perchlorate in martian soil, which is nonreactive under the conditions of the Viking biology experiments. We show that calcium perchlorate exposed to gamma rays decomposes in a CO2 atmosphere to form hypochlorite (ClO−), trapped oxygen (O2), and chlorine dioxide (ClO2). Our results show that the release of trapped O2 (g) from radiation-damaged perchlorate salts and the reaction of ClO− with amino acids that were added to the martian soils can explain the results of the Viking biology experiments. We conclude that neither hydrogen peroxide nor superoxide is required to explain the results of the Viking biology experiments. Key Words: Mars—Radiolysis—Organic degradation—in situ measurement—Planetary habitability and biosignatures. Astrobiology 13, 515–520.
1. Introduction
Experiments performed during the Viking Mars mission indicated the presence of low levels of reactive oxidants in the Viking soils (Klein et al., 1976). In the Viking Gas Exchange (GEx) experiment, the release of 70–700 nmol of O2 (g) was measured when 1 cm3 soil samples were exposed to water (Oyama and Berdahl, 1977). In the Viking Labeled Release (LR) experiment, ∼30 nmol of 14C-labeled gas was produced due to the partial decomposition of an aqueous mixture of 14C-labeled organics that was added to 0.5 cm3 of soil (Levin and Straat, 1976). Heat treatment indicated that the origin of GEx and LR results is likely different; by using a protocol intended to sterilize the soil, the GEx oxygen release was determined to be thermally stable, while the species responsible for the LR results was decomposed by heat (Klein, 1978). Since the return of the Viking results, explanations have focused on the possible UV-induced formation and presence of hydrogen peroxide as the thermally unstable LR oxidant and superoxide as the thermally stable GEx oxidant (Oyama et al., 1977). It has also been suggested that the diffusion of these species in the regolith is responsible for subsurface oxidation (Bullock et al., 1994; Yen et al., 2000) and explains the apparent lack of organics in samples measured by the Viking Gas Chromatograph Mass Spectrometer (GCMS) (Biemann et al., 1977). Although numerous variations on these hypotheses have been presented since the Viking mission, none have been demonstrated to be completely consistent with all aspects of these two experiments (Zent and McKay, 1994; ten Kate, 2010).
Three decades after Viking, the Wet Chemistry Laboratory on the NASA Phoenix Mars Lander measured perchlorate anions (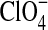 ) at ∼0.5 wt % levels, likely present in the soil as Ca(ClO4)2 and/or Mg(ClO4)2 (Hecht et al.,
2009). Recently, samples collected from the Rocknest site by the Mars Science Laboratory (MSL) and analyzed by using the Sample Analysis at Mars (SAM) instrument suite have been found to contain perchlorate (Archer et al.,
2013). Although perchlorate salts can behave as strong oxidants at elevated temperatures, their stability under the conditions of the GEx and LR preclude them from directly explaining the results of these experiments. However, we demonstrate that products formed during the decomposition of perchlorate by ionizing radiation can explain the results of the LR and GEx experiments. Due to the thin atmosphere and lack of a planetary magnetosphere on Mars, galactic cosmic rays and solar energetic particles are only slightly attenuated before reaching the surface. The adsorbed radiation dose in the martian regolith at the Viking sampling depth of 10–20 cm has been estimated to be ∼0.1 Gy/year, and galactic cosmic ray penetration extends down to ∼2 m in the regolith (Dartnell et al.,
2007).
) at ∼0.5 wt % levels, likely present in the soil as Ca(ClO4)2 and/or Mg(ClO4)2 (Hecht et al.,
2009). Recently, samples collected from the Rocknest site by the Mars Science Laboratory (MSL) and analyzed by using the Sample Analysis at Mars (SAM) instrument suite have been found to contain perchlorate (Archer et al.,
2013). Although perchlorate salts can behave as strong oxidants at elevated temperatures, their stability under the conditions of the GEx and LR preclude them from directly explaining the results of these experiments. However, we demonstrate that products formed during the decomposition of perchlorate by ionizing radiation can explain the results of the LR and GEx experiments. Due to the thin atmosphere and lack of a planetary magnetosphere on Mars, galactic cosmic rays and solar energetic particles are only slightly attenuated before reaching the surface. The adsorbed radiation dose in the martian regolith at the Viking sampling depth of 10–20 cm has been estimated to be ∼0.1 Gy/year, and galactic cosmic ray penetration extends down to ∼2 m in the regolith (Dartnell et al.,
2007).
We have characterized radiolysis products of Ca(ClO4)2 that were partially decomposed, in CO2 (g) at Mars-like pressures with a 60Co gamma-ray source. We show that ionizing radiation decomposes perchlorate into lower-oxidation-state oxychlorine and oxygen species, including hypochlorite (ClO−), chlorine dioxide (ClO2), and oxygen gas (O2), that can explain the Viking GEx and LR results. We demonstrate that the primary release of 14CO2 observed in the LR experiment can be reproduced by the decomposition of chloroalanine formed by the reaction of soil hypochlorite with the 14C-labeled alanine present in the LR organic medium. We also show that release of O2 (g) trapped in radiation-damaged perchlorate salt can explain the GEx results.
2. Methods
Anhydrous calcium perchlorate was prepared from calcium perchlorate tetrahydrate (Sigma Aldrich, 99%; CAS 15627-86-8) by dehydrating 0.5 g samples, in 5 mL glass ampules (Wheaton, 651509), under vacuum at 200°C for 8–10 h. After the dehydrated samples were allowed to cool under vacuum to room temperature, the ampules were backfilled to 7.5±0.2 mbar with CO2 gas (Scott, UHP 99.999%) and flame sealed. The sealed samples were exposed to gamma radiation at room temperature with a 60Co source at a rate of 50 kGy h−1 for a total dose of 500 kGy. The samples remained at ambient temperature for ∼2 days during transit from the radiation facility (Sterigenics Inc., CA). Since heat can accelerate product (e.g., hypochlorite) decomposition, upon return the samples were stored in the still sealed ampules at −20°C until just prior to analysis.
After irradiation, hypochlorite and chlorine dioxide concentrations were determined by measuring the UV absorbance spectra of the gamma-irradiated samples in aqueous solution. Concentrations were determined as a function of time by using a molar extinction coefficient of 350 L mol−1 cm−1 at 290 nm for hypochlorite and 1250 L mol−1 cm−1 at 360 nm for chlorine dioxide. Absorbance measurements were made with an Ocean Optics HR2000 UV-vis-NIR spectrometer, an Ocean Optics HD2000 UV-vis-NIR light source, a 1 cm cuvette, and associated optical fibers. Since the pH of Phoenix and Viking soils is slightly basic (Quinn and Orenberg, 1993; Kounaves et al., 2010), the irradiated perchlorate products were measured in a slightly basic aqueous solution (pH 9 borax buffer).
Hypochlorite and chlorine dioxide concentrations were also independently quantified by measurement of the UV absorbance of tri-iodide formed from the quantitative oxidation of iodide to tri-iodide in aqueous solution (Prince, 1964). Hypochlorite and chlorine dioxide were first measured by adding irradiated perchlorate to KI (1%) in sodium borate (pH 9), followed by measurement of the 350 nm tri-iodide absorption. At pH 9, hypochlorite is completely reduced to chloride, and chlorine dioxide undergoes a one-electron reduction to chlorite. After measuring the 350 nm absorbance at pH 9, the solution was acidified to a pH of 1.5. Under acidic conditions, chlorite (in this case formed from chlorine dioxide reduction at pH 9) reacts with iodide and is reduced to chloride. Hypochlorite and chlorine dioxide concentrations were then calculated from the difference in the pH 9 and pH 1.5 tri-iodide absorbances.
To measure the production of O2 (g) and CO2 (g) by the irradiated perchlorate samples upon addition of aqueous organics, ∼100 mg samples were transferred under dry-He into 10 cc glass vials and crimp sealed with rubber septa. The quantity of O2 (g) and 13CO2 (g) produced after the addition of 13C-labeled alanine (2.5×10−3 M) was determined by periodically sampling the vial headspace with a gas-tight syringe, followed by gas chromatography–mass spectroscopic analysis of the extracted gases. 13C-labeled alanine was used to differentiate CO2 production due to alanine decomposition from background CO2 (primarily 12CO2).
Ultraviolet spectroscopy was used to confirm that observed rate of labeled-CO2 production corresponded to N-chloroamino acid decomposition. The 255 nm absorbance due to N-chloroamino acids formed after the addition of reagent Ca(ClO)2 to a 5.0×10−4 M alanine and 2.5×10−4 M glycine solution was measured to determine the decomposition rates. During experimental runs, sample temperature was held at 10°C to approximate the temperature of the Viking biology experiments. The thermal stability of the perchlorate decomposition products was evaluated by heating sealed samples at 160°C for 3 h to mimic the LR heat treatment protocol. After cooling to 10°C, the heat-treated samples were analyzed in the same manner as the other samples. Ca(ClO4)2 samples, prepared as described above, that were not exposed to gamma irradiation served as control samples.
The Viking LR data was obtained from the NASA Planetary Data System. The data sets contain the counts recorded by a solid-state beta detector as a function of time resulting from the presence of 14C-labeled gas in the sample cell headspace.
3. Results
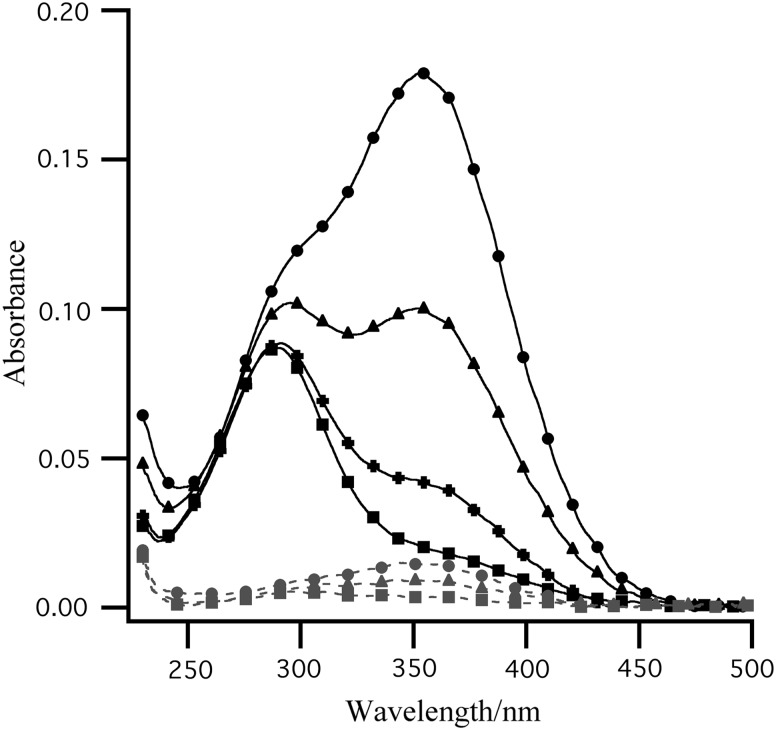
Figure 1 shows UV spectra of Ca(ClO4)2 added to pH 9 solution after a ∼500 kGy irradiation dose. Both ClO− and ClO2 are identified by their absorbances at 290 and 360 nm, respectively. We attribute the 360 nm absorbance decrease over the duration of the measurements to the loss of ClO2 (g) from solution, rather than decomposition. In basic conditions, the reduction of ClO2 (g) results in the formation of chlorite (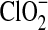 ), which has an absorbance at 260 nm. The absence of a 260 nm absorbance indicates that chlorite is not formed in solution and that
), which has an absorbance at 260 nm. The absence of a 260 nm absorbance indicates that chlorite is not formed in solution and that 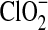 is not an end product of the Ca(ClO4)2 radiolysis.
is not an end product of the Ca(ClO4)2 radiolysis.
FIG. 1.
UV spectra of gamma-irradiated Ca(ClO4)2 in an aqueous pH 9 buffered solution (20°C). ɛmax=360 nm for ClO2 (g), and ɛmax=290 nm for ClO− (aq). A decrease absorbance at 360 nm occurs due to the loss of ClO2 (g) from solution. Only residual levels of ClO2 (g) remain in samples heated to 160°C (unheated: • 0 min; ▴ 40 min;+80 min; ▪ 120 min; heated:  0 min;
0 min;  40 min;
40 min;  120 min).
120 min).
Table 1 reports the levels of ClO− and ClO2 measured in irradiated Ca(ClO4)2 and in irradiated Ca(ClO4)2 that was heated for 3 h at 160°C prior to analysis. No ClO− and only trace levels of ClO2 were measured in samples that were heat treated. Also shown in Table 1 is the quantity of O2 (g) and 13CO2 (g) released from irradiated perchlorate samples upon wetting with 13C-labeled LR organics. The quantity of 13CO2 produced is equal to the amount of ClO− present in the irradiated sample, suggesting that ClO2 does not contribute to organic decomposition. The amount of O2 released from samples that were heat treated prior to analysis was only slightly lower than the amount from samples that were not heated, a result that is consistent with the Viking GEx experiment.
Table 1.
Calcium Perchlorate Radiolysis Products and Yieldsa
| |
Ca(ClO4)2 |
(CaClO4)2 heated |
||||||
|---|---|---|---|---|---|---|---|---|
| Product | mol % | σb | Gc | σ | mol % | σ | Gc | σ |
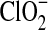 |
0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | N.A. | N.A |
| ClO− | 0.020 | 0.003 | 0.032 | 0.005 | 0.00 | 0.00 | N.A. | N.A. |
| ClO2 | 0.068 | 0.005 | 0.109 | 0.007 | 0.02 | 0.002 | N.A. | N.A |
| O2 | 1.0 | 0.1 | 1.6 | 0.2 | 0.83 | 0.02 | N.A. | N.A. |
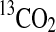 d d
|
0.022 | 0.003 | N.A. | N.A. | 0.00 | 0.00 | N.A. | N.A. |
Reported as mol % relative to initial 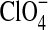 .
.
Standard deviation.
Number of molecules produced per 100 eV of absorbed energy.
Based on the 13CO2 released after addition of 13C labeled LR organics to irradiated Ca(ClO4)2.
N.A., not applicable.
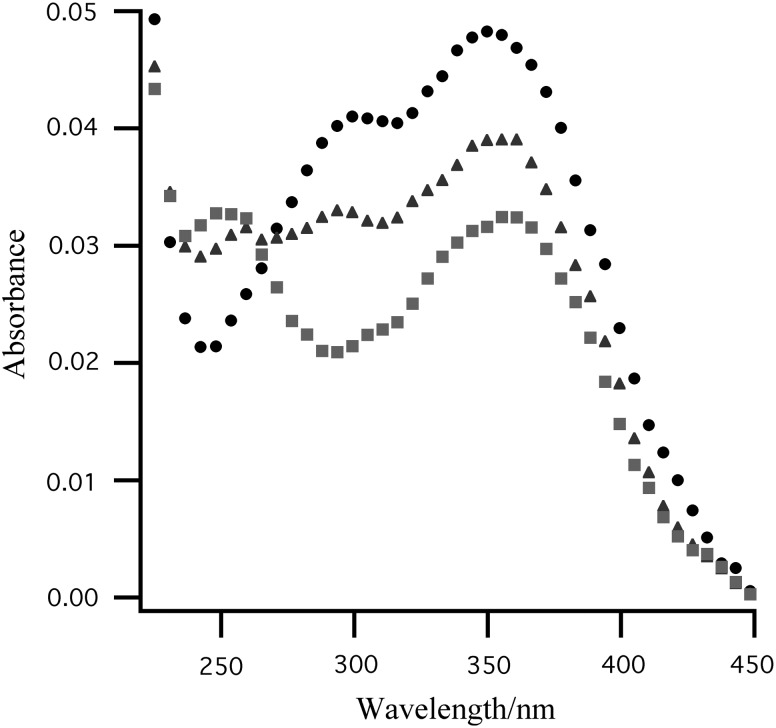
The Viking LR nutrient medium contained seven isotopically labeled organic compounds (sodium formate, D-alanine, L-alanine, sodium D-lactate, sodium L-lactate, glycine, and calcium glycolate), each at a concentration of 2.5×10−4 M (Klein et al., 1976). Upon addition of irradiated Ca(ClO4)2 to the aqueous LR organics, an absorption at 255 nm rapidly appears due to the formation of N-chloroamino acids (Fig. 2). Under the Viking LR experiment conditions, based on the second-order rate constants for the chlorination of alanine and glycine, ∼30 nmol of ClO− will react to 99% completion with the alanine and glycine in the LR nutrient in less than 210 ms (Pattison et al., 2003). The rapid scavenging of ClO− by the amino acids prevents its reaction with formate, lactate, or glycolate. When aqueous alanine solution is added to gamma-irradiated Ca(ClO4)2, the observed increase in headspace CO2 closely matches the Viking LR results (Fig. 3). As was the case in the Viking LR experiments, no CO2 release was observed from samples heated to 160°C. However, the heat-treated samples did release O2 (g), as was observed in the Viking GEx experiment (Table 1).
FIG. 2.
The rapid formation of N-chloroamino acids (255 nm) upon addition of Viking LR organics to gamma-irradiated Ca(ClO4)2 in aqueous solution (• 0 s; ▴ 0.16 s; ▪ 0.32 s). The absorbance by ClO− at 290 nm decreases as N-chloroamino acids form (260 nm). The absorbance by ClO2 (g) at 360 nm is attenuated due to dilution by the addition of the LR organics. No reaction between the LR organics and ClO2 (g) is observed. The LR aqueous organic solution contains sodium formate, D-alanine, L-alanine, sodium D-lactate, sodium L-lactate, glycine, and calcium glycolate, each at a concentration of 2.5×10−4 M.
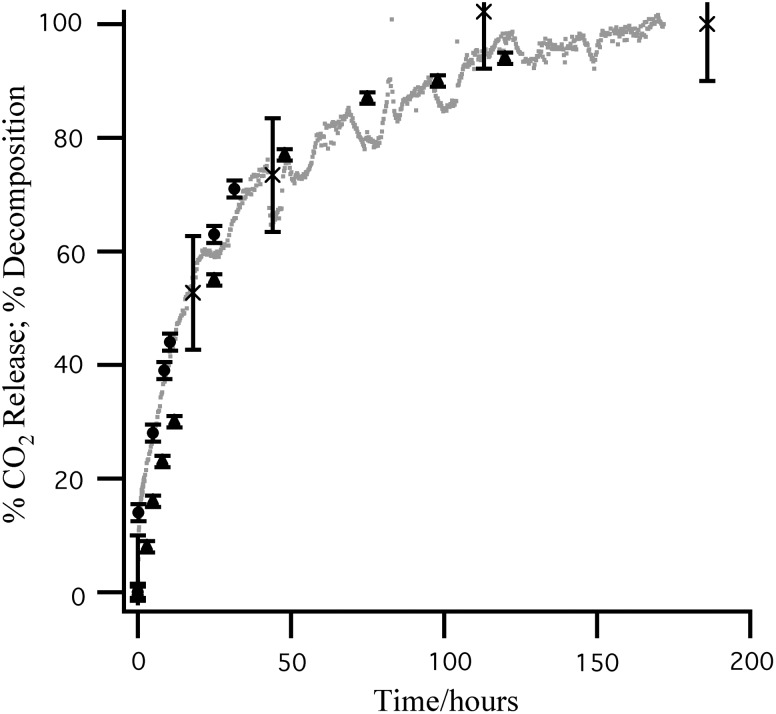
FIG. 3.
Viking LR gas release (VL2C3  ) compared to 13CO2 (g) released from aqueous 13C-alanine after addition to gamma-irradiated Ca(ClO4)2 (×). No 13CO2 release was observed from samples heated to 160°C. Also shown is the decomposition of chloroamino acids [initial concentrations 4.0×10−4
M (▴) and 2.0×10−4
M (•)] formed after the addition of Ca(ClO)2 to a 5.0×10−4
M alanine and 2.5×10−4
M glycine solution. The chloroamino acid decomposition rate compared to the 13CO2 release rate suggests that chloroalanine is the primary chlorination product.
) compared to 13CO2 (g) released from aqueous 13C-alanine after addition to gamma-irradiated Ca(ClO4)2 (×). No 13CO2 release was observed from samples heated to 160°C. Also shown is the decomposition of chloroamino acids [initial concentrations 4.0×10−4
M (▴) and 2.0×10−4
M (•)] formed after the addition of Ca(ClO)2 to a 5.0×10−4
M alanine and 2.5×10−4
M glycine solution. The chloroamino acid decomposition rate compared to the 13CO2 release rate suggests that chloroalanine is the primary chlorination product.
Following rapid formation, N-chloroalanine and N-chloroglycine decompose through pH-independent (from pH 5 to 9) first-order reaction mechanisms with the rate of N-chloroglycine decomposition being ∼2% relative to the rate of N-chloroalanine decomposition (Hand et al., 1983). Our tests in which reagent Ca(ClO)2 was added to solutions containing both alanine and glycine at ∼10°C indicated that N-chloroalanine is the primary chlorination product (Fig. 3). However, it may be possible that in the LR experiment on Mars N-chloroglycine formation was higher. If this is the case, N-chloroglycine decomposition may contribute to the slower secondary labeled gas release that was observed in the Viking LR. This slow secondary gas release in the LR has previously been attributed to the decomposition of organics by iron oxide (Levin and Straat, 1979).
4. Discussion
Prior to the Phoenix mission, the possible presence of perchlorates or other oxyhalides on Mars in the context of the Viking results and the preservation of biomarkers had not been rigorously considered. The possibility that Mars soil may contain perchlorates was recognized, but the instruments on missions prior to Phoenix lacked the capability to distinguish between different forms of chlorine (Clark et al., 2005). It has been suggested that thermodynamically unstable species formed on UV-activated surfaces of halide evaporites could be responsible for O2 generation in the GEx experiment; however, the presence of bulk O2 (g) in martian soil was discounted (Zent and McKay, 1994). O2 (g) trapped in soil pore space and released upon humidification by the Rebiner effect has been previously proposed as an explanation for the GEx experiment (Nussinov et al., 1978). However, perchlorate radiolysis has not previously been proposed as the origin and trapping mechanism of O2 (g) in the martian regolith. Our results suggest that the O2 (g) is trapped in the radiation-damaged salt and is released upon water-induced dissolution.
Plumb et al. (1989) proposed that nitrate partially photolyzed by UV light in the presence of calcite could explain the GEx and LR results. Although calcite has been identified at the Phoenix site, nitrates have not been identified on Mars. However, if present, nitrate like perchlorate is decomposed by ionizing radiation (e.g., Hennig et al., 1953) and may contribute to the reactive nature of the martian soil.
In addition to chlorine dioxide and hypochlorite formation, radiolysis of alkali and alkaline Earth perchlorates [e.g., (MgClO4)2] can also result in the formation of chlorate and chlorite (Prince and Johnson, 1965). The presence of perchlorate radiolysis products in martian soils presents a number of important ramifications for Mars exploration, the habitability of martian soil, and the search for organic compounds. Additionally, processes involving superoxide and peroxide that have been previously proposed based on interpretations of Viking biology results may play a less important role in the oxidative alteration of organics than has previously been suggested. A number of shortcomings of the hypotheses that hydrogen peroxide and superoxide were responsible for the LR and GEx results have been recognized. In particular, due to pH-dependant reaction rates and competitive loss mechanisms, implausibly large amounts of hydrogen peroxide have been required to simulate the LR results (Levin and Straat, 1981).
Hypochlorite and other potentially reactive oxyhalide species can decompose some classes of organic compounds and act as antimicrobial agents (Deborde and von Gunten, 2008); however, on Mars these effects may be limited to areas in close proximity to soils that contain perchlorate. However, given the possible atmospheric origin of perchlorate (Catling et al., 2010), it is likely to be a component of globally distributed dust and may interfere with soil analyses. The presence of perchlorate at the Phoenix (Hecht et al., 2009) and MSL (Archer et al., 2013) landing sites suggests a global distribution. Additionally, although this suggestion is controversial (Biemann and Bada, 2011), reevaluation of the Viking GCMS results after the Phoenix mission suggests that perchlorate interfered with the detection of soil organics (Navarro-González et al., 2010). Likewise, the presence of more reactive lower-oxidation-state oxychlorine species in martian soils may interfere with soil analyses including analyses performed with the SAM instrument on the MSL Curiosity rover (Mahaffy et al., 2012). For example, during thermal analysis, the release of oxygen and reactive chlorine species from the martian soil may occur at temperatures below the decomposition temperature of perchlorate. The detection of chlorohydrocarbons when using the Viking 1 GCMS (Biemann et al., 1977) and MSL SAM instrument suite (Glavin et al., 2013) from martian soils heated to 200°C supports this possibility. Additionally, our results suggest that fluidic-based derivatization approaches intended to enhance in situ amino acid detection may be inhibited by the rapid chlorination of amino acids by ClO− (Skelley et al., 2006; Mahaffy et al., 2012).
5. Conclusions
We conclude that on Mars galactic cosmic rays and solar energetic particles should result in the formation of hypochlorite, other lower-oxidation-state oxychlorine species, and trapped O2 (g) in perchlorate-containing soils. Based on our experimental results, we conclude that the decomposition of N-chloroalanine formed by the reaction of hypochlorite with the alanine in Viking LR nutrient medium was the primary source of labeled gas (14CO2) production in the LR experiment. We also conclude that the O2 (g) trapped in radiation-damaged perchlorate salts was the origin of the O2 (g) release observed in the Viking GEx experiments. Based on our results, it appears that neither hydrogen peroxide nor superoxide is required to explain Viking LR and GEx experiments.
Acknowledgments
Funding was provided by the NASA Astrobiology: Exobiology and Evolutionary Biology Program (grant NNX09AM93G). The authors thank C.P. McKay, C.R. Stoker and Inge ten Kate for their reviews and comments. The authors also acknowledge Dr. Cynthia B. Phillips (SETI Institute) and Dr. Monika Kress (SJSU). Partial funding for H. Martucci was provided by the NASA Education and Public Outreach in Earth and Space Science (EPOESS) program.
Abbreviations
GCMS, Gas Chromatograph Mass Spectrometer; GEx, Gas Exchange; LR, Labeled Release; MSL, Mars Science Laboratory; SAM, Sample Analysis at Mars.
References
- Archer P.D. Sutter B. Ming D.W. McKay C.P. Navarro-González R. Franz H.B. McAdam A. Mahaffy P.R the MSL Science Team. Possible detection of perchlorates by evolved gas analysis of Rocknest soils: global implications [abstract 2168]. In 44th Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute; Houston. 2013. [Google Scholar]
- Biemann K. Bada J.L. Comment on “Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars” by Rafael Navarro-González et al. J Geophys Res. 2011;116 doi: 10.1029/2011JE003869. [DOI] [Google Scholar]
- Biemann K. Oro J. Toulmin P. Orgel L.E. Nier A.O. Anderson D.M. Simmonds P.G. Flory D. Diaz A.V. Rushneck D.R. Biller J.E. Lafleur A.L. The search for organic substances and inorganic volatile compounds in the surface of Mars. J Geophys Res. 1977;82:4641–4658. doi: 10.1126/science.194.4260.72. [DOI] [PubMed] [Google Scholar]
- Bullock M.A. Stoker C.R. McKay C.P. Zent A.P. A coupled soil-atmosphere model of H2O2 on Mars. Icarus. 1994;107:142–154. doi: 10.1006/icar.1994.1012. [DOI] [PubMed] [Google Scholar]
- Catling D.C. Claire M.W. Zahnle K.J. Quinn R.C. Clark B.C. Hecht M.H. Kounaves S. Atmospheric origins of perchlorate on Mars and in the Atacama. J Geophys Res. 2010;115 doi: 10.1029/2009JE003425. [DOI] [Google Scholar]
- Clark B.C. Morris R.V. McLennan S.M. Gellert R. Jolliff B. Knoll A.H. Squyres S.W. Lowenstein T.K. Ming D.W. Tosca N.J. Yen A. Christensen P.R. Gorevan S. Brückner J. Calvin W. Dreibus G. Farrand W. Klingelhoefer G. Waenke H. Zipfel J. Bell J.F., III Grotzinger J. McSween H.Y. Rieder R. Chemistry and mineralogy of outcrops at Meridiani Planum. Earth Planet Sci Lett. 2005;240:73–94. [Google Scholar]
- Dartnell L.R. Desorgher L. Ward J.M. Coates A.J. Modelling the surface and subsurface martian radiation environment: implications for astrobiology. Geophys Res Lett. 2007;34 doi: 10.1029/2006GL027494. [DOI] [Google Scholar]
- Deborde M. von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res. 2008;42:3–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- Glavin D. Archer D. Brunner A. Buch A. Cabane M. the MSL Science Team. Investigating the origin of chlorohydrocarbons detected by the Sample Analysis at Mars (SAM) instrument at Rocknest [abstract 1080]. In 44th Lunar and Planetary Science Conference Abstracts, Lunar and Planetary Institute; Houston. 2013. [Google Scholar]
- Hand V.C. Snyder M.P. Margerum D.W. Concerted fragmentation of N-chloro-α-amino acid anions. J Am Chem Soc. 1983;105:4022–4025. [Google Scholar]
- Hecht M.H. Kounaves S.P. Quinn R.C. West S.J. Young S.M.M. Ming D.W. Catling D.C. Clark B.C. Boynton W.V. Hoffman J. DeFlores L.P. Gospodinova K. Kapit J. Smith P.H. Detection of perchlorate and the soluble chemistry of martian soil at the Phoenix Lander site. Science. 2009;325:64–67. doi: 10.1126/science.1172466. [DOI] [PubMed] [Google Scholar]
- Hennig G. Lees R. Matheson M.S. The decomposition of nitrate crystals by ionizing radiations. J Chem Phys. 1953;21:664–668. [Google Scholar]
- Klein H.P. The Viking biological experiments on Mars. Icarus. 1978;34:666–674. [Google Scholar]
- Klein H.P. Horowitz N.H. Levin G.V. Oyama V.I. Lederberg J. Rich A. Hubbard J.S. Hobby G.L. Straat P.A. Berdahl B.J. Carle G.C. Brown F.S. Johnson R.D. The Viking biological investigation: preliminary results. Science. 1976;194:99–105. doi: 10.1126/science.194.4260.99. [DOI] [PubMed] [Google Scholar]
- Kounaves S.P. Hecht M.H. Kapit J. Gospodinova K. DeFlores L. Quinn R.C. Boynton W.V. Clark B.C. Catling D.C. Hredzak P. Ming D.W. Moore Q. Shusterman J. Stroble S. West S.J. Young S.M.M. Wet chemistry experiments on the 2007 Phoenix Mars Scout Lander mission: data analysis and results. J Geophys Res. 2010;115 doi: 10.1029/2009JE003424. [DOI] [Google Scholar]
- Levin G.V. Straat P.A. Viking labeled release biology experiment: interim results. Science. 1976;194:1322–1329. doi: 10.1126/science.194.4271.1322. [DOI] [PubMed] [Google Scholar]
- Levin G.V. Straat P.A. Laboratory simulations of the Viking labeled release experiment: kinetics following second nutrient injection and the nature of the gaseous end product. J Mol Evol. 1979;14:185–197. doi: 10.1007/BF01732377. [DOI] [PubMed] [Google Scholar]
- Levin G.V. Straat P.A. A search for a nonbiological explanation of the Viking Labeled Release life detection experiment. Icarus. 1981;45:494–516. [Google Scholar]
- Mahaffy P.R. Webster C.R. Cabane M. Conrad P.G. Coll P. Atreya S.K. Arvey R. Barciniak M. Benna M. Bleacher L. Brinckerhoff W.B. Eigenbrode J.L. Carignan D. Cascia M. Chalmers R.A. Dworkin J.P. Errigo T. Everson P. Franz H. Farley R. Feng S. Frazier G. Freissinet C. Glavin D.P. Harpold D.N. Hawk D. Holmes V. Johnson C.S. Jones A. Jordan P. Kellogg J. Lewis J. Lyness E. Malespin C.A. Martin D.K. Maurer J. McAdam A.C. McLennan D. Nolan T.J. Noriega M. Pavlov A.A. Prats B. Raaen E. Sheinman O. Sheppard D. Smith J. Stern J.C. Tan F. Trainer M. Ming D.W. Morris R.V. Jones J. Gundersen C. Steele A. Wray J. Botta O. Leshin L.A. Owen T. Battel S. Jakosky B.M. Manning H. Squyres S. Navarro-González R. McKay C.P. Raulin F. Sternberg R. Buch A. Sorensen P. Kline-Schoder R. Coscia D. Szopa C. Teinturier S. Baffes C. Feldman J. Flesch G. Forouhar S. Garcia R. Keymeulen D. Woodward S. Block B.P. Arnett K. Miller R. Edmonson C. Gorevan S. Mumm E. The Sample Analysis at Mars investigation and instrument suite. Space Sci Rev. 2012;170:401–478. [Google Scholar]
- Navarro-González R. Vargas E. de la Rosa J. Raga A.C. McKay C.P. Reanalysis of the Viking results suggests perchlorate and organics at midlatitudes on Mars. J Geophys Res. 2010;115 doi: 10.1029/2010JE003599. [DOI] [Google Scholar]
- Nussinov M.D. Chernyak Y.B. Ettinger J.L. Model of the fine-grain component of martian soil based on Viking Lander data. Nature. 1978;274:859–861. [Google Scholar]
- Oyama V.I. Berdahl B.J. The Viking gas exchange experiment results from Chryse and Utopia surface samples. J Geophys Res. 1977;82:4669–4676. [Google Scholar]
- Oyama V.I. Berdahl B.J. Carle G.C. Preliminary findings of the Viking gas exchange experiment and a model for martian surface chemistry. Nature. 1977;265:110–114. [Google Scholar]
- Pattison D.I. Hawkins C.L. Davies M.J. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis, and computational modeling. Chem Res Toxicol. 2003;16:439–449. doi: 10.1021/tx025670s. [DOI] [PubMed] [Google Scholar]
- Plumb R.C. Tantayanon R. Libby M. Xu W.W. Chemical model for Viking biology experiments: implications for the composition of the martian regolith. Nature. 1989;338:633–635. [Google Scholar]
- Prince L.A. Determination of chloride, hypochlorite, chlorite, chlorate, perchlorate, and chlorine dioxide in composite mixtures. Anal Chem. 1964;36:613–616. [Google Scholar]
- Prince L.A. Johnson E.R. The radiation-induced decomposition of the alkali and alkaline Earth perchlorates, I. Product yields and stoichiometry. J Phys Chem. 1965;69:359–377. [Google Scholar]
- Quinn R. Orenberg J. Simulations of the Viking Gas Exchange experiment using palagonite and Fe-rich montmorillonite as terrestrial analogs: implications for the surface composition of Mars. Geochim Cosmochim Acta. 1993;57:4611–4618. doi: 10.1016/0016-7037(93)90186-z. [DOI] [PubMed] [Google Scholar]
- Skelley A.M. Cleaves H.J. Jayarajah C.N. Bada J.L. Mathies R.A. Application of the Mars organic analyzer to nucleobase and amine biomarker detection. Astrobiology. 2006;6:824–837. doi: 10.1089/ast.2006.6.824. [DOI] [PubMed] [Google Scholar]
- ten Kate I.L. Organics on Mars? Astrobiology. 2010;10:589–603. doi: 10.1089/ast.2010.0498. [DOI] [PubMed] [Google Scholar]
- Yen A.S. Kim S.S. Hecht M.H. Frant M.S. Murray B. Evidence that the reactivity of the martian soil is due to superoxide ions. Science. 2000;289:1909–1912. doi: 10.1126/science.289.5486.1909. [DOI] [PubMed] [Google Scholar]
- Zent A.P. McKay C.P. The chemical reactivity of the martian soil and implications for future missions. Icarus. 1994;108:146–157. [Google Scholar]