Abstract
Objective:
To identify clinical and nonclinical factors associated with time from epilepsy onset to surgical evaluation and treatment among a cohort of children having epilepsy surgery.
Methods:
Data were abstracted from records of 430 children (younger than 18 years) who had epilepsy neurosurgery at the University of California, Los Angeles from 1986 to 2010. Multivariable Cox proportional hazards models were used to analyze unique associations of clinical severity, pre-referral brain MRI, and sociodemographic characteristics with time to surgery.
Results:
Shorter time to surgery was associated with active (hazard ratio [HR] 5.67, 95% confidence interval [CI] 3.74–8.70) and successfully treated infantile spasms (HR 2.20, 95% CI 1.63–2.96); daily or more seizures (HR 2.09, 95% CI 1.58–2.76); MRI before referral regardless of imaging findings (HR 1.95, 95% CI 1.47–2.58); private insurance (HR 1.54, 95% CI 1.14–2.09); and Hispanic ethnicity (HR 1.38, 95% CI 1.01–1.87). There were race/ethnicity by insurance interactions (log-rank p = 0.049) with shortest time to surgery for Hispanic children with private insurance.
Conclusions:
Shorter intervals to surgical treatment were associated with greater epilepsy severity and insurance type, consistent with existing literature. However, associations of shorter times to treatment with having a brain MRI before referral and Hispanic ethnicity were unexpected and warrant further investigation. More knowledgeable referring providers and parents with greater help-seeking capability may explain obtaining an MRI before referral. Shorter intervals to surgery among Hispanic children may relate to the same factors yielding an increased volume of Hispanic children receiving surgery at the University of California, Los Angeles since 2000.
Epilepsy surgery is recognized as a safe and effective treatment option for children with medically intractable epilepsies associated with structural lesions.1–8 Timely referral may be important because preliminary evidence suggests that shorter intervals from epilepsy onset to surgery are associated with better short-term cognitive outcomes.9,10 However, a substantial proportion of children do not receive surgery in a timely manner.11,12 Furthermore, during testimony for the Institute of Medicine report on epilepsy, parents of children with epilepsy reported concern regarding delays in receipt of epilepsy specialty care, including surgery.13
We previously found that children with infantile spasms (IS) had shorter intervals from seizure onset to surgery compared with those without spasms.9 However, the impact of other clinical and nonclinical factors with time to surgery for pediatric patients has not been explored. Although studies have demonstrated sociodemographic disparities in the utilization of temporal lobectomy, none have examined whether such factors mediate delays in time to surgery in children.14–17 To elucidate factors associated with timing of referral and surgery among children with epilepsy, we utilized a large cohort from the University of California, Los Angeles (UCLA) who had surgery over the past 25 years. Based on prior literature in children and adults, we hypothesized that increased epilepsy severity as documented by a history of IS and a greater seizure frequency, abnormal pre-referral brain MRI, private insurance, and non-Hispanic, white race/ethnicity would be associated with shorter time to surgery.
METHODS
Sample.
This sample was drawn from a consecutive series of 605 children (younger than 18 years at surgery) who had epilepsy surgery at UCLA (January 1986 to December 2010). Excluded were children who had palliative procedures (vagus nerve stimulation and corpus callosotomy; n = 143), craniotomy without resection (biopsy only; n = 4), diagnostic intracranial electrodes without resection (n = 4), and multiple subpial transections without cortical resection (n = 1); patients who were “self-pay” (uninsured but with financial means to pay, primarily of international residence; n = 23) were also excluded. The final cohort for our analytic sample was 430 children. If a child had multiple procedures, data regarding only the first resection were analyzed. Children who underwent presurgical evaluation but were not deemed candidates for resective surgery and those who were offered surgery but whose parents declined the procedure were not captured in this surgical database.
Standard protocol approvals, registrations, and patient consents.
The UCLA Institutional Review Board approved this study. Patients or families signed research informed consents and Health Insurance Portability and Accountability Act (HIPAA) authorizations. Before HIPAA enactment, this study was considered exempt from requiring research informed consent. This study is not a clinical trial.
Data collection.
Clinical, sociodemographic, and pre-referral diagnostic data were obtained by abstraction of outpatient and inpatient medical records, EEG including video-EEG telemetry reports, UCLA and non-UCLA radiology studies, operative/scheduling reports, histopathology reports, and UCLA encounter/registration forms. Encounter and registration forms include home address, insurance, race/ethnicity, and preferred language, obtained by an administrative assistant before a visit.
Clinical and diagnostic variables.
Details regarding the standardized presurgical evaluation protocols, surgical procedures, and clinical variables have been previously described.3,9,12 Clinical variables included date of epilepsy onset and surgery; seizure frequency before surgery (daily seizures or more vs less than daily); type of surgery (hemispherectomy, multilobar, and lobar/focal resection); presurgical history of IS as determined by clinical history and EEG criteria of hypsarrhythmia or modified hypsarrhythmia (history of active spasms at surgery, successfully treated spasms, or no history of spasms); number of antiepileptic drugs (AEDs) at the time of presurgical evaluation (0–1, 2, or ≥3); side of surgery; and etiology (determined by UCLA neuroimaging and histopathology). Etiology was defined as neurodevelopmental (cortical dysplasia, hemimegalencephaly, and tuberous sclerosis complex) or non-neurodevelopmental (cortical atrophy, hippocampal sclerosis, infection, Rasmussen encephalitis, tumor, and nondiagnostic). We abstracted whether a child had a pre-referral brain MRI (yes vs no/unknown), and whether the prior imaging was normal or abnormal related to epilepsy etiology/surgical target.
“Time to surgery” was calculated as the interval from date of epilepsy onset to date of epilepsy surgery, and “time to referral” was the interval from date of epilepsy onset to the date of the first appointment at UCLA for evaluation for refractory epilepsy (with a neurologist or neurosurgeon). Because UCLA is a tertiary care center, nearly all children in this cohort had treatment initiated before referral to UCLA.
Sociodemographic variables.
Insurance type at the time of referral, race/ethnicity, sex, primary residence, and preferred language were abstracted from UCLA records. Insurance was categorized as Preferred Provider Organization, Health Maintenance Organization, indemnity, Medicaid (MediCal in California), and California Children's Services (CCS). Insurance type was dichotomized into private (Preferred Provider Organization, Health Maintenance Organization, indemnity) or government insurance (Medicaid/CCS). There were no uninsured children in this cohort (UCLA does not offer pro bono care). Race/ethnicity was classified as non-Hispanic white, Hispanic, or other (African American, Asian, or Middle Eastern). Geographic residence was classified using zip codes (Los Angeles [LA] County or not).
Analysis.
Bivariate comparisons were made using appropriate statistical tests (χ2, Pearson correlations for dichotomous and ordered variables, analysis of variance, and log-rank test with Kaplan-Meier survival analysis). Multivariable survival analysis using Cox proportional hazards models was used to examine the relative association of clinical, diagnostic, and sociodemographic variables with dependent variables (time to surgery, time to referral, and time from referral to surgery) in separate regression models, controlling for date of surgery, grouped into 5-year intervals. Dummy variables were created for categorical nondichotomous independent variables (history of IS, number of AEDs, race/ethnicity). Dichotomous and ordered covariates had correlations of r ≤ |0.50| with each other. The relative associations of the following clinical, pre-referral diagnostic, and sociodemographic variables with dependent variables were examined: history of active IS or treated IS (reference: no history of IS), daily seizures or more (reference: less than daily seizures), neurodevelopmental etiology (reference: non-neurodevelopmental etiology), 0 to 2 AEDs (reference: ≥3 AEDs), age at epilepsy onset, pre-referral MRI (reference: no/unknown MRI), male (reference: female), Hispanic or other ethnicity (reference: non-Hispanic white), and private insurance (reference: Medicaid/CCS).
To assess potential mediating effects of associations (hazard ratios [HRs]) of race/ethnicity with time to surgery and to referral, LA County residence and language were each separately added as a covariate to the models described previously. The magnitude of these HRs was compared with those models without that corresponding variable. The relationships of ethnicity and insurance with time to surgery and time to referral were further examined using a log-rank test to assess significance of time to surgery and of time to referral across 4 race-insurance categories (Hispanic-private, Hispanic-government, non-Hispanic white–private, and non-Hispanic white–government; n = 389); between-category differences were tested using a separate Cox proportional hazards test.
To elucidate whether having a pre-referral MRI vs having an abnormal finding on a pre-referral MRI was associated with dependent variables, we reanalyzed the main models replacing whether or not the child had a pre-referral MRI with dummy variable indicators for whether the MRI was normal or abnormal regarding epilepsy etiology/surgical target (reference: no/unknown pre-referral MRI). Because all children in this cohort by definition had surgery before age 18 years, we conducted a sensitivity analysis to determine whether any observed association of age at onset to time to surgery or to referral was a function of study design; models were reanalyzed excluding children aged 10 years or older at epilepsy onset.
All analyses were performed using Stata software (11.0; StataCorp, College Station, TX), setting an a priori p value ≤0.05 for significance.
RESULTS
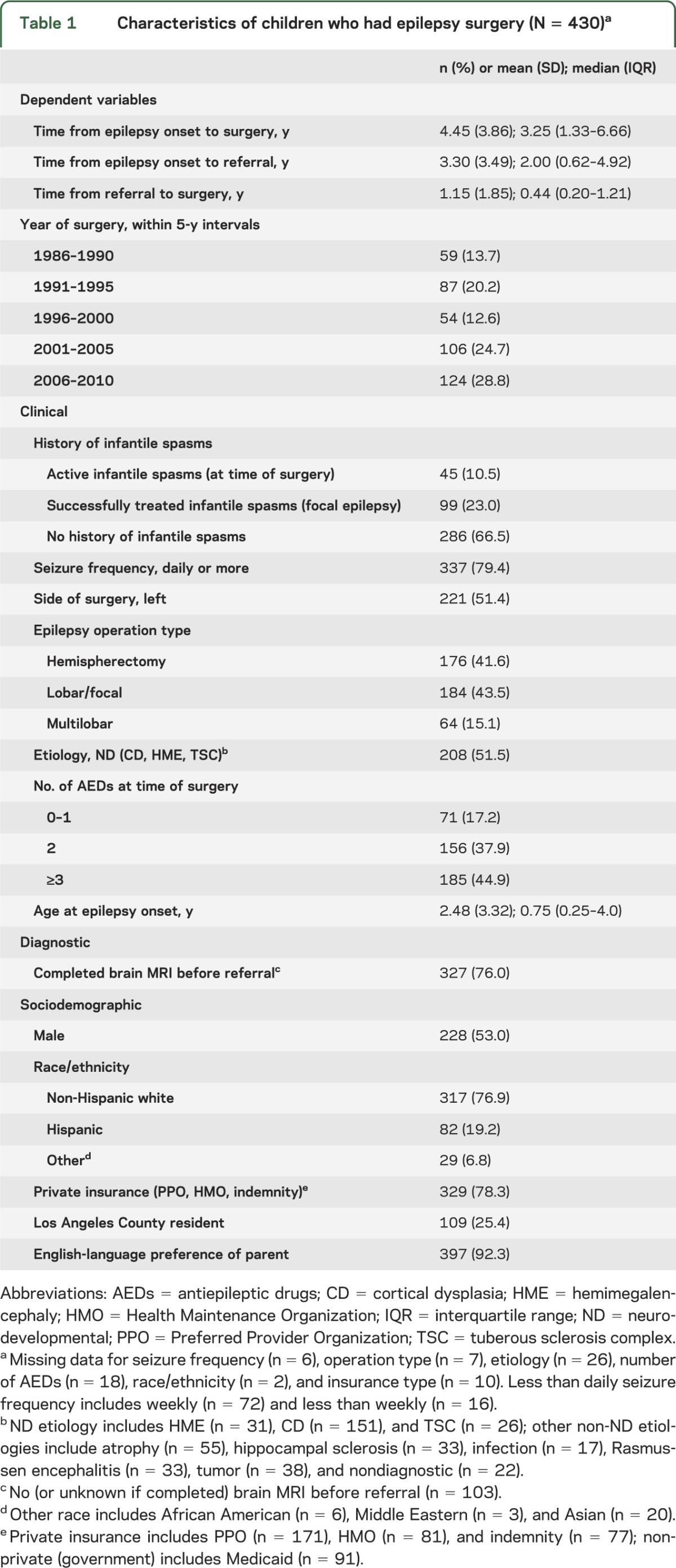
Clinical, diagnostic, and sociodemographic characteristics of this cohort are shown in table 1 and in table e-1 on the Neurology® Web site at www.neurology.org. Children with IS (active and successfully treated in 1 group) had median times to referral (0.88 vs 2.99 years) and to surgery (1.41 vs 4.42 years) that were shorter compared with those without a history of IS.
Table 1.
Characteristics of children who had epilepsy surgery (N = 430)a
In multivariate analysis, shorter time from epilepsy onset to surgery was associated with active (HR 5.67, 95% confidence interval [CI] 3.74–8.60) and successfully treated IS (HR 2.20, 95% CI 1.63–2.96) (vs no history of IS); daily or more seizure frequency (HR 2.09, 95% CI 1.58–2.76) (vs less than daily seizure); MRI before referral (HR 1.95, 95% CI 1.47–2.58) (vs no/unknown MRI); private insurance (HR 1.54, 95% CI 1.14–2.09) (vs Medicaid); Hispanic ethnicity (HR 1.38, 95% CI 1.01–1.87) (vs non-Hispanic white); and older age of epilepsy onset (HR 1.09, 95% CI 1.05–1.14; table 2).
Table 2.
Associations of time to surgery, time to referral, and time from referral to surgery with clinical, diagnostic, and demographic variablesa
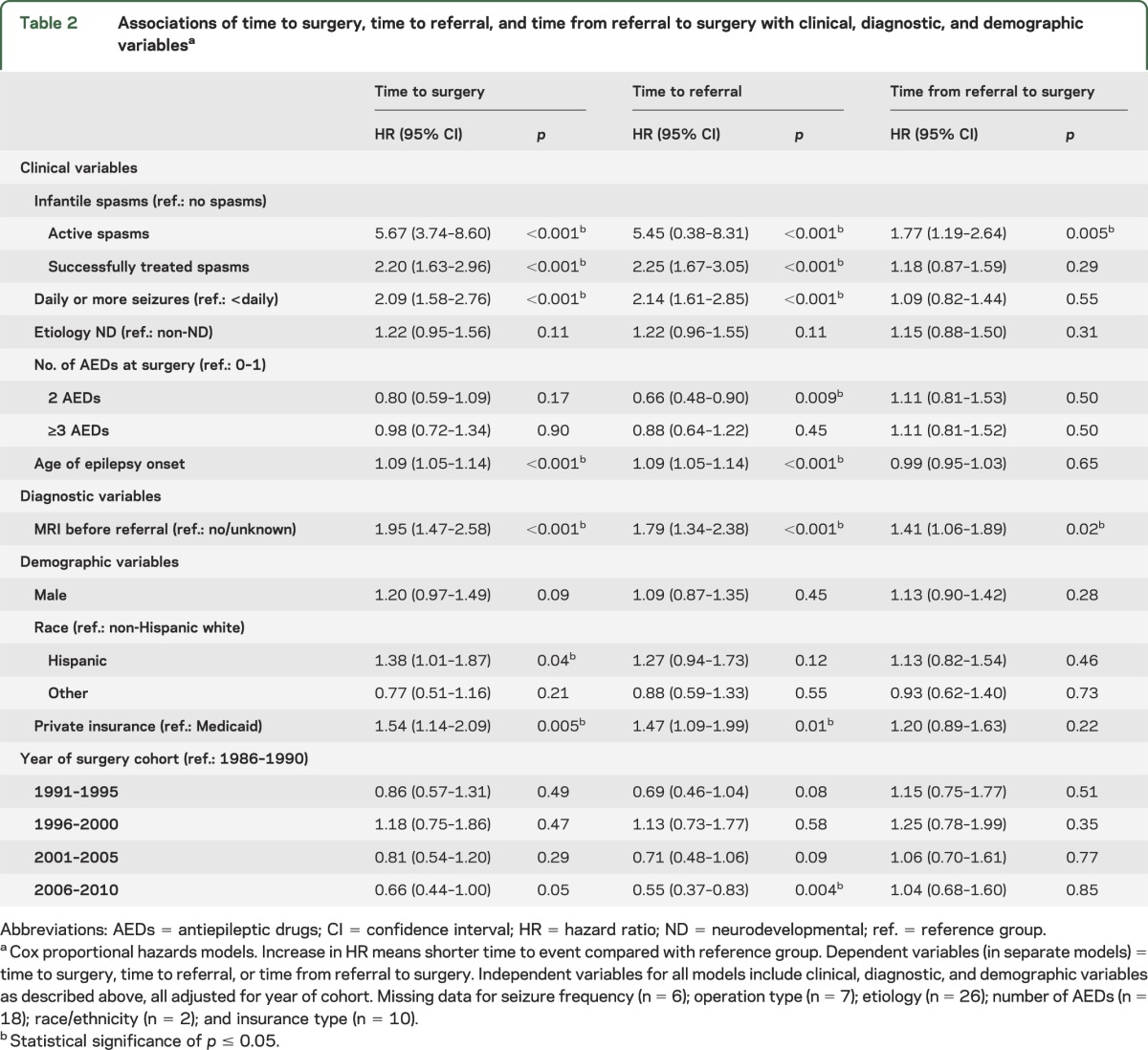
A similar pattern was seen for factors associated with time to referral except for 3 differences (table 2). Longer time to referral was associated with operations from 2006 to 2010 (vs 1986–1990) (HR 0.55, 95% CI 0.37–0.83; p = 0.004) and taking 2 AEDs (vs 0 or 1 AED) (HR 0.66, 95% CI 0.48–0.90; p = 0.009). In addition, the association of Hispanic ethnicity with shorter time to referral was of a smaller magnitude and not significant (HR 1.27, 95% CI 0.94–1.73; p = 0.12). Shorter times from referral to surgery were associated with active IS (vs no history of IS) and having a pre-referral MRI (table 2).
Because Hispanic children were more likely to live in LA County (χ2; p < 0.001) and have parents whose preferred language was not English (χ2; p < 0.001), we examined whether geographic residence and language might be mediators of the associations we observed between Hispanic race/ethnicity and shorter time to surgery. There was a slight lessening of the association between Hispanic ethnicity and time to surgery in the model comparing LA with non-LA County residence (HR 1.33 vs 1.38; table e-2). Adding preferred language resulted in higher magnitudes of HRs for the associations of Hispanic ethnicity with shorter time to surgery (HR 1.46 vs 1.38) and to referral (HR 1.31 vs 1.27). Children living in LA County had longer times from referral to surgery (table e-2).
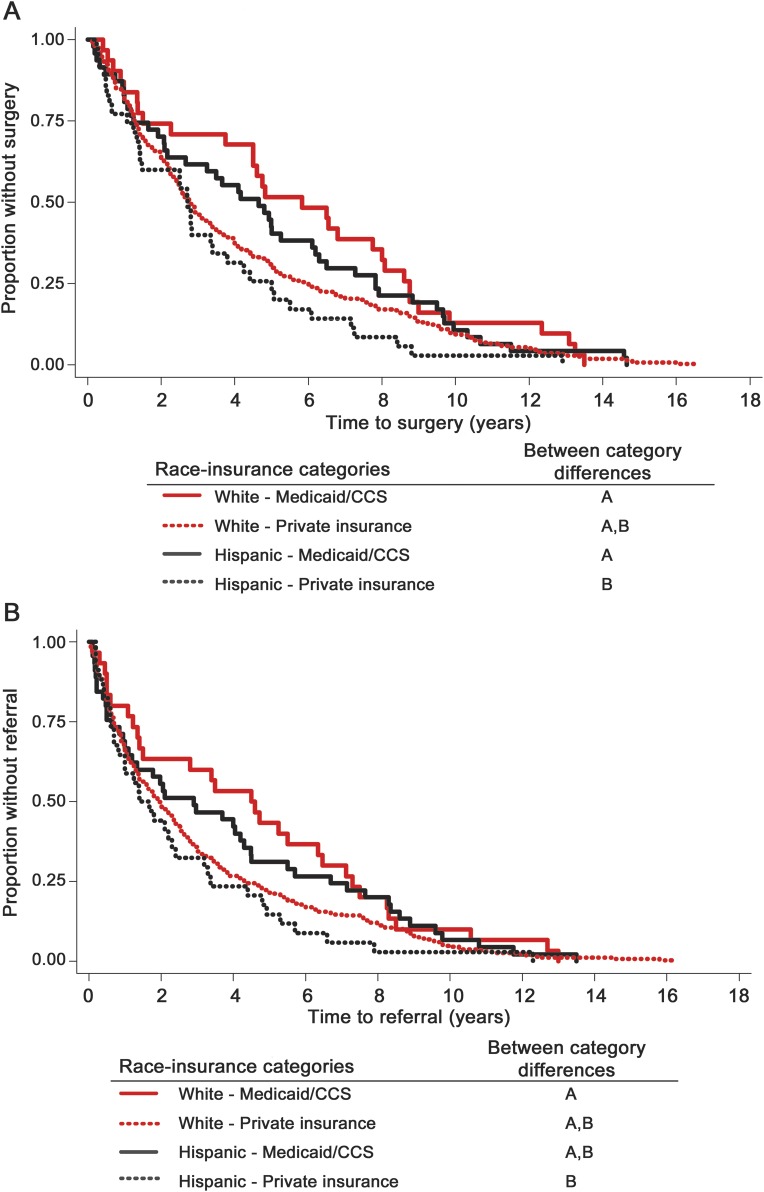
The relationships of ethnicity and insurance were further examined across 4 race-insurance categories (Hispanic-private, Hispanic-government, non-Hispanic white–private, and non-Hispanic white–government; n = 389). There were ethnicity-insurance group differences in time to surgery (log-rank test p = 0.049) and to referral (log-rank test p = 0.097; table 3). Regarding specific between-group differences, shorter times were associated with Hispanic ethnicity and private insurance compared with non-Hispanic whites with Medicaid (time to surgery and to referral) and compared with Hispanics with Medicaid (time to surgery; figure 1). This finding was observed concomitant with an increase in the proportion of Hispanic children having surgery at UCLA, with the largest increase since 2000 (figure 2).
Table 3.
Times to referral and surgery (years) by race and insurance status (n = 389)a
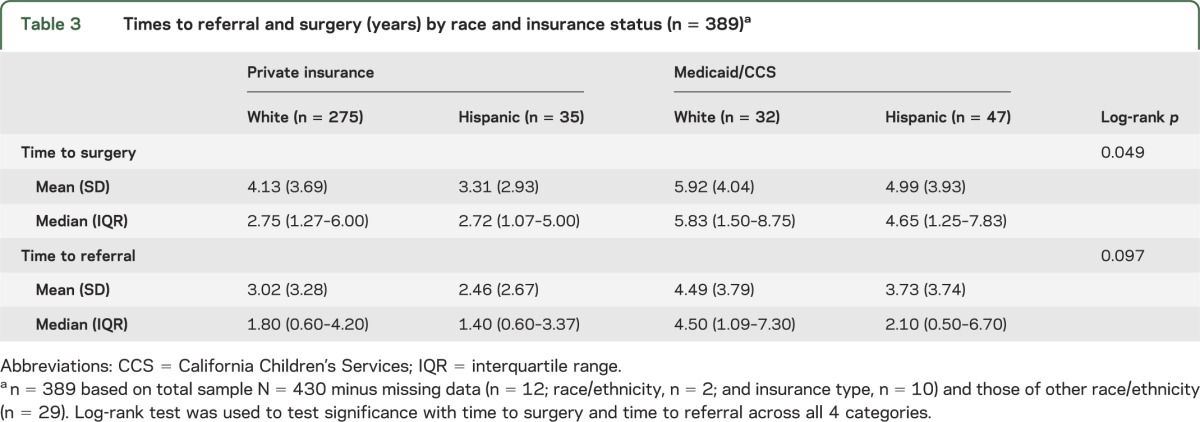
Figure 1. Kaplan-Meier analysis for (A) time to surgery and (B) time to referral by race and insurance.
Race-insurance categories with different letters are significantly different from one another using Cox proportional hazards test. Log-rank test across all 4 categories for (A) time to surgery (p = 0.049) and (B) time to referral (p = 0.097); n = 389 based on total sample N = 430 minus missing data (n = 12; race/ethnicity, n = 2; and insurance type, n = 10) and those of other race/ethnicity (n = 29). CCS = California Children's Services.
Figure 2. Number of children who had epilepsy surgery by year and race at University of California, Los Angeles (UCLA) (1986–2010).
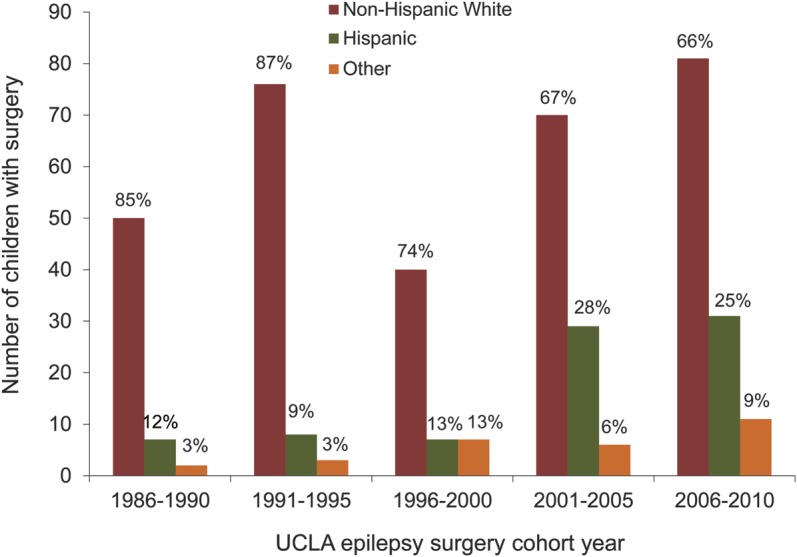
Percentages represent percent of the total number of children having surgery within each 5-year cohort period.
We investigated whether the associations of having a pre-referral MRI with shorter times to referral, surgery, and referral to surgery were attributable to having the test or to an abnormal result. We found that children who had an MRI before referral had a shorter time to surgery compared with those without an MRI regardless of whether the MRI was normal (HR 1.67, 95% CI 1.16–2.40) or abnormal (HR 2.03, 95% CI 1.52–2.71; table e-3). A similar finding was seen for time to referral. Children who had an abnormal pre-referral MRI had a shorter time from referral to surgery compared with children without an MRI (HR 1.51, 95% CI 1.12–2.03; table e-3). Older age of epilepsy onset was no longer associated with a shorter time to surgery or to referral in sensitivity analyses excluding children older than 10 years at epilepsy onset.
DISCUSSION
In a large cohort from a single institution in southern California, we found that shorter times from epilepsy onset to pediatric epilepsy surgery were associated with clinical severity including history of IS and seizure frequency (daily or more), and with sociodemographic factors such as having private insurance and Hispanic ethnicity and having had a brain MRI before referral irrespective of whether the imaging was positive for an epileptogenic lesion. We did not find an association of time to surgery with etiology. Furthermore, we found an interaction between ethnicity and insurance whereby the shortest time to surgery was associated with being Hispanic with private insurance compared with being Hispanic or non-Hispanic white with Medicaid.
Associations of time from epilepsy onset to surgery with having a history of IS and with insurance type were anticipated based on prior studies of pediatric epilepsy surgery patients and literature associating poorer specialty care access with having no insurance or “underinsurance.”9,16,18 The associations of shorter time to surgery with prior MRI and with Hispanic ethnicity were previously unknown and unexpected. Even in the literature on adult epilepsy surgery, meta-analyses of predictors of outcome have focused on clinical variables, sex, and age.15,19 Our study was able to probe additional sociodemographic factors as well as pre-referral diagnostic testing because of the range of variables available through record abstraction.
Epilepsy surgery in children differs from adults in several ways. The etiologies and operations are more heterogeneous, with cortical dysplasia and tumor being the most common, and mesial temporal sclerosis occurring less frequently in children.6,11 Young children and infants with “catastrophic” epilepsy are at highest risk for epilepsy-induced developmental encephalopathy, and early surgical intervention may be critical in ameliorating adverse developmental outcomes, particularly given the neuroplasticity of young brains.1,3,9 Although the natural history of mesial temporal sclerosis often includes a “quiescent period,” potentially contributing to the observed 20-year epilepsy duration before adult surgery, hippocampal sclerosis accounts for a small proportion of pediatric epilepsy procedures.20 Unlike adults, children with intellectual disability, psychiatric disease, and those of very young age are potential surgical candidates suggesting, hypothetically, that there are fewer barriers to referral for pediatric epilepsy surgery.21
Similar to a previous study from our group, a history of IS was strongly associated with shorter intervals to surgical treatment.9 We note that mean time to surgery for the IS group of 2.6 years (median 1.41 years) may still be perceived as too long although more definitive evidence is needed regarding the impact of time to surgery on developmental and quality-of-life outcomes.
We found that having had a brain MRI before referral, and not what the MRI showed, was associated with shorter intervals from epilepsy onset to referral and to surgery. It is possible that having an MRI is a proxy for unmeasured factors, such as the referring physician type and knowledge about epilepsy and surgical treatment options, and parents' knowledge and advocacy for obtaining testing and for identifying treatment options.22–25 The number of AEDs at the time of surgery may similarly be a proxy for referring physician knowledge given our finding that children taking 2 AEDs compared with 0 to 1 had longer times to referral. Limited provider knowledge regarding caring for patients with epilepsy may mediate referral delays.26–30 In a survey of Michigan neurologists, nearly 20% reported that patients had to fail all AEDs to be considered medication refractory.26 Another study found that 39% of adults who had epilepsy surgery at a university center came self-referred without having discussed surgery with their neurologists, and 14% were specifically told by their provider not to consider surgery.27 In a separate study, only a small proportion of primary care providers report feeling very comfortable caring for patients with epilepsy.30
In the United States, lack of insurance or Medicaid insurance is more common among socioeconomically disadvantaged minority groups. In adults, there is evidence suggesting that African Americans and Hispanics are less likely to undergo temporal lobectomy compared with non-Hispanic whites.14,17,31 Conversely, a retrospective study using the Kids' Inpatient Database found that African American children with temporal lobe epilepsy were no less likely to receive temporal lobectomy than non–African American children.16 Although we have no data on those who were eligible for surgery but not referred, we expected and found that the California equivalent of Medicaid was associated with longer intervals to surgery than private insurance. We did not anticipate that Hispanic ethnicity would be associated with shorter intervals relative to non-Hispanic whites, even after accounting for insurance type. We explored whether this finding was mediated by the higher likelihood of Hispanic patients living locally, but that only minimally attenuated the magnitude of the association of ethnicity and time to surgery. Given the increase in the number of Hispanic children who had surgery over the last decade at UCLA, regional outreach may account for the higher number and the shorter times to surgery. Furthermore, we cannot rule out the possibility that these unexpected findings may be attributable to providers who tended to care for and refer children of Hispanic ethnicity to UCLA. We are currently conducting interviews with Hispanic and non-Hispanic parents to assess referring physician type, parental level of education and employment, epilepsy knowledge, the impact of advocacy groups and social media, and other factors that contribute to patient referral for pediatric epilepsy surgery.
Regarding limitations, our analysis was limited to 1 pediatric epilepsy center having a relatively higher proportion of younger patients, more cases of cerebral hemispherectomy, and fewer temporal lobe resections compared with other cohorts.7,11,32–36 Nonetheless, this is one of the largest and longest followed pediatric epilepsy surgery cohorts. This region serves a very large and ethnically diverse US region, allowing us to meaningfully explore both clinical and nonclinical factors among Hispanic children. Additionally, the exact timing of medical intractability was not available from our data sources. Furthermore, our sample is limited to those children who underwent surgery; we have no data on children who were referred but to whom surgery was declined. Because we do not have data on children who were never referred, including those who may have been uninsured, we cannot directly address access to care.
For parents of children with epilepsy, pediatricians, and general neurologists, this study identified not only clinical severity (spasms and greater seizure frequency) but also pre-referral diagnostic imaging, private insurance, and Hispanic ethnicity as factors associated with shorter time from epilepsy onset to surgery for pediatric epilepsy surgery patients. Although our results need to be substantiated with prospective, multicenter studies, our findings highlight the need for standardized protocols regarding prompt timing of referral of children with medically refractory epilepsy to specialty care, and underscore the importance of the development of clinical interventions, in addition to policy and advocacy strategies to remediate such delays.
Supplementary Material
ACKNOWLEDGMENT
Dr. Vinters was supported in part by the University of California Pediatric Neuropathology Consortium. The authors acknowledge the contribution of the following individuals over the lifetime of this program: Drs. Robert Asarnow, Alan Shewmon, Harry Chugani, W. Donald Shield, Joyce Wu, Joyce Matsumoto, Jason Lerner, Youseff Comair, Warwick Peacock, Christopher C. Giza, and Susan Koh. The authors also give special thanks to Sue Yudovin, NP, who has been with the program since 1986.
GLOSSARY
- AED
antiepileptic drug
- CCS
California Children's Services
- CI
confidence interval
- HIPAA
Health Insurance Portability and Accountability Act
- HR
hazard ratio
- IS
infantile spasm
- LA
Los Angeles
- UCLA
University of California, Los Angeles
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.B. Baca: concept and study design, data and statistical analysis plan, interpretation of data, drafting of initial and final manuscript. B.G. Vickrey: data and statistical analysis plan, interpretation of data, edited the manuscript. S. Vassar: statistical data management and programming. J.S. Hauptman, A. Dadour, T. Oh: collection of original data and edited the manuscript. R. Sankar: clinical epilepsy assessments and edited the manuscript. N. Salamon: neuroradiology assessments and edited the manuscript. H.V. Vinters: supervised histopathology evaluations and edited the manuscript. G.W. Mathern: concept and study design, data and statistical analysis plan, interpretation of data, and edited final version of the manuscript.
STUDY FUNDING
This study was supported by Epilepsy Foundation grant 213971 (PI: Dr. Baca) and NIH grant R01 NS38992 (PI: Dr. Mathern).
DISCLOSURE
C. Baca receives salary support from Epilepsy Foundation grant 213971 for this project and also receives research support from the NIH/National Institute of Neurological Disorders and Stroke (R37-NS031146-11). B. Vickrey serves on scientific advisory boards for the Sports Concussion Institute, American Heart Association, and the NIH; receives research support from the NIH (NIA: RC4AG038804; National Institute of Neurological Disorders and Stroke: R37-NS031146-11 and 1U54 NS081764), the US Veterans Administration Health Services Research and Development Service (NRI-11-126 and VA QUERI PRP), the American Heart Association (AHA/PRT 0875133N), and the Food and Drug Administration (R01 FD003923); and is a consultant to EMD Serono Canada. S. Vassar, J. Hauptman, A. Dadour, T. Oh, and N. Salamon report no disclosures relevant to the manuscript. H. Vinters: the H.V. Vinters trust owns shares in and receives dividends from the following makers of drugs and medical equipment: GE, 3M, Pfizer, Teva Pharma, Becton Dickinson, and GlaxoSmithKline PLC. The Vinters laboratory has received research funding in the past from Neuropace, Inc., and currently receives NIH funds for research on stroke and dementia (P50 AG16570, P01 AG12435, NS 044378). R. Sankar serves on scientific advisory boards for and has received honoraria and funding for travel from UCB Pharma, Sunovion, Upsher-Smith, and Lundbeck Pharma; serves on speakers' bureaus for and has received speaker honoraria from UCB, GlaxoSmithKline, and Lundbeck. He receives funding from NIH-MH079933 (coinvestigator) and from Pfizer (Lyrica pediatric partial seizures trial). G. Mathern received research funding from NIH grant R01 NS38992, which partially supported this study and he serves on the Data Management Committee for NeuroPace, Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Asarnow RF, LoPresti C, Guthrie D, et al. Developmental outcomes in children receiving resection surgery for medically intractable infantile spasms. Dev Med Child Neurol 1997;39:430–440 [DOI] [PubMed] [Google Scholar]
- 2.Cossu M, Lo Russo G, Francione S, et al. Epilepsy surgery in children: results and predictors of outcome on seizures. Epilepsia 2008;49:65–72 [DOI] [PubMed] [Google Scholar]
- 3.Jonas R, Nguyen S, Hu B, et al. Cerebral hemispherectomy: hospital course, seizure, developmental, language, and motor outcomes. Neurology 2004;62:1712–1721 [DOI] [PubMed] [Google Scholar]
- 4.Lendt M, Helmstaedter C, Kuczaty S, Schramm J, Elger CE. Behavioural disorders in children with epilepsy: early improvement after surgery. J Neurol Neurosurg Psychiatry 2000;69:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinclair DB, Aronyk KE, Snyder TJ, et al. Pediatric epilepsy surgery at the University of Alberta: 1988–2000. Pediatr Neurol 2003;29:302–311 [DOI] [PubMed] [Google Scholar]
- 6.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol 2008;7:525–537 [DOI] [PubMed] [Google Scholar]
- 7.Terra-Bustamante VC, Fernandes RM, Inuzuka LM, et al. Surgically amenable epilepsies in children and adolescents: clinical, imaging, electrophysiological, and post-surgical outcome data. Childs Nerv Syst 2005;21:546–551 [DOI] [PubMed] [Google Scholar]
- 8.van Empelen R, Jennekens-Schinkel A, van Rijen PC, Helders PJ, van Nieuwenhuizen O. Health-related quality of life and self-perceived competence of children assessed before and up to two years after epilepsy surgery. Epilepsia 2005;46:258–271 [DOI] [PubMed] [Google Scholar]
- 9.Jonas R, Asarnow RF, LoPresti C, et al. Surgery for symptomatic infant-onset epileptic encephalopathy with and without infantile spasms. Neurology 2005;64:746–750 [DOI] [PubMed] [Google Scholar]
- 10.Loddenkemper T, Holland KD, Stanford LD, Kotagal P, Bingaman W, Wyllie E. Developmental outcome after epilepsy surgery in infancy. Pediatrics 2007;119:930–935 [DOI] [PubMed] [Google Scholar]
- 11.Harvey AS, Cross JH, Shinnar S, Mathern GW. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008;49:146–155 [DOI] [PubMed] [Google Scholar]
- 12.Hemb M, Velasco TR, Parnes MS, et al. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986–2008. Neurology 2010;74:1768–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine Epilepsy Across the Spectrum. Washington, DC: The National Academies Press; 2012 [Google Scholar]
- 14.Burneo JG, Black L, Knowlton RC, Faught E, Morawetz R, Kuzniecky RI. Racial disparities in the use of surgical treatment for intractable temporal lobe epilepsy. Neurology 2005;64:50–54 [DOI] [PubMed] [Google Scholar]
- 15.Englot DJ, Wang DD, Rolston JD, Shih TT, Chang EF. Rates and predictors of long-term seizure freedom after frontal lobe epilepsy surgery: a systematic review and meta-analysis. J Neurosurg 2012;116:1042–1048 [DOI] [PubMed] [Google Scholar]
- 16.McClelland S, III, Curran CC, Davey CS, Okuyemi KS. Intractable pediatric temporal lobe epilepsy in the United States: examination of race, age, sex, and insurance status as factors predicting receipt of resective treatment. J Neurosurg 2007;107:469–473 [DOI] [PubMed] [Google Scholar]
- 17.McClelland S, III, Guo H, Okuyemi KS. Racial disparities in the surgical management of intractable temporal lobe epilepsy in the United States: a population-based analysis. Arch Neurol 2010;67:577–583 [DOI] [PubMed] [Google Scholar]
- 18.Bisgaier J, Rhodes KV. Auditing access to specialty care for children with public insurance. N Engl J Med 2011;364:2324–2333 [DOI] [PubMed] [Google Scholar]
- 19.Haneef Z, Stern J, Dewar S, Engel J., Jr Referral pattern for epilepsy surgery after evidence-based recommendations: a retrospective study. Neurology 2010;75:699–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg AT. Understanding the delay before epilepsy surgery: who develops intractable focal epilepsy and when? CNS Spectr 2004;9:136–144 [DOI] [PubMed] [Google Scholar]
- 21.Cross JH, Jayakar P, Nordli D, et al. Proposed criteria for referral and evaluation of children for epilepsy surgery: recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006;47:952–959 [DOI] [PubMed] [Google Scholar]
- 22.Chadwick D. Why are so few patients with epilepsy treated surgically? A United Kingdom perspective. Acta Neurochir Suppl 1990;50:117–118 [DOI] [PubMed] [Google Scholar]
- 23.Committee on the Public Health Dimensions of the Epilepsies Vision 20/20: Pediatrics Working Group written testimony: Impact of the Epilepsies on Infants and Children. Presented at the 1st Meeting of the Committee on the Public Health Dimensions of the Epilepsies; January 10, 2010; Washington, DC
- 24.Dlugos DJ. The early identification of candidates for epilepsy surgery. Arch Neurol 2001;58:1543–1546 [DOI] [PubMed] [Google Scholar]
- 25.Trevathan E. Epilepsy Public Health Surveillance: Current State, Gaps and Opportunities. Presented at the Workshop on Public Health Surveillance, Population Health Research, and Data Collection for the Epilepsies; March 21, 2011; Beverly Hills, CA
- 26.Hakimi AS, Spanaki MV, Schuh LA, Smith BJ, Schultz L. A survey of neurologists' views on epilepsy surgery and medically refractory epilepsy. Epilepsy Behav 2008;13:96–101 [DOI] [PubMed] [Google Scholar]
- 27.Benbadis SR, Heriaud L, Tatum WO, Vale FL. Epilepsy surgery, delays and referral patterns: are all your epilepsy patients controlled? Seizure 2003;12:167–170 [DOI] [PubMed] [Google Scholar]
- 28.de Flon P, Kumlien E, Reuterwall C, Mattsson P. Empirical evidence of underutilization of referrals for epilepsy surgery evaluation. Eur J Neurol 2010;17:619–625 [DOI] [PubMed] [Google Scholar]
- 29.Speechley KN, Levin SD, Wiebe S, Blume WT. Referral patterns of family physicians may allow population-based incidence studies of childhood epilepsy. Epilepsia 1999;40:225–231 [DOI] [PubMed] [Google Scholar]
- 30.Moore JL, McAuley JW, Mott D, Reeves AL, Bussa B. Referral characteristics of primary care physicians for seizure patients. Epilepsia 2000;41:744–748 [DOI] [PubMed] [Google Scholar]
- 31.Berg AT, Vickrey BG, Langfitt JT, et al. The multicenter study of epilepsy surgery: recruitment and selection for surgery. Epilepsia 2003;44:1425–1433 [DOI] [PubMed] [Google Scholar]
- 32.Gilliam F, Wyllie E, Kashden J, et al. Epilepsy surgery outcome: comprehensive assessment in children. Neurology 1997;48:1368–1374 [DOI] [PubMed] [Google Scholar]
- 33.Munari C, Lo Russo G, Minotti L, et al. Presurgical strategies and epilepsy surgery in children: comparison of literature and personal experiences. Childs Nerv Syst 1999;15:149–157 [DOI] [PubMed] [Google Scholar]
- 34.Paolicchi JM, Jayakar P, Dean P, et al. Predictors of outcome in pediatric epilepsy surgery. Neurology 2000;54:642–647 [DOI] [PubMed] [Google Scholar]
- 35.Van Oijen M, De Waal H, Van Rijen PC, Jennekens-Schinkel A, van Huffelen AC, Van Nieuwenhuizen O. Resective epilepsy surgery in childhood: the Dutch experience 1992–2002. Eur J Paediatr Neurol 2006;10:114–123 [DOI] [PubMed] [Google Scholar]
- 36.Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol 1998;44:740–748 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.