Abstract
Objective:
We hypothesized that infectious burden (IB), a composite serologic measure of exposure to common pathogens (i.e., Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus 1 and 2) associated with vascular risk in the prospective Northern Manhattan Study (NOMAS), would also be associated with cognition.
Methods:
Cognition was assessed using the Mini-Mental State Examination (MMSE) at enrollment and the modified Telephone Interview for Cognitive Status (TICS-m) at annual follow-up visits. Adjusted linear and logistic regressions were used to measure the association between IB index and MMSE. Generalized estimating equation models were used to evaluate associations with TICS-m and its change over time.
Results:
Serologies and cognitive assessments were available in 1,625 participants of the NOMAS cohort. In unadjusted analyses, higher IB index was associated with worse cognition (change per standard deviation [SD] of IB for MMSE was −0.77, p < 0.0001, and for first measurements of TICS-m was −1.89, p < 0.0001). These effects were attenuated after adjusting for risk factors (for MMSE adjusted change per SD of IB = −0.17, p = 0.06, for TICS-m adjusted change per SD IB = −0.68, p < 0.0001). IB was associated with MMSE ≤24 (compared to MMSE >24, adjusted odds ratio 1.26 per SD of IB, 95% confidence interval 1.06–1.51). IB was not associated with cognitive decline over time. The results were similar when IB was limited to viral serologies only.
Conclusion:
A measure of IB associated with stroke risk and atherosclerosis was independently associated with cognitive performance in this multiethnic cohort. Past infections may contribute to cognitive impairment.
Basic and clinical research provide evidence that inflammation triggered by infectious agents may play a role in the pathogenesis of ischemic stroke,1,2 atherosclerosis,3,4 and dementia.5 Bacterial and viral infections may invade vessel walls, provoke cytokine release, influence lipid metabolism, and contribute in other ways to vascular dysfunction. Both viral infections, including herpes simplex virus type 1 (HSV-1)6 and cytomegalovirus (CMV),7 and bacteria, such as Chlamydia pneumoniae8 and Helicobacter pylori,9 have been associated with cognitive impairment and Alzheimer disease (AD). In prior analyses of the Northern Manhattan Study (NOMAS), a weighted measure of infectious burden (IB) including these pathogens was associated with stroke risk and carotid artery atherosclerosis.2,4
Accumulating evidence suggests that cerebrovascular injury and vascular risk factors are associated with cognitive decline and increased risk for AD and other dementias.10–14 As the population continues to age, health care needs associated with treating and caring for elderly individuals with cognitive decline are projected to pose significant public health and economic burdens.15 We hypothesized that a measure of chronic infection incorporating several common infections (i.e., C pneumoniae, H pylori, CMV, and HSV-1 and -2) associated with stroke would also be associated with cognition and cognitive decline in our stroke-free multiethnic cohort.
METHODS
Standard protocol approvals, registrations, and patient consents.
The institutional review boards at Columbia University Medical Center and the University of Miami approved the study. All participants gave informed consent to participate in the study.
Description of study population and baseline data collection.
The cohort derived from 3,298 multiethnic stroke-free participants enrolled in NOMAS between 1993 and 2001, as previously described.2 Briefly, participants were ≥40 years of age at enrollment and resided in northern Manhattan, New York, for ≥3 months in a household with a telephone.
Data collection at baseline included vital signs, demographic data, medical history, vascular risk factors, and clinical laboratory parameters including glucose levels and fasting lipid levels. In a subset (n = 984) the number of APOE ε4 alleles carried by each subject was determined by HhaI digestion of PCR products amplified from genomic DNA. For detailed covariate assessment, see appendix e-1 on the Neurology® Web site at www.neurology.org.
Assessment of IB.
Blood samples collected at enrollment were centrifuged and frozen at −70°C in 1 mL aliquots until the time of analysis. Since not all participants had blood available for the measurement of all 5 serologies, a subsample of 1,625 was used for the present analysis, as previously described.2 Serologies were measured using ELISA for the following: C pneumoniae (Savyon Diagnostics, Ashdod, Israel), H pylori, CMV (Wampole Laboratories, Princeton, NJ), and HSV-1 and 2 (Focus Diagnostics, Cypress, CA). Immunoglobulin G titers were used for all pathogens except C pneumoniae, for which immunoglobulin A titers were used based on our previous results.16,17 All testing was performed in a batch analysis blinded to clinical outcome.
Cognitive assessment.
Cognitive status was assessed at baseline using the 30-point Mini-Mental State Examination (MMSE)18 and at annual telephone interview follow-up using the modified Telephone Interview for Cognitive Status (TICS-m).19 Testing was performed by bilingual trained research assistants in English or Spanish, depending on language spoken at home.
Statistical analyses.
We used IB as the main predictor. Our development of a weighted IB index based on the relationship of individual serologic test results to risk of stroke was described previously.2 In brief, parameter estimates from Cox proportional hazards model for risk of stroke associated with each serologic result (positive or negative), adjusted for the other serologies, were used to derive a weighted index designated as IB. We constructed models unadjusted and adjusted for demographic and social factors (age, sex, race-ethnicity, education, and insurance status) and vascular risk factors (hypertension, diabetes mellitus, cardiac disease, smoking status, depressive symptoms, reported alcohol consumption, physical activity, cholesterol medication, and lipid levels). Besides age and high-density lipoprotein, all covariates were treated categorically. The rationale for choice of covariates and interactions was based on the literature, biological plausibility, and previous experience with the cohort. We analyzed MMSE as both a continuous and binary outcome (MMSE ≤24 vs >24) based on previously defined thresholds in order to facilitate clinical interpretation.20 TICS-m was treated as a continuous variable. We fitted linear regression with continuous MMSE to calculate the slope and 95% confidence interval (CI), and logistic regression with MMSE categories to calculate odds ratio (OR) and 95% CI. Generalized estimating equation (GEE) models with exchangeable covariance structure were used to evaluate associations with TICS-m and its change over time. We tested for interactions between IB and all demographic or medical risk factors. Moreover, in a post hoc analysis, we created a viral burden index (VIB) in the same manner as the overall IB index and tested the association of the VIB index with cognition.
We used the inverse probability weighted method to correct for potential selection bias that may have been introduced when the analyzed group was selected from the whole cohort. All hypothesis testing was 2-tailed and p values less than 0.05 were considered significant. All analyses were performed using SAS v9.1.3 (SAS Institute, Cary, NC).
RESULTS
Study population characteristics.
The analyzed population consisted of 1,625 subjects (65% women, mean age 69 ± 10 years, 58% Hispanic) with serology measurements (table 1). Characteristics among these participants were similar to those in the overall NOMAS cohort.2
Table 1.
Baseline characteristicsa
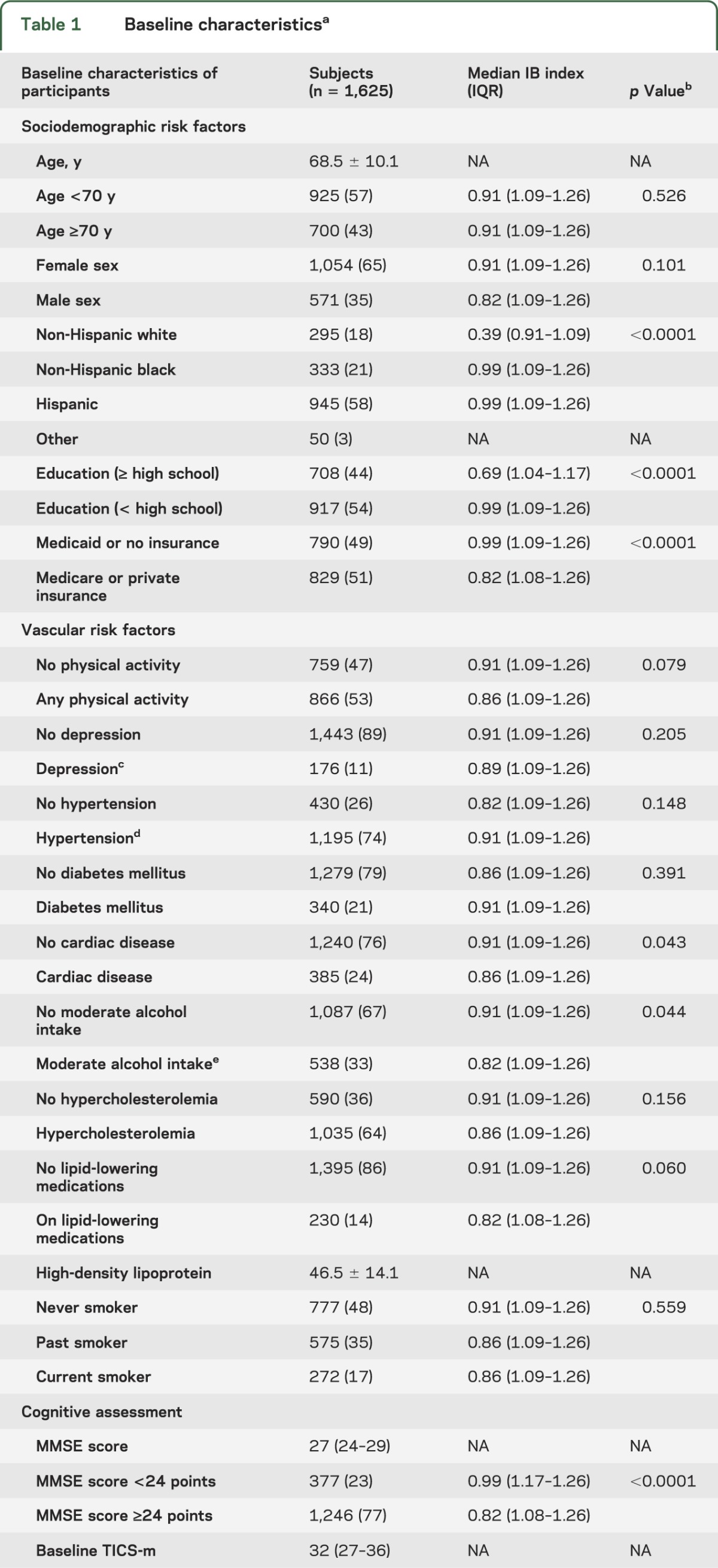
Median MMSE was 27 (interquartile range [IQR] 24–29) and median TICS-m over all follow-up measurements was 32 (IQR 27–36). The mean IB index (±SD) was 1.00 ± 0.33 and median 1.08 (IQR 0.91–1.26). The IB index was higher in non-Hispanic black and Hispanic subjects, and in participants with less than high school education, no alcohol intake, and without cardiac disease (table 1).
Association of IB index with MMSE.
In unadjusted analysis, higher IB index was associated with lower MMSE as a continuous measure (change in MMSE per SD IB index = −0.77; p < 0.0001). After adjusting for demographics and vascular risk factors, the effect was attenuated (change in MMSE per SD IB index = −0.17; p = 0.06).
The IB index was associated with greater odds of having MMSE ≤24 compared to MMSE >24 (unadjusted OR per SD IB = 1.58, 95% CI 1.36–1.82). The association persisted after adjusting for demographic and risk factors (adjusted OR per SD IB 1.26, 95% CI 1.06–1.51; table 2).
Table 2.
Association of the infectious burden index with cognitive functiona
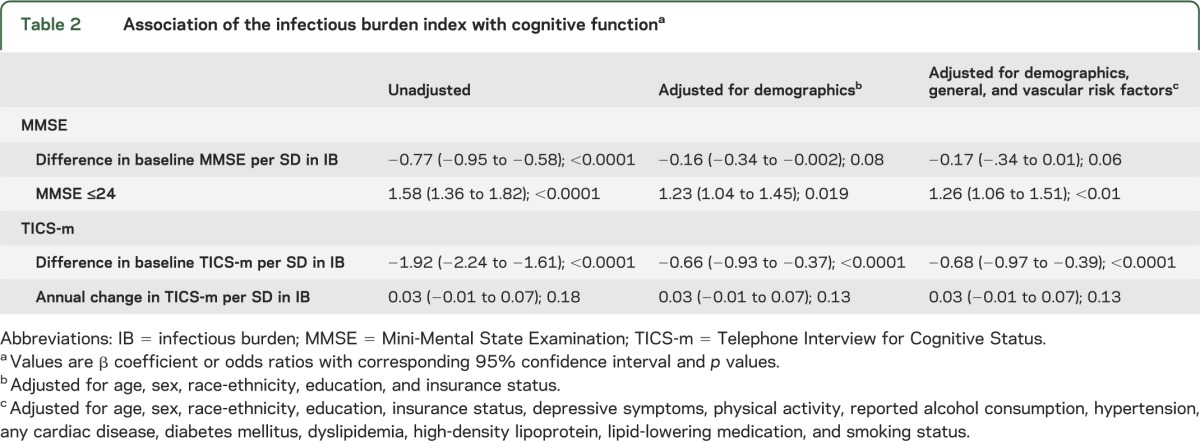
In subgroup analyses for those with APOE genotype available (n = 984), we found that the association between the IB index and MMSE did not change after further adjusting for APOE genotype (change in MMSE per SD of IB index = −0.20; p = 0.06), and the effect of the IB index on MMSE did not differ by APOE genotype (p for interaction = 0.61).
Association of IB index with TICS-m.
There was an association of higher IB index and impaired cognition assessed with the TICS-m (difference in baseline TICS-m per SD IB = −1.92; p < 0.0001). The effect was attenuated after adjusting for demographics and vascular risk factors, but the association persisted (adjusted change in baseline TICS-m per SD IB index = −0.68; p < 0.0001; table 2). We found no association, however, of IB with change in TICS-m over time in analyses either unadjusted or adjusting for demographics and risk factors (p = 0.13).
Modification of the association of IB index with cognition.
We found interactions of several baseline risk factors (i.e., sex, physical activity, education, and insurance status) with IB index relating to MMSE. The associations between IB index and MMSE were prominent among those who were physically inactive (adjusted change in MMSE per SD IB index = −0.43; p = 0.001), women (adjusted change in MMSE per SD IB index = −0.32; p = 0.007), those with Medicaid or no insurance (adjusted change in MMSE per SD IB index = −0.44; p = 0.003), and those with less than high school education (adjusted change in MMSE per SD IB index = −0.44; p = 0.002), while there were no associations among their counterparts. Only physical activity remained an effect modifier when all these interaction terms were included in the final model (p for interaction in the adjusted model = 0.01).
No interaction of demographic or risk factors with IB index, however, was found with regard to a change in TICS-m over time.
Exploratory analyses of viral burden and its association with cognition.
Limiting the IB serologies to viral serologies (VIB) resulted in similar findings as for the overall IB index. The VIB index was associated with MMSE ≤24 compared to MMSE >24 (adjusted OR per SD of VIB index = 1.22; p = 0.04), as well as with TICS-m (adjusted difference in baseline TICS-m per SD of VIB index = −0.70; p < 0.0001), but was not associated with change in TIC-m over time (p = 0.24).
DISCUSSION
In this multiethnic population-based sample, we found that a measure of IB previously associated with vascular disease risk is independently associated with cognitive performance in cross-sectional analyses. We used well-known and validated measures of cognitive function. Cognitive status was assessed at baseline using the 30-point MMSE18 and also during annual follow-up using the TICS-m.19 These results are consistent with prior studies identifying an association between chronic infections and both cognition5–8,21–27 and vascular risk,2–4,28,29 but we further extend prior findings by using a novel weighted average of IB associated with vascular disease risk and by using complementary measures of cognitive function. Our study results point to the possibility that the same cumulative index of pathogens may be a common risk factor for both stroke and cognitive impairment.
Interestingly, we found a more pronounced association of IB with MMSE when treated as a dichotomous variable compared to MMSE as a continuous variable. This might point to a threshold effect or that only a major change in MMSE is associated with a higher IB. The use of the 24-point threshold to define cognitive impairment in Hispanic patients has been a matter of debate since it is not very well documented in this ethnic group,20 but this threshold has been used widely in the literature to describe cognitive impairment and therefore permits comparison with previous studies.
Additionally, we found that the effect of IB on cognitive impairment may depend on certain demographic and vascular risk factors. In general, we found that the magnitude of effects of IB was greater among women, those with lower socioeconomic status (lower levels of education and health insurance), and the physically inactive. The association was most prominently modified by participants' physical activity levels. Among physically inactive participants, a higher IB index was associated with a lower MMSE, while there was no association among physically active participants. This result should be considered exploratory, however, as we investigated multiple interactions. Nevertheless, this observation provides some indirect evidence that the negative effects of chronic infection might be mitigated by beneficial behaviors such as physical activity, and evidence is accumulating that exercise has anti-inflammatory effects.30
IB was not associated, however, with cognitive decline over time, and we did not find any interactions for this with baseline risk factors. The absence of an association with cognitive decline over time could reflect the relatively advanced state of cognitive impairment at the time participants in our relatively elderly cohort were enrolled, limiting our ability to detect further decline with time. Our duration of follow-up, moreover, may have been insufficient to detect a change. Finally, it is possible that a practice effect, such that participants improve scores on cognitive tests over time, may have limited our ability to detect an effect.
Human and animal studies provide evidence that chronic infections with HSV-1, HSV-2, CMV, HIV-1, C pneumoniae, and H pylori are associated with an elevated risk of cognitive impairment and different forms of dementia.5–8,21–27 Most of these previous studies were focused on one specific pathogen and the few studies that examined multiple pathogens simultaneously assumed equal weight for each investigated pathogen. For example, one study investigated 400 randomly selected home-dwelling individuals with cardiovascular diseases.5 The primary endpoint was cognitive impairment, defined as MMSE score <24 points at baseline and after 1 year of follow-up. Viral burden at baseline was defined as the number of seropositivities toward HSV-1, HSV-2, and CMV equally divided into 3 categories (0 to 1, 2, or 3). The lowest category was a combination because few people had zero viral seropositivities. Bacterial burden was defined as seropositivities toward C pneumoniae and Mycoplasma pneumoniae divided into 3 categories (0, 1, or 2). Logistic regression was performed with the categories of viral or bacterial burden, various risk factors, and demographic data as independent variables. In subjects with 3 viral seropositivities (compared to 1), the adjusted hazard ratio for cognitive impairment at baseline was 2.5 (1.3–4.7) and after 1 year it was 2.3 (1.2–4.6). However, no association was observed between cognition and bacterial burden. Another investigator studied a subset (n = 1,204) of the participants in the Sacramento Area Latino Study on Aging.24 Baseline serum samples were assayed for levels of immunoglobulin G antibodies to CMV and HSV-1. Participants were screened annually over a 4-year period for cognitive function (by MMSE) and episodic memory (by Delayed Recall Scale scores). They found a higher rate of cognitive decline over the 4-year period in subjects with the highest CMV antibody levels at baseline than in individuals with the lowest levels (p = 0.003). Unlike these studies, we did not assume that each infection should be associated with a similar risk of cerebrovascular disease or cognition, and we implemented a novel weighted average of IB that was previously associated with cerebrovascular events. However, since previous studies showed associations primarily with viruses, we also performed an exploratory analysis to assess whether the driving force behind the association of IB index with cognition might be the viral pathogen burden. The estimates for the associations with cognition using viral serologies alone were almost identical to those using a combined bacterial and viral score, providing support for the notion that most of the effect of IB on stroke and cognition is mediated through viral rather than bacterial serologies.
The mechanism for this association remains uncertain. It may be that chronic infection due to these pathogens contributes to the overall inflammatory milieu and, together with other risk factors, leads to atherosclerosis, subclinical stroke, and dementia. Inflammation in brain vessels has been postulated to play an important role in both vascular dementia and AD.31 In addition, a direct toxic effect of some neurotropic agents may play a role in the development of cognitive impairment.32,33 Further studies are required to establish the pathogenic mechanisms.
In our univariate analysis, we found an association of IB index with insurance status and education, both proxies for socioeconomic status (SES), as well as race-ethnicity. In the multivariate analysis after adjusting for these demographic variables, the association of IB index with cognition was attenuated. It might be that the known socioeconomic gradients concerning the incidence of cognitive impairment34 are partly explained by a higher IB.35 For example, data from the National Health and Nutrition Examination Survey III addressed socioeconomic and race-ethnic differences in infection status in the United States.36 H pylori, CMV, HSV-1, and hepatitis B virus were used for the infection burden analyses.36 The authors found that individuals with less than high school education had roughly 50% increased risk of having an additional infection compared with high school graduates, whereas those with postsecondary education had 50% lower odds.36 Income had similar effects, whereby low income was associated with 33% higher odds of an additional infection, and high income with 45% lower odds, compared with the middle-income group.36 This association might be due to a higher exposure rate but it might also be due to an initial susceptibility to infections triggered by socioeconomic stressors. There is evidence of an association between low SES, increased stress, and enhanced susceptibility to several viruses.37
Our study has limitations. Because it is cross-sectional, we cannot make conclusions about the direction of associations. The infections we investigated, however, most likely preceded the development of cognitive impairment, since the antibody pattern reflects chronic infectious status and representative studies have shown that the majority of CMV and HSV-1 infections occur in childhood.38,39 Second, it was not possible to examine the relationship between infection and specific forms of cognitive impairment. For example, earlier research has shown that CMV DNA is found in a higher proportion of brains of people with vascular dementia than in age-matched controls.7 Third, we did not use detailed neuropsychological testing in our analyses. However, both the MMSE as well as the TICS-m are well-established tools to assess cognitive performance in large cohorts. Fourth, we did not have APOE genotype information in all subjects. However, in the subgroup analyses, we found that IB index was independently associated with cognition even after adjusting for APOE genotype, and the effects of IB index on cognition did not differ by APOE genotype. These findings should be interpreted with caution due to potential selection bias. Finally, a few factors may have reduced the ability to detect a strong association between IB index and cognitive decline over time in our study. For example, a moderate practice effect over the follow-up period might have played a role, or a threshold effect, indicating that when the damage is already done there is no further decline.
The strengths of this study include the population-based multiethnic cohort, including a large proportion of Hispanic subjects, who are frequently underrepresented in studies of cognition, and the ability to adjust for numerous potential covariates. In addition, GEE analyses were used to model corresponding trajectories of cognitive decline.
Our results need to be validated in independent populations before they can be generalized. If confirmed, however, these findings could have potential clinical implications. For example, treatment and eradication of these pathogens might have a positive impact on cognition as well as stroke, thereby addressing 2 major causes of neurologic disease burden worldwide. In the case of viral pathogens, early childhood vaccination or antiviral treatment could decrease stroke risk and cognitive impairment. Based on our data, early intervention might be more promising, since there was no association with decline over time in this elderly cohort.
Our results extend the findings of previous studies aimed at investigating the association of chronic infections and cognitive performance and specifically point to IB as a common risk factor of both stroke and cognitive impairment.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- CMV
cytomegalovirus
- GEE
generalized estimating equation
- HSV
herpes simplex virus
- IB
infectious burden
- IQR
interquartile range
- MMSE
Mini-Mental State Examination
- NOMAS
Northern Manhattan Study
- OR
odds ratio
- SES
socioeconomic status
- TICS-m
modified Telephone Interview for Cognitive Status
- VIB
viral burden index
Footnotes
Supplemental data at www.neurology.org
Editorial, page 1182
AUTHOR CONTRIBUTIONS
Study idea and planning of analysis: Mira Katan and Mitchell S.V. Elkind. Statistical analysis: Yeseon P. Moon, Myunghee C. Paik. Interpretation of the data: Mira Katan, Yeseon P. Moon, Myunghee C. Paik, Ralph L. Sacco, Clinton Wright, and Mitchell S.V. Elkind. Drafting of the manuscript: Mira Katan. Critical revision of the manuscript: Mira Katan, Yeseon P. Moon, Myunghee C. Paik, Ralph L. Sacco, Clinton B. Wright, and Mitchell S.V. Elkind. Obtaining funding: Mira Katan, Ralph L. Sacco, and Mitchell S.V. Elkind.
STUDY FUNDING
Supported by the NIH/NINDS (R37 NS 29993 [R.L.S., M.S.V.E.] and R01 NS 48134 [M.S.V.E.]), the Swiss National Science Foundation (PBZHP3-130982 [M.K.]), and the Fondation Leducq (career development grant [M.K.]).
DISCLOSURE
M. Katan received within the last 3 years research grants from BRAHMS/Thermo Fisher Scientific, the Swiss National Science Foundation (PBZHP3-130982), and the Fondation Leducq. Y.P. Moon and M.C. Paik report no disclosures. R. Sacco received research support from NINDS for the Northern Manhattan Study (R37 NS 29993) and support from the Evelyn McKnight Brain Institute. C.B. Wright has been supported over the last 2 years by the NIH (K02 NS 059729, R01 HL 108623) and the American Heart Association (SDG 0735387N). He receives royalties from UpToDate for 2 chapters and has done legal consulting for the law firms of Abali, Milne and Faegre, Baker, Daniels. He is a consultant for Merck and does stroke adjudication for a clinical trial. M.S. Elkind receives compensation for serving on an event adjudication committee for Jarvik Heart; receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/NINDS; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation) and GlaxoSmithKline (Avandia and stroke litigation); and serves as Resident & Fellow Section Editor for Neurology®, for which he receives compensation from the American Academy of Neurology. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Heuschmann PU, Neureiter D, Gesslein M, et al. Association between infection with Helicobacter pylori and Chlamydia pneumoniae and risk of ischemic stroke subtypes: results from a population-based case-control study. Stroke 2001;32:2253–2258 [DOI] [PubMed] [Google Scholar]
- 2.Elkind MS, Ramakrishnan P, Moon YP, et al. Infectious burden and risk of stroke: the Northern Manhattan study. Arch Neurol 2010;67:33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein SE, Zhou YF, Zhu J. Infection and atherosclerosis: emerging mechanistic paradigms. Circulation 1999;100:e20–e28 [DOI] [PubMed] [Google Scholar]
- 4.Elkind MS, Luna JM, Moon YP, et al. Infectious burden and carotid plaque thickness: the Northern Manhattan study. Stroke 2010;41:e117–e122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke 2003;34:2126–2131 [DOI] [PubMed] [Google Scholar]
- 6.Itzhaki RF, Dobson CB, Shipley SJ, Wozniak MA. The role of viruses and of APOE in dementia. Ann NY Acad Sci 2004;1019:15–18 [DOI] [PubMed] [Google Scholar]
- 7.Lin WR, Wozniak MA, Wilcock GK, Itzhaki RF. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol Dis 2002;9:82–87 [DOI] [PubMed] [Google Scholar]
- 8.Balin BJ, Gerard HC, Arking EJ, et al. Identification and localization of Chlamydia pneumoniae in the Alzheimer's brain. Med Microbiol Immunol 1998;187:23–42 [DOI] [PubMed] [Google Scholar]
- 9.Kountouras J, Tsolaki M, Gavalas E, et al. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 2006;66:938–940 [DOI] [PubMed] [Google Scholar]
- 10.Luchsinger JA, Mayeux R. Cardiovascular risk factors and Alzheimer's disease. Curr Atheroscler Rep 2004;6:261–266 [DOI] [PubMed] [Google Scholar]
- 11.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006;37:2220–2241 [DOI] [PubMed] [Google Scholar]
- 12.Richards SS, Emsley CL, Roberts J, et al. The association between vascular risk factor-mediating medications and cognition and dementia diagnosis in a community-based sample of African-Americans. J Am Geriatr Soc 2000;48:1035–1041 [DOI] [PubMed] [Google Scholar]
- 13.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med 2003;348:1215–1222 [DOI] [PubMed] [Google Scholar]
- 14.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997;277:813–817 [PubMed] [Google Scholar]
- 15.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elkind MS, Lin IF, Grayston JT, Sacco RL. Chlamydia pneumoniae and the risk of first ischemic stroke: the Northern Manhattan Stroke Study. Stroke 2000;31:1521–1525 [DOI] [PubMed] [Google Scholar]
- 17.Elkind MS, Tondella ML, Feikin DR, Fields BS, Homma S, Di Tullio MR. Seropositivity to Chlamydia pneumoniae is associated with risk of first ischemic stroke. Stroke 2006;37:790–795 [DOI] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198 [DOI] [PubMed] [Google Scholar]
- 19.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1988;1:111–117 [Google Scholar]
- 20.Black SA, Espino DV, Mahurin R, et al. The influence of noncognitive factors on the Mini-Mental State Examination in older Mexican-Americans: findings from the Hispanic EPESE: Established Population for the Epidemiologic Study of the Elderly. J Clin Epidemiol 1999;52:1095–1102 [DOI] [PubMed] [Google Scholar]
- 21.Honjo K, van Reekum R, Verhoeff NP. Alzheimer's disease and infection: do infectious agents contribute to progression of Alzheimer's disease? Alzheimers Dement 2009;5:348–360 [DOI] [PubMed] [Google Scholar]
- 22.Strandberg TE, Pitkala KH, Linnavuori K, Tilvis RS. Cognitive impairment and infectious burden in the elderly. Arch Gerontol Geriatr Suppl 2004;9:419–423 [DOI] [PubMed] [Google Scholar]
- 23.Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci 2008;63:610–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc 2006;54:1046–1054 [DOI] [PubMed] [Google Scholar]
- 25.Bilbo SD. Early-life infection is a vulnerability factor for aging-related glial alterations and cognitive decline. Neurobiol Learn Mem 2010;94:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kountouras J, Tsolaki M, Boziki M, et al. Association between Helicobacter pylori infection and mild cognitive impairment. Eur J Neurol 2007;14:976–982 [DOI] [PubMed] [Google Scholar]
- 27.Letenneur L, Peres K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS One 2008;3:e3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein SE. The multiple mechanisms by which infection may contribute to atherosclerosis development and course. Circ Res 2002;90:2–4 [PubMed] [Google Scholar]
- 29.Smieja M, Gnarpe J, Lonn E, et al. Multiple infections and subsequent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003;107:251–257 [DOI] [PubMed] [Google Scholar]
- 30.Stewart LK, Flynn MG, Campbell WW, et al. Influence of exercise training and age on CD14+ cell-surface expression of toll-like receptor 2 and 4. Brain Behav Immun 2005;19:389–397 [DOI] [PubMed] [Google Scholar]
- 31.Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol Aging 2001;22:837–842 [DOI] [PubMed] [Google Scholar]
- 32.Li H, Gang Z, Yuling H, et al. Different neurotropic pathogens elicit neurotoxic CCR9- or neurosupportive CXCR3-expressing microglia. J Immunol 2006;177:3644–3656 [DOI] [PubMed] [Google Scholar]
- 33.Ringheim GE, Conant K. Neurodegenerative disease and the neuroimmune axis (Alzheimer's and Parkinson's disease, and viral infections). J Neuroimmunol 2004;147:43–49 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan GA, Turrell G, Lynch JW, Everson SA, Helkala EL, Salonen JT. Childhood socioeconomic position and cognitive function in adulthood. Int J Epidemiol 2001;30:256–263 [DOI] [PubMed] [Google Scholar]
- 35.Strandberg TE, Pitkala KH, Tienari PJ, Tilvis RS. Re: Education and dementia: what lies behind the association? Neurology 2008;71:1555–1556; author reply 1556 [DOI] [PubMed] [Google Scholar]
- 36.Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci 2009;64:272–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen S. Social status and susceptibility to respiratory infections. Ann NY Acad Sci 1999;896:246–253 [DOI] [PubMed] [Google Scholar]
- 38.Schillinger JA, Xu F, Sternberg MR, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis 2004;31:753–760 [DOI] [PubMed] [Google Scholar]
- 39.Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 2006;43:1143–1151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


