Abstract
Objective:
Resting-state functional connectivity MRI (rs-fcMRI) may provide insight into the neurophysiology of HIV and aging.
Methods:
In this cross-sectional study, we used rs-fcMRI to investigate intra- and internetwork connectivity among 5 functional brain networks in 58 HIV-infected (HIV+) participants (44% receiving highly active antiretroviral therapy) and 53 HIV-uninfected (HIV−) controls. An analysis of covariance assessed the relationship among age, HIV laboratory markers, or degree of cognitive impairment and brain networks.
Results:
Individuals who were HIV+ had decreased rs-fcMRI intranetwork correlations in the default mode (DMN, p = 0.01), control (CON, p = 0.02), and salience (SAL, p = 0.02) networks, but showed no changes in the sensorimotor (SMN) or dorsal attention (DAN) network. Compared with HIV− controls, participants who were HIV+ had a significant loss of internetwork correlations between the DMN-DAN (p = 0.02), trending loss in DMN-SAL (p = 0.1) and CON-SMN (p = 0.1), and trending increase in CON-SAL (p = 0.1). Neither HIV markers (plasma HIV viral load or CD4+ cell count) nor degree of cognitive impairment correlated with rs-fcMRI measures. Aging correlated with a decrease in the magnitude of intranetwork functional connectivity within the DMN (p = 0.04) and SAL (p = 0.006) and with decreased magnitude of internetwork functional connectivity between DMN and SAL (p = 0.009) for both HIV+ and HIV− participants. No interaction was observed between HIV and aging.
Conclusions:
HIV and aging may cause independent decreases in rs-fcMRI. HIV may lead to a baseline decrease in brain function similar to deterioration that occurs with aging.
HIV directly invades the brain, causing glial and neuronal dysfunction. Approximately half of all individuals infected with HIV (HIV+) develop HIV-associated neurocognitive disorder (HAND).1 Although the prevalence of severe forms of HAND has decreased because of highly active antiretroviral therapy (HAART), the incidence of mild forms of HAND continues to increase. Additionally, individuals who are HIV+ are surviving longer with these mild impairments, possibly making them more susceptible to age-related neurodegeneration. Cognitive dysfunction is primarily assessed by neuropsychological performance (NP) tests,1,2 which may miss subtle brain changes.3 New methods for understanding the pathophysiology of HIV-associated neurodegeneration are needed.
Neuroimaging allows assessment of the neurophysiologic effects of HIV and aging on brain function. An established tool for investigating large-scale brain networks is blood oxygen level–dependent (BOLD) resting-state functional connectivity MRI (rs-fcMRI). Multiple networks have been identified including the default mode (DMN), dorsal attention (DAN), salience (SAL), executive control (CON), and sensorimotor (SMN). These networks recapitulate the spatial topographies evoked under BOLD functional task–based paradigms.4 However, rs-fcMRI avoids performance confounds of task-based imaging,5 making it more suitable for potentially impaired clinical populations such as individuals who are HIV+.
We examined the neurobiological effects of HIV and aging on 5 functionally defined brain networks in HIV+ and HIV− participants using rs-fcMRI. We hypothesized that HIV and aging would independently affect rs-fcMRI brain networks.
METHODS
Patient characteristics.
A total of 111 participants provided consent and had a clinical examination, NP testing, and neuroimaging. This cohort included 58 HIV− controls and 53 HIV+ participants. Twenty-three of the HIV+ individuals (40%) were taking HAART, and all of these participants were on a stable regimen for at least 6 months before enrollment. Individuals with a history of other neurologic illnesses, major psychiatric disorders, or active substance abuse were excluded from participation. The serologic status of all HIV+ individuals was confirmed by documented positive HIV enzyme-linked immunoassay and Western blot or detection of plasma HIV RNA by PCR. All HIV+ participants had laboratory evaluations (plasma CD4 cell count and plasma HIV RNA viral load [VL]) performed within 3 months of imaging.
Assessment for active substance use.
To assess active substance use, urine was collected from each subject and tested for the presence of marijuana, cocaine, opiates, methamphetamines, barbiturates, benzodiazepines, and phencyclidine. Oral history was obtained with all participants asked to disclose frequency and magnitude of use of any of these drugs within the last 6 months. We defined an individual as substance positive (substance+) if either assessment revealed substance use. Within the HIV+ group, 13 subjects were substance+ and 35 were substance−. Substance use was predominantly marijuana.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Institutional Review Board at Washington University in St. Louis. All participants were consented according to the Institutional Review Board protocol. The recommendations of the Strengthening the Reporting of Observational Studies in Epidemiology criteria were followed whenever applicable.6
Neuropsychological evaluation.
A common battery of NP tests (including Trail Making Tests A and B, the Hopkins Verbal Learning Test, and Symbol Digit Modalities Test) was administered. These tests examine multiple cognitive domains and have been used for diagnosing neurocognitive impairment.7 Raw test scores from each test were standardized by using demographic-adjusted normative means.8 A standardized z score was calculated by subtracting the appropriate normative mean from the raw score and then dividing by the normative SD for each test. Averaging z scores for each of the tests yielded a summary z score (NPZ-4).
Image acquisition.
All imaging was performed using a 3.0-tesla Tim-Trio scanner (Siemens, Erlangen, Germany) equipped with the standard 12-channel head coil. A high-resolution, 3-dimensional, sagittal, T1-weighted, magnetization-prepared rapid gradient echo scan (MPRAGE) was acquired (echo time [TE] = 16 milliseconds, repetition time [TR] = 2,400 milliseconds, inversion time = 1,000 milliseconds, flip angle = 8°, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). In addition, a 2-dimensional multislice oblique axial spin density/T2-weighted fast spin echo (FSE) scan was obtained (TE = 455 milliseconds, TR = 3,200 milliseconds, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). rs-fcMRI scans were collected using a gradient spin-echo sequence (TE = 27 milliseconds, TR = 2.2 seconds, 64 × 64 acquisition matrix, flip angle = 90°) that was sensitive to the BOLD contrast (T2* weighting). A total of 36 contiguous, 4.0-mm-thick slices were acquired parallel to the anterior commissure/posterior commissure plane. Each participant had two 6-minute rs-fcMRI scans. During rs-fcMRI scans, participants were instructed to fixate on a visual cross-hair.
Preprocessing of rs-fcMRI.
rs-fcMRI preprocessing followed methods described previously.9 Briefly, this included compensation for slice-dependent time shifts, elimination of systematic odd-even slice intensity differences caused by interleaved acquisition, and rigid body correction for head movement within and across runs. Data were intensity scaled (1 multiplicative factor applied to all voxels of all frames within each run) to obtain a mode value of 1,000.10 This scaling facilitated assessment of voxel-wise variance for purposes of quality assurance (QA) but did not affect computed correlations. Atlas normalization was achieved by computing 3 affine transforms with rs-fcMRIs aligned to the FSE; the FSE images aligned to each individual's MPRAGE and the MPRAGE aligned to a group atlas. The resulting 3 affine transforms allowed for transformation of the original rs-fcMRI data to 3-mm3 atlas space. Head-motion correction was included in this resampling step.
Additional preprocessing in preparation for correlation mapping included spatial smoothing (6-mm, full-width, half-maximum Gaussian blur in each direction), voxel-wise removal of linear trends over each rs-fcMRI scan, and temporal low-pass filtering that retained frequencies below 0.1 Hz. Spurious variance was reduced by regression of nuisance waveforms derived from head-motion correction with the time series extracted from regions (of noninterest) in white matter and CSF.11 The whole-brain average time series was also removed as a nuisance regressor.12
Quality assurance.
QA measures included analysis of root mean square of head displacement (in millimeters) derived from motion correction and the voxel-wise time series SD averaged over the whole brain.11 QA-based exclusion criteria were empirically determined with the objective of maximizing the number of included subjects while achieving QA parameter distribution equivalence across groups. If the SD of the mean rs-fcMRI signal for an individual was >2.2% (after nuisance regression) or if the root mean square movement exceeded 2 mm, then the individual was excluded from further analysis. A total of 1 HIV− and 6 HIV+ individuals were removed because of excessive movement.
Postprocessing of rs-fcMRI.
A library of 36 previously defined regions of interest (ROIs) spanning 5 distinct functional networks (DMN, DAN, CON, SAL, and SMN) was used.5,13 Statistical tests of rs-fcMRI correlations were computed after applying Fisher z transformation:
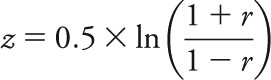 |
(1) |
to the computed Pearson correlation coefficients, yielding approximately normally distributed values. Next, cross-correlation matrices were constructed between the 36 ROIs for each subject. As in the case of the whole-brain correlations, Pearson correlation coefficients were Fisher z-transformed. Within-network (intranetwork) correlation average composite scores were computed for each of the 5 networks. For instance, the composite DMN score for a subject k was computed as:
| (2) |
where i and j refer to an ROI pair. Between-network (internetwork) correlation average composite scores were computed similarly for each subject for each of the 10 possible between-network composites.
Differences in inter- and intranetwork composite scores were assessed using a 1-way analysis of covariance (ANCOVA) with HIV status and sex included as cofactors. The independent effects of age and HIV serostatus on rs-fcMRI outcomes were determined for inter- and intranetwork correlations. If no interaction between HIV and age on the composite value was observed, an additive model was used. All results were corrected for multiple comparisons using a false discovery rate threshold of 0.05. Intranetwork composite scores were corrected for 5 comparisons. Internetwork composite scores were corrected for 10 comparisons. The effect size was computed as the regression effect divided by the residual SD. The age-equivalent effect of HIV infection was determined by dividing the HIV effect size by the yearly effect size of age. The age effect was expressed per 10 years of aging for comparison.
RESULTS
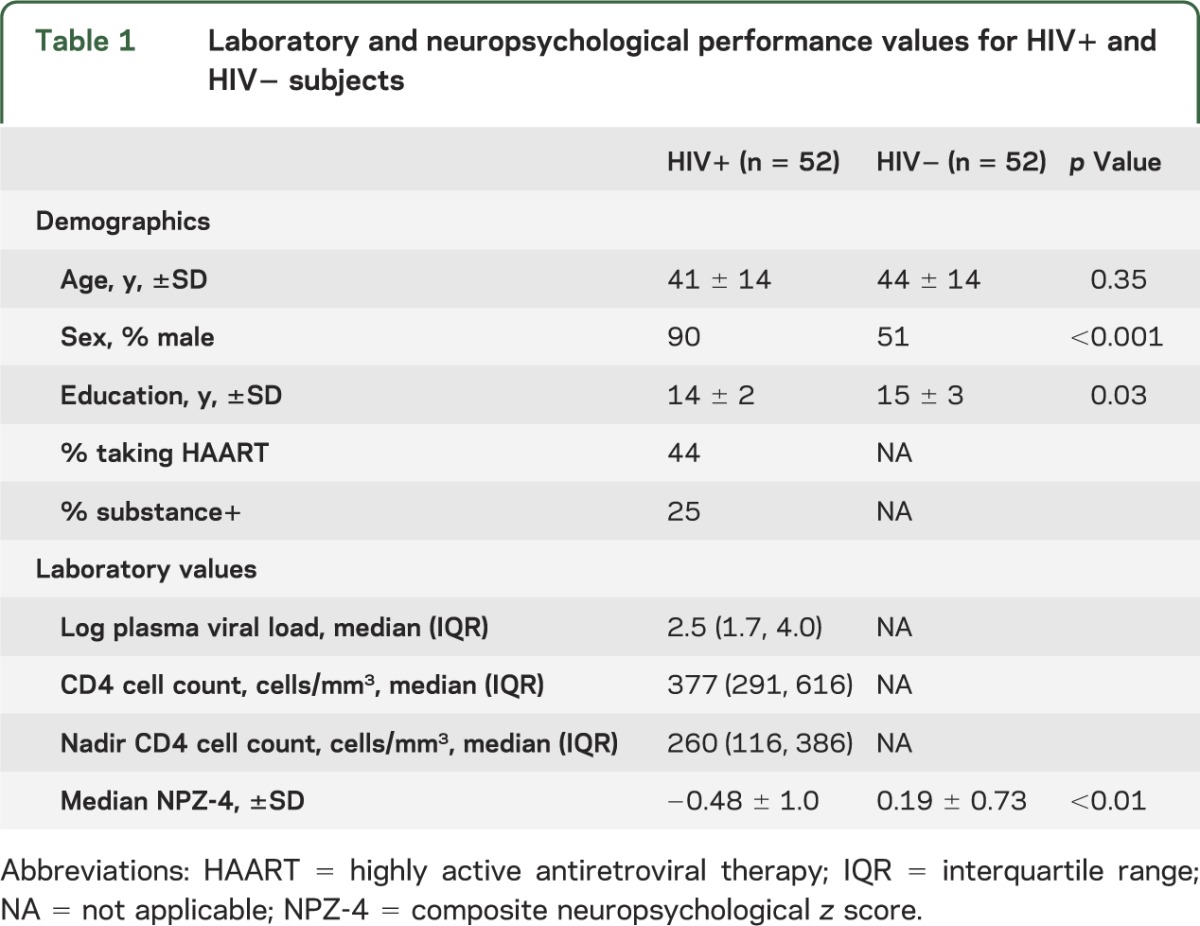
A total of 52 HIV+ and 52 HIV− participants were included in the final analysis. The 2 groups were similar in age distribution. However, the HIV− cohort had significantly fewer males and was more educated (table 1). Some of the HIV+ participants (n = 12) were impaired on simple neuropsychological examinations (NPZ-4) but no participant met criteria for dementia.1
Table 1.
Laboratory and neuropsychological performance values for HIV+ and HIV− subjects
The right lateral parietal (rLP) cortex, a node of the DMN, was used as a seed region to compare correlation patterns for HIV+ and HIV− subjects. Studies have shown HIV-associated functional deficits in this region.14,15 rs-fcMRI maps were produced by computing the z-transformed Pearson correlation coefficient (r) between the time series extracted from this ROI and all other brain voxels (figure 1, A and B). Patients who were HIV+ had a loss of positive resting-state correlations between the rLP cortex and other DMN nodes including the medial prefrontal cortex (green oval) and the posterior cingulate cortex (blue oval). In addition, participants who were HIV+ had a loss of negative correlations between the rLP cortex and more anterior portions of the LP that comprise the SAL network (red circles) (figure 1C).
Figure 1. DMN rs-fcMRI connectivity (HIV−) − (HIV+).
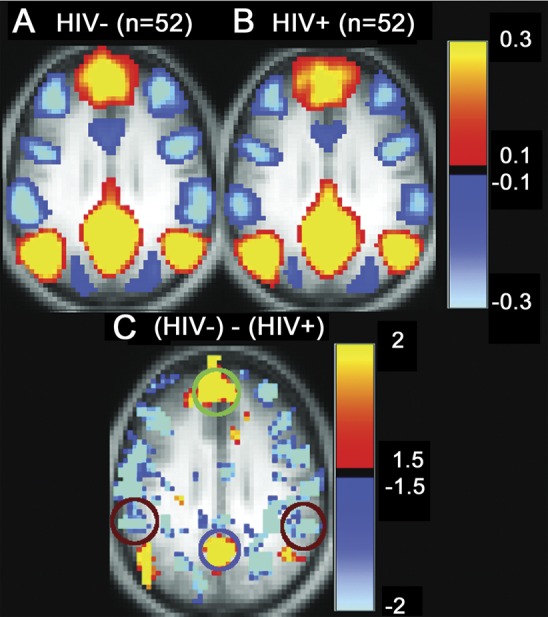
Whole-brain blood oxygen level–dependent resting-state functional connectivity (rs-fc) correlation maps using the right lateral parietal cortex as a seed region: (A) HIV uninfected (HIV−), (B) HIV infected (HIV+), and (C) difference between HIV− and HIV+ subjects. Using a threshold of p < 0.01 and a voxel cluster size of 5, a reduction in functional correlations was seen within default mode network (DMN) regions including the medial prefrontal cortex (green oval) and posterior cingulate cortex (purple oval; DMN). A loss of anticorrelations was seen in anterior portions of the right lateral parietal cortex belonging to the salience network (red circles).
Based on the loss of correlations within the DMN and reduction in negative correlations within task-positive regions (SAL), additional networks were explored. Average matrices for each group were computed representing pairwise correlations among the 36 ROIs (figure e-1 on the Neurology® Web site at www.neurology.org). Intranetwork correlations are arrayed in blocks along the diagonal, whereas internetwork correlations appear in off-diagonal cells. Internetwork correlations may be positive or negative (negative rs-fcMRI correlations are called anticorrelations). Although correlation patterns appeared to be similar between groups, subtraction of the average group matrices revealed an effect of HIV on correlation strength between nodes from particular networks (figure e-1C). In the subtraction matrix, warmer hues within the DMN, CON, and SAL network represent a loss of positive correlations within network. Cooler hues within the DAN suggest that some intranetwork rs-fcMRI correlations may be higher in HIV+ compared with HIV− individuals. However, this increase did not result in a significantly higher DAN composite score. For the off-diagonal internetwork correlations in the subtraction matrix, cooler hues between DMN and DAN and between DMN and SAL represent a loss of negative correlations between anticorrelated networks. Cooler hues between CON and SAL represent loss of positive internetwork correlations between these networks.
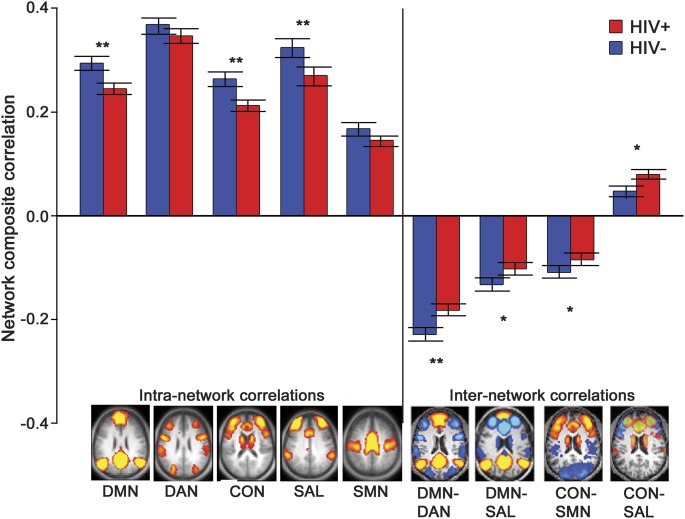
To quantify observed changes within brain networks, we computed both intra- and internetwork composite scores. After correcting for multiple comparisons, intranetwork correlation values were diminished in HIV+ participants compared with HIV− controls within the DMN (p = 0.01), CON (p = 0.02), and SAL network (p = 0.02). After correcting for multiple comparisons, internetwork correlations were lost in the DMN-DAN composite (p = 0.02), with trending loss in DMN-SAL (p = 0.1) and CON-SMN (p = 0.1) composites. A trending increase in positive internetwork correlations was seen in CON-SAL (p = 0.1) (figure 2).
Figure 2. Intra- and internetwork rs-fcMRI connectivity (HIV−) − (HIV+).
Intranetwork composite correlation values for each of the 5 major networks (DMN, DAN, CON, SAL, SMN) were computed for each HIV-infected (HIV+) (red) and HIV-uninfected (HIV−) (blue) subject by averaging all within-network correlation pairs for each network. Internetwork composite scores were computed by averaging between-network correlation pairs. ** Significant effects (p < 0.05). * Trend-level (p < 0.1) effects. Among the intranetwork composites, DMN (p = 0.006), CON (p = 0.005), and SAL (p = 0.03) showed significant decreases between the 2 groups. Among the internetwork composites, DMN-DAN (p = 0.02), and trending loss in DMN-SAL (p = 0.1), SAL-CON (p = 0.1), and CON-SMN (p = 0.1). A trending increase in positive internetwork correlations was observed in CON-SAL (p = 0.1). CON = control network; DAN = dorsal attention network; DMN = default mode network; rs-fcMRI = resting-state functional connectivity MRI; SAL = salience; SMN = sensorimotor network.
We assessed whether observed changes in brain networks were associated with either common laboratory markers of HIV infection or NP measures of cognition. Of the 3 intranetwork and 4 internetwork relationships that showed an HIV effect, none was associated with plasma markers (i.e., log HIV VL or CD4 cell count) (table e-1). These rs-fcMRI measures were also similar in HIV+ participants with (n = 12) or without (n = 40) cognitive impairment. The presence of HAART did not affect composite scores because patients who were HIV+ and receiving HAART (n = 23) had similar rs-fcMRI measures to individuals who were HIV+ but HAART naive (n = 29). In addition, active substance use did not affect correlations because patients who were HIV+ and substance+ (n = 13) had similar rs-fcMRI measures to those who were substance− (n = 35) (table e-1).
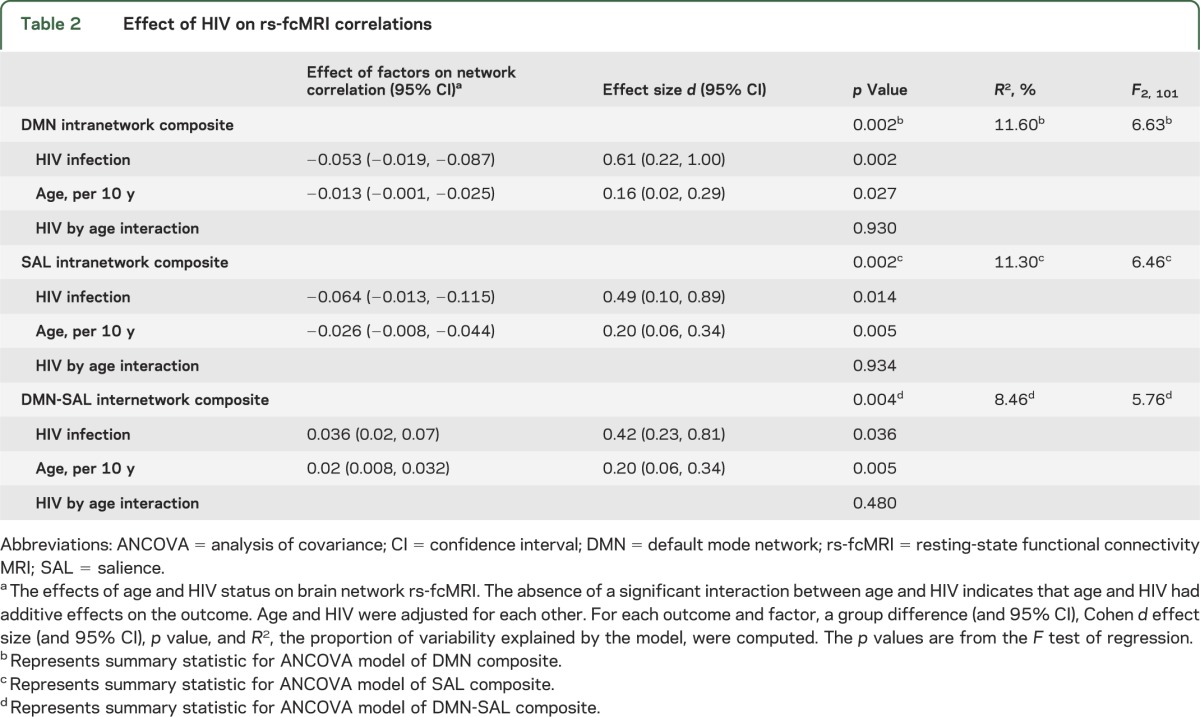
Each of the 3 intranetwork and 4 internetwork relationships showing an HIV effect was regressed on age and corrected for sex for both HIV+ and HIV− participants. HIV and aging both caused significant decreases in rs-fcMRI intranetwork composite values within the DMN (figure 3A) and SAL network (figure 3B). The same age and HIV effects were seen in the internetwork composite DMN-SAL (figure 3C). In an ANCOVA model, no interaction was observed between HIV and aging. HIV contributed to greater decreases in rs-fcMRI than aging. The decreases in rs-fcMRI caused by HIV were equivalent to decreases caused by 30 to 40 years of aging (table 2).
Figure 3. Independent effects of HIV and aging on rs-fcMRI correlations.
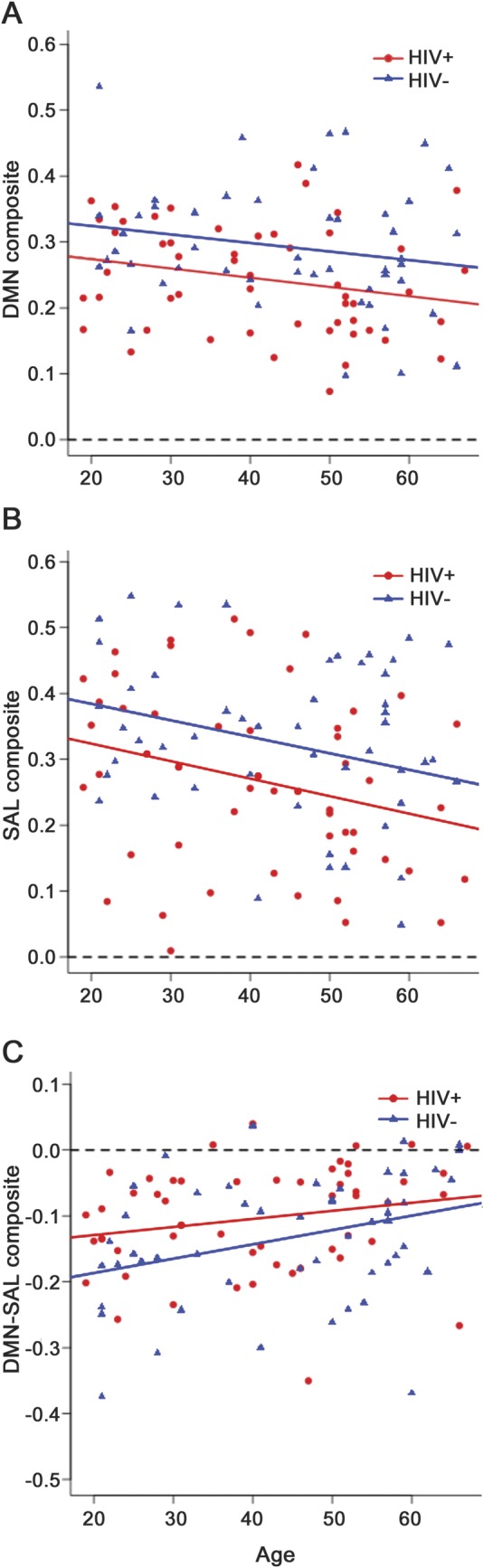
Parallel best-fit lines with differing intercepts model the effects of HIV and aging on intranetwork composite correlation value in both (A) the default mode network (DMN) and (B) the salience (SAL) network showing that the effects of HIV and aging are independent for each network. (C) Similar lines model the independent effects of HIV and aging on internetwork DMN-SAL anticorrelation. For all composite values, the magnitude of functional correlations diminishes toward zero (dashed line) due to HIV and aging. However, no interaction was seen between HIV and aging.
Table 2.
Effect of HIV on rs-fcMRI correlations
DISCUSSION
This work investigated the effects of HIV and aging on brain functional networks using rs-fcMRI. Resting-state functional connectivity was largely diminished in intra- and internetwork connections known to be affected by aging and neurodegenerative disorders.9,16 Observed loss of correlation strength did not correlate with plasma laboratory markers of disease (log HIV VL or CD4+ cell count), degree of cognitive impairment, or HAART use. In addition, resting-state functional connectivity within particular networks was lower in HIV+ individuals than HIV− controls, but decreased at the same rate as a function of age for both groups. Our results suggest that HIV may lead to patterns of degradation in brain function similar to those associated with advancing age, possibly placing older patients who are HIV+ at an increased risk for developing cognitive impairment.
The etiology of observed decreases in rs-fcMRI networks due to HIV remains unknown, but existing pathology and neuroimaging studies inform this question. HIV enters the brain through infected monocytes, which trigger an inflammatory release of cytokines, neurotoxins, and toxic viral proteins.17 This inflammatory response can cause synaptic neuronal pruning and lead to an overall decrease in neuronal function.18 We hypothesize these changes in neuronal structure could diminish glutamate cycling,19 reducing metabolic demand.20 A tight coupling exists between metabolic demands and cerebral blood flow that could explain observed HIV-related reductions in both resting cerebral blood flow21,22 and rs-fcMRI.
In the pre-HAART era, pathologic changes were primarily observed within subcortical brain structures. Associated cognitive deficits included psychomotor slowing, diminished concentration, and mood changes.23 However, the introduction of HAART has greatly altered the clinical presentation of HAND. Cortical abnormalities have been described3 in recent years with deficits observed in verbal memory, executive control, and attention. Our current results using rs-fcMRI complement existing NP and neuroimaging studies. Neuroimaging methods such as volumetric measurement,24 magnetic resonance spectroscopy,19 arterial spin labeling,21,22 and BOLD functional imaging14,25 have demonstrated that HIV causes cortical changes even in the HAART era.
HIV also causes alterations within resting networks (DMN, CON, and SAL), possibly helping to explain reported cognitive impairments. The DMN is thought to be involved in using past experiences to plan for the future and navigate social interactions26; the SAL network may provide emotional stimuli to neural systems16; and the CON network may assist in selecting a proper response to an array of possibilities.16 Detailed neuropsychological studies in HIV+ individuals have reported deficits in working memory (engaging CON), prospective memory (engaging DMN), and emotional-conflict processing (engaging SAL).27–29 It may be that loss of functional connectivity within the DMN, SAL, and CON undermines the ability of an individual who is HIV+ to successfully process and integrate various external and internal stimuli.28
Additionally, although the regional specificity of HIV impact at the molecular level in the neocortex remains to be fully elucidated, recent molecular evidence does suggest that HIV may show some preference for DMN, CON, and SAL. HIV affects the dorsolateral prefrontal regions important for CON processing,30 temporal areas important for SAL processing,31 and middle frontal and posterior cingulate areas important for DMN processing.32,33 It may be that HIV reduces intranetwork integrity by affecting important hub regions.
HIV may also affect correlations between networks. Internetwork relationships have been hypothesized to reflect the brain's ability to integrate information between opposing networks while coordinating network-level competition for limited brain resources.34 Regions from the DMN are thought to form a cross-network integrating and coordinating center.35 Our observed loss of intranetwork correlation in the DMN suggests that this integrating role might be deteriorated in HIV, possibly causing wider dysfunction in the internetwork coordination of other highly cross-connected networks such as DAN, SAL, and CON.
The multinetwork decrease in intra- and internetwork rs-fcMRI observed in HIV+ patients suggests that the virus may cause large-scale disruption of cortical communication similar to that seen in other neurodegenerative disorders.36 rs-fcMRI studies of patients with Alzheimer disease have demonstrated a whole-brain loss of inter- and intranetwork correlations (although, notably, increased correlations have been observed in the SAL network in Alzheimer disease).37 We only observed reductions in intranetwork functional connectivity in our cohort of HIV+ participants, although we did see a trending increase in positive internetwork relationship in CON-SAL.
Additive effects of HIV and aging were observed in rs-fcMRI measures of the DMN and SAL. These results complement recent neuroimaging14,19,22 and neuropsychological3 studies demonstrating that independent decreases in brain function occur with HIV and aging. The networks in which we observed independent effects of HIV and aging (DMN and SAL) are among the networks thought to be most affected by aging and neurodegenerative disorders.37 We extend existing research on rs-fcMRI in aging by showing that the internetwork relationship between DMN and SAL also disintegrates with both age and HIV. Thus, available evidence suggests that HIV may lead to an advanced-age, brain-function phenotype. Our results extend previous studies that have suggested that HIV can cause premature aging in multiple organ systems,38 including the brain.39 However, the effects of HIV and aging may be differentiable because aging has been shown to lead to increases in functional connectivity in the SMN, where we observed no significant changes in the SMN composite in individuals who were HIV+.40
Our analysis has limitations. The generalizability of our results is somewhat limited because of sex differences between the HIV− and HIV+ groups. Although corrections were performed to control for possible differences because of sex, a larger cohort of HIV+ females is required.
We performed only a limited NP battery that provides a gross assessment of overall performance. A more complete battery may show associations between rs-fcMRI and particular tests. Although substance use was not observed to affect rs-fcMRI measures, a more carefully controlled study of possible substance effects is needed. The observation that plasma VL and HAART status did not modulate functional connectivity is limited by 3 factors. First, we did not collect VL in the CSF. Plasma VL may not adequately reflect changes that occur in the brain. Second, this study did not examine the effects of CNS penetrating effectiveness of HAART regimens. Finally, this study is cross-sectional. A longitudinal study assessing CSF VL and CNS penetrating effectiveness may better assess the effects of HAART intervention.
We primarily observed a loss in resting-state correlations within and between particular networks due to HIV. HIV and aging effects were independent of each other, suggesting that HIV prematurely ages the brain and possibly increases susceptibility to neurodegenerative disorders. rs-fcMRI may be able to both assess early changes in brain function caused by HIV and influence treatment strategies targeted to the brain.
Supplementary Material
GLOSSARY
- ANCOVA
analysis of covariance
- BOLD
blood oxygen level dependent
- CON
control network
- DAN
dorsal attention network
- DMN
default mode network
- FSE
fast spin echo
- HAART
highly active antiretroviral therapy
- HAND
HIV-associated neurocognitive disorder
- MPRAGE
magnetization-prepared rapid gradient echo
- NP
neuropsychological performance
- NPZ-4
composite neuropsychological z score
- QA
quality assurance
- rLP
right lateral parietal
- ROI
region of interest
- rs-fcMRI
resting-state functional connectivity MRI
- SAL
salience
- SMN
sensorimotor network
- TE
echo time
- TR
repetition time
- VL
viral load
Footnotes
Supplemental data at www.neurology.org
Editorial, page 1178
AUTHOR CONTRIBUTIONS
Mr. Thomas and Mr. Brier: analysis and interpretation. Dr. Snyder: critical revision of manuscript for intellectual content. Dr. Vaida: analysis and interpretation. Dr. Ances: study concept, design, and supervision.
STUDY FUNDING
This work was supported by the NIH (grants NINR R01NR012907 to B.M.A., NINR R01NR012657 to B.M.A., NIMH K23MH081786 to B.M.A., National Institute of Neurological Disorders and Stroke P30 NS048056 to A.Z.S.).
DISCLOSURE
Mr. Thomas, Mr. Brier, Dr. Snyder, Dr. Vaida, and Dr. Ances report no disclosures. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011;17:3–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75:2087–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cysique LA, Brew BJ. Prevalence of non-confounded HIV-associated neurocognitive impairment in the context of plasma HIV RNA suppression. J Neurovirol 2011;17:176–183 [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 2009;106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang D, Raichle ME. Disease and the brain's dark energy. Nat Rev Neurol 2010;6:15–28 [DOI] [PubMed] [Google Scholar]
- 6.Vandenbroucke JP, Elm Von E, Altman DG. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007;4:e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gorp WG, Miller EN, Satz P, Visscher B. Neuropsychological performance in HIV-1 immunocompromised patients: a preliminary report. J Clin Exp Neuropsychol 1989;11:763–773 [DOI] [PubMed] [Google Scholar]
- 8.Carey CL, Woods SP, Rippeth JD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment. Clin Neuropsychol 2004;18:234–248 [DOI] [PubMed] [Google Scholar]
- 9.Brier MR, Thomas JB, Snyder AZ. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J Neurosci 2012;32:8890–8899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ojemann JG, Akbudak E, Snyder AZ, et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 1997;6:156–167 [DOI] [PubMed] [Google Scholar]
- 11.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20:2852–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol 2009;101:3270–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shannon BJ, Raichle ME, Snyder AZ, et al. Premotor functional connectivity predicts impulsivity in juvenile offenders. Proc Natl Acad Sci USA 2011;108:11241–11245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol 2004;56:259–272 [DOI] [PubMed] [Google Scholar]
- 15.Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 2002;59:1343–1349 [DOI] [PubMed] [Google Scholar]
- 16.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabriel G, Marcus K. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masliah E, Heaton RK, Marcotte TD, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann Neurol 1997;42:963–972 [DOI] [PubMed] [Google Scholar]
- 19.Harezlak J, Buchthal S, Taylor M, et al. Persistence of HIV-associated cognitive impairment, inflammation, and neuronal injury in era of highly active antiretroviral treatment. AIDS 2011;25:625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rottenberg DA, Sidtis JJ, Strother SC, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med 1996;37:1133–1141 [PubMed] [Google Scholar]
- 21.Ances B, Vaida F, Ellis R, Buxton R. Test–retest stability of calibrated BOLD-fMRI in HIV− and HIV+ subjects. Neuroimage 2011;54:2156–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ances BM, Vaida F, Yeh MJ, et al. HIV infection and aging independently affect brain function as measured by functional magnetic resonance imaging. J Infect Dis 2010;201:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navia BA, Rostasy K. The AIDS dementia complex: clinical and basic neuroscience with implications for novel molecular therapies. Neurotox Res 2005;8:3–24 [DOI] [PubMed] [Google Scholar]
- 24.Thompson PM, Dutton RA, Hayashi KM, et al. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proc Natl Acad Sci USA 2005;102:15647–15652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melrose RJ, Tinaz S, Castelo JMB, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: an fMRI investigation of semantic event sequencing. Behav Brain Res 2008;188:337–347 [DOI] [PubMed] [Google Scholar]
- 26.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL. Evidence for the default network's role in spontaneous cognition. J Neurophysiol 2010;104:322–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carey CL, Woods SP, Rippeth JD, Heaton RK, Grant I; HIV Neurobehavioral Research Center (HNRC) Group Prospective memory in HIV-1 infection. J Clin Exp Neuropsychol 2006;28:536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novara C, Casari S, Compostella S, Dorz S, Sanavio E, Sica C. Coping and cognitive processing style in HIV-positive subjects. Psychother Psychosom 2000;69:316–321 [DOI] [PubMed] [Google Scholar]
- 29.Schulte T, Müller-Oehring E, Sullivan EV, Pfefferbaum A. Disruption of emotion and conflict processing in HIV infection with and without alcoholism comorbidity. J Int Neuropsychol Soc 2011;17:537–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman BB, Lisinicchia JG, Chen T, et al. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. J Neuroimmune Pharmacol 2012;7:686–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adel-Biassette H, Cretien F, Wingertsmann L, Gray F. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol Appl Neurobiol 1999;25:123–133 [DOI] [PubMed] [Google Scholar]
- 32.Winston A, Duncombe C, Li PC, et al. Two patterns of cerebral metabolite abnormalities are detected on proton magnetic resonance spectroscopy in HIV-infected subjects commencing antiretroviral therapy. Neuroradiology 2012;54:1331–1339 [DOI] [PubMed] [Google Scholar]
- 33.Kołtowska A, Hendrich B, Knysz B, et al. Analysis of metabolic changes of brain in HIV-1 seropositive patients with proton magnetic resonance spectroscopy. Pol J Radiol 2010;75:27–32 [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008;39:527–537 [DOI] [PubMed] [Google Scholar]
- 35.de Pasquale F, Penna Della S, Snyder AZ, Marzetti L. A cortical core for dynamic integration of functional networks in the resting human brain. Neuron 2012;24:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He BJ, Shulman GL, Snyder AZ, Corbetta M. The role of impaired neuronal communication in neurological disorders. Curr Opin Neurol 2007;20:655–660 [DOI] [PubMed] [Google Scholar]
- 37.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol 2011;46:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ances BM, Ortega M, Vaida F, Heaps J. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012;59:469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fling BW, Peltier SJ, Bo J, Welsh RC. Age differences in interhemispheric interactions: callosal structure, physiological function, and behavior. Front Neurosci 2011;5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.