Abstract
Stress reactivity is a disposition that underlies individual differences in stress responses, thereby affecting vulnerability for the development of disease. Besides genetic and early postnatal environmental factors, stress reactivity has been shown to be influenced by an adverse prenatal developmental environment, but it is unclear if such effects persist into older age. We tested associations between fetal growth and perceived stress reactivity in 421 participants from the Hertfordshire Cohort at age 66 to 75 years. Regression analysis showed a U-shaped association between birth weight and perceived stress reactivity, with increased levels of stress reactivity at the lower and upper end of the birth weight distribution. These effects were stable after adjustment for markers of early adversity and recent adversity and chronic stress. Although the effects were small, they are consistent with findings from studies in younger cohorts, and demonstrate that such effects can persist into older age.
Keywords: Stress reactivity, Perceived Stress Reactivity Scale (PSRS), Fetal growth, Prenatal adversity, Fetal programming, Developmental Origins of Health and Disease (DOHaD), Birth weight, Hertfordshire Cohort Study, Older age, Elderly
People respond differently when exposed to the same stressor. Such differences can be observed in all four major stress response domains, namely physiology, behavior, subjective experience and cognitive function (Steptoe, 2007). Stress reactivity reflects an individual’s capacity or tendency to respond to a stressor (Schlotz, in press). It is a disposition that underlies individual differences in stress responses and is assumed to be a vulnerability factor for the development of diseases (Cohen & Manuck, 1995; Lovallo & Gerin, 2003). For example, stress reactivity has been linked with an increased risk for cardiovascular disease (Chida & Steptoe, 2010; Hamer & Steptoe, 2012; Treiber et al., 2003), infectious disease (Cohen et al., 2002), and psychosis (Myin-Germeys, van Os, Schwartz, Stone, & Delespaul, 2001). Stress reactivity is thought to be relatively stable, with situational changes varying around a person-specific set-point that is determined by heritable and early environmental factors. A number of studies have provided evidence for effects of genetic and early environmental factors, as well as their interaction, on the stress process in general, and stress reactivity in particular (Manuck & McCaffery, 2010), probably shaped by natural selection (Ellis, Jackson, & Boyce, 2006).
Besides early postnatal environmental factors, the prenatal developmental environment may affect a person’s development later in life. It has been suggested that prenatal factors may have permanent effects on organ development, thereby influencing the risk for later physical and mental health and disease (Barker, 1995; Gillman, 2005; Gluckman, Hanson, Cooper, & Thornburg, 2008; Schlotz & Phillips, 2009). For example, lower birth weight, a marker of reduced fetal growth due to prenatal adversity, has been associated with ischemic heart disease in later life (Huxley et al., 2007). Stress reactivity might play a mechanistic role in such associations (Phillips, 2007). Consistent with this notion, a number of studies found evidence for changes in stress reactivity in association with an adverse prenatal developmental environment. Lower birth weight has been shown to be associated with increased cortisol responses to psychosocial stress in children (Jones et al., 2006) and young adults (Wüst, Entringer, Federenko, Schlotz, & Hellhammer, 2005), and maternal stress during pregnancy was associated with an increased cortisol stress response (Entringer, Kumsta, Hellhammer, Wadhwa, & Wust, 2009). In a large study of 18-year-old Swedish military recruits, a curvilinear association between birth weight and psychological stress susceptibility has been found, with high stress reactivity at lowest birth weights, and the effect leveling off at very high birth weights (Nilsson, Nyberg, & Ostergren, 2001). Similarly, lower birth weight was associated with increased lower limb movement during stress, but not in a non-stress situation (Schlotz, Jones, Phillips, Godfrey, & Phillips, 2007). Indirect evidence comes from cohort studies where distress was assessed. In the National Child Development Study, lower birth weight has been found to be linearly associated with increased psychological distress (Cheung, Khoo, Karlberg, & Machin, 2002), and lower birth weight was similarly associated with higher distress in the Aberdeen Children of the 1950s study (Wiles, Peters, Leon, & Lewis, 2005). In the 1970 British Birth Cohort Study a curvilinear association between birth weight and distress was found, with highest levels of distress at lower birth weights (Cheung, 2002). In contrast to these findings, the only study investigating stress reactivity in an elderly cohort found an inverse U-shaped association between cortisol responses to a standardized psychosocial stressor and birth weight (Kajantie et al., 2007). Investigating psychological traits in elderly participants at approximately 63 years of age, Lahti and colleagues found an inverse J-shaped association between birth weight and harm avoidance (Lahti et al., 2008), and an inverse linear association between birth weight and trait anxiety (J. Lahti et al., 2010), both representing potential vulnerability factors for high stress reactivity.
In sum, most of the available evidence points at inverse associations between birth weight and stress reactivity in children and adults. Some studies found curvilinear association with slight increases in stress reactivity or distress at high levels of birth weight. Therefore, fetal growth seems to have an impact on stress reactivity that can be detected up to middle adulthood. Whereas one study investigated cortisol stress responses in the elderly (Kajantie et al., 2007), it has not been established whether an association between birth weight and perceived stress reactivity persists into older age. We used a longitudinal birth cohort study, the Hertfordshire Cohort Study, to investigate if this association can be detected in 66-to-75-year-old adults. Based on the evidence presented above and the assumption of relative stability of stress reactivity we hypothesized that stress reactivity would be associated with birth weight in a curvilinear shape. As fluctuations in stress reactivity can result from recent stress exposures and to account for the potential influence of continued adversity, we controlled for chronic stress and socioeconomic status (SES) of the participants at the time of the assessment of stress reactivity. In addition, in order to account for potential effects of early postnatal environmental adversity, we controlled for the father’s SES when the participant was born. This adjustment helps separating effects due to prenatal adversity from effects due to continued adversity (Schlotz & Phillips, 2009). We also adjusted the models for the participant’s sex to account for sex differences in birth weight and perceived stress reactivity. The study was based on the following hypotheses:
H1: In this cohort of elderly participants, stress reactivity is associated with birth weight in a curvilinear shape, with increased stress reactivity at relatively low and relatively high birth weights.
H2: This association does not change when potential confounders of the association are controlled, namely sex, recent chronic stress experience, SES of the participant, and SES of the participant’s father at the time of the participant’s birth.
Methods
Participants
The Hertfordshire Cohort Study (HCS) comprises several thousand men and women born in the English county of Hertfordshire between 1931 and 1939, and still resident there in 1998. More than 20000 persons were identified using the National Health Service Central Register (NHSCR), and a cohort of 3000 men and women was recruited to the HCS. The full cohort and the procedures are described in detail elsewhere (Syddall et al., 2005). To prevent excessive burden to the cohort questionnaires were sent to the sub-sample of West Hertfordshire, which consisted of 609 men and women at the time of the study. This sub-sample was chosen as the participants did not participate in other cohort-related studies close in time. The questionnaire was returned by 421 participants (response rate = 69 %). The responders were not substantially different from non-responders with regard to birth weight (mean difference = −0.04 kg; p = .48), sex (46 % versus 48 % female; p = .68), father’s SES (20 % versus 16 % I-IIIn; p = .30), participant’s current SES (44 % versus 39 % I-IIIn; p = .31), but responders were slightly older than non-responders (mean difference = 1.2 years; p < .001). The study was approved by the Hertfordshire Local Research Ethics Committee.
Measurements
Birth weight
Birth weight was recorded by a midwife and a health visitor, who transcribed the information into ledgers at the Hertfordshire county office (cf. Syddall et al., 2005).
Stress reactivity
Stress reactivity was assessed by the Perceived Stress Reactivity Scale (PSRS; Schlotz, Yim, Zoccola, Jansen, & Schulz, 2011). Each PSRS item considers an individual’s exposure to stress and a typical response to the stressor, for example (item 9): When I am unsure what to do or say in a social situation... (0) I generally stay cool; (1) I often feel warm; (2) I often begin to sweat. Another example (item 23): When I have many tasks and duties to fulfill... (0) In general, I stay calm; (1) I usually get impatient; (2) I often get irritable. Items represent different potentially stressful situations in everyday life and three ordered options of perceived physiological, behavioral, cognitive or emotional responses, with different responses between items. Scores are aggregated across similar stressors to compute situation-specific scale scores (reliability estimator reported is Cronbach’s α): Perceived Reactivity to Work Overload (feeling nervous, agitated, irritated in response to high work load; α = .81), Perceived Reactivity to Social Conflicts (feeling affected, annoyed, upset in response to social conflict, criticism, rejection; α = .77), Perceived Reactivity to Failure (feeling annoyed, disappointed, down in response to failure; α = .74) and Perceived Reactivity to Social Evaluation (feeling nervous, losing self-confidence in response to social evaluation; α = .72), and Prolonged Reactivity (difficulty relaxing/unwinding after high workload; α = .67). In line with the assumption of relative consistency across situations, these sub-scales are highly correlated and can be aggregated to yield an overall score of perceived stress reactivity (PSRS total score; α = .91). Reliability and validity of the PSRS have been demonstrated (Schlotz, Hammerfald, Ehlert, & Gaab, 2011; Schlotz, Yim, et al., 2011; Schulz, Jansen, & Schlotz, 2005).
Chronic stress
Chronic stress was assessed by the Short Trier Inventory for Chronic Stress (STICS; Schlotz & Schulz, 2008). The STICS is a short form derived from the well-validated Trier Inventory for Chronic Stress (Schulz & Schlotz, 1999; Schulz, Schlotz, & Becker, 2004). We used 21 items that assess chronic stress resulting from excessive demands such as work overload, social overload (too much of responsibility and care for other people), and excessive demands at work (experience of failure; unsuccessful effort); from dissatisfaction such as work discontent (no meaningful work to do) and lack of social recognition (being rejected, criticized, depreciated, or ignored despite best effort); and from performance pressure in social and work situations (pressure to be successful, perform well, make no mistakes, meet expectations). For each item, the respondent indicates the frequency of a stressful experience in the last three months on a five point rating scale, ranging from 0 (never) to 4 (very often). Cronbach’s α of .84 reflects satisfactory internal consistency and supports aggregation of items to yield a chronic stress total score.
Socioeconomic status (SES)
Participants’ current SES was assessed using the UK Registrar General’s scale of Social Class, based on current (or most recent) occupation of the head of household. The six major classes were collated into 2 categories, professional, managerial/technical, and non-manual skilled occupations (classes I, II and IIIn), and manual skilled, partly skilled, and unskilled occupations (classes IIIm, IV, and V). Using the same classification system, the SES of a participant’s father at the time of the participant’s birth as reported by the participant was assessed. Data on occupation was collected as part of a home interview (Syddall et al., 2005).
Statistical analysis
Associations between birth weight and stress reactivity were tested using ordinary least squares linear regression models, regressing stress reactivity on both linear and quadratic components of birth weight. In a second model, the SES of the participant’s father, and current SES and chronic stress levels of the participant were included in the model to control for potential effects of early and more recent adversity on stress reactivity. That model was also adjusted for sex. In addition, sex differences in the association between birth weight and perceived stress reactivity were explored by including interactions with sex in the regression model. The total score of Perceived Stress Reactivity was the primary outcome variable. Additional analyses focused on PSRS subscales to examine potential domain specificity. Statistical analyses were done using Stata 11.2 (StataCorp, 2009, College Station, TX, USA).
Results
The sample consisted of 228 men and 193 women, who had a mean age of 70 years (range: 66-75) when they filled out the questionnaires. Table 1 shows the characteristics of the participants for the complete sample. Mean birth weight, and mean scores of perceived stress reactivity and chronic stress were in the normal range (comparison values not reported here).
Table 1.
Characteristics of the study sample (N = 421).
| Characteristic | Descriptive statistic |
|---|---|
| Birth weight (kg)a | 3.4 (0.5) |
| Maleb | 228 (54 %) |
| Age at study datea | 70.5 (2.5) |
| Socioeconomic status participant | |
| High (I, II, or IIIn) b | 184 (44 %) |
| Low (IIIm, IV, or V) b | 237 (56 %) |
| Socioeconomic status participant’s father at participant’s birthc | |
| High (I, II, or IIIn) b | 80 (20 %) |
| Low (IIIm, IV, or V) b | 320 (80 %) |
| PSRS-tota | 18.0 (8.3) |
| STICS-tota | 26.0 (9.7) |
Note. PSRS-tot: Perceived Stress Reactivity Scale total score; STICS-tot: Short Trier Inventory for Chronic Stress total score.
Mean (SD)
Frequency (%).
Lower n due to missing values
Table 2 shows correlations between the major variables. As can be seen from the table, there was no linear association between birth weight and the PSRS scales.
Table 2.
Correlations between major variables (tetrachoric correlations between dichotomous variables, otherwise Pearson or point biserial correlations).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Birth weight |
||||||||||
| 2. Sexa | −.13** | |||||||||
| 3. SES father | −.01 | .18* | ||||||||
| 4. SES current | −.05 | .11 | .33*** | |||||||
| 5. STICS-tot | −.08 | .01 | −.11* | .04 | ||||||
| 6. PSRS-tot | −.02 | .27*** | .04 | .12* | .35*** | |||||
| 7. PSRS-RWO |
−.02 | .16*** | .05 | .14** | .26*** | .83*** | ||||
| 8. PSRS-RSC | −.01 | .23*** | .03 | .08 | .27*** | .81*** | .56*** | |||
| 9. PSRS-RSE | −.04 | .30*** | .04 | .14** | .23*** | .79*** | .60*** | .49*** | ||
| 10. PSRS-RFA |
.02 | .10* | −.01 | −.01 | .27*** | .76*** | .53*** | .62*** | .50*** | |
| 11. PSRS-PRR |
−.01 | .27*** | .11* | .11* | .36*** | .76*** | .54*** | .54*** | .51*** | .41*** |
Note. PSRS-tot: Perceived Stress Reactivity Scale total score; STICS-tot: Short Trier Inventory for Chronic Stress total score.
0 = male; 1 = female
p < .05
p < .01
p < .001
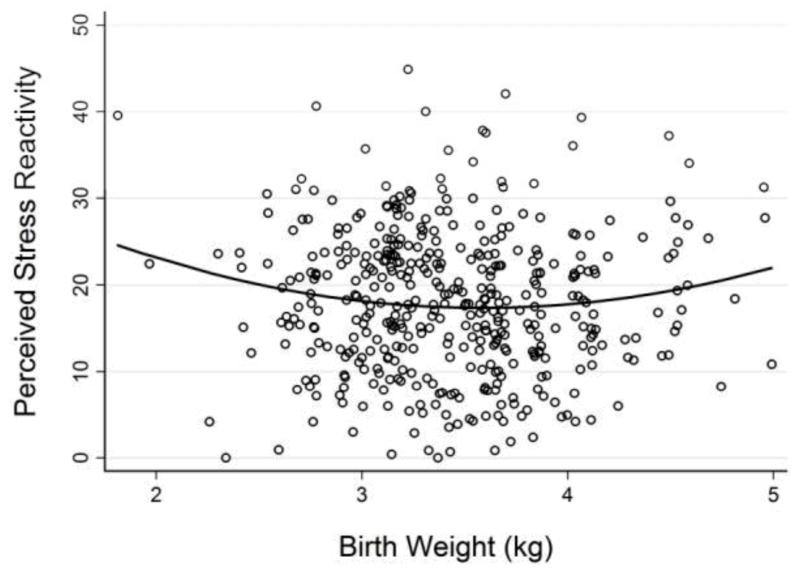
Curvilinear associations were tested by including linear and quadratic predictors in a regression model with overall Perceived Stress Reactivity (PSRS total score) as the outcome. This model showed that overall Perceived Stress Reactivity was predicted by birth weight (see Model 1 in Table 3), with significant effects of both, linear and quadratic component. Perceived Stress Reactivity initially decreased by 16.5 scale points with birth weight increasing by 1 kg (p = .023). However, this inverse association was qualified by a positive quadratic component (p = .025), meaning that the decreasing trend leveled out around average birth weights and started to increase again for higher birth weights. Figure 1 illustrates this U-shaped association. The model explained approximately 1% of the variance in Perceived Stress Reactivity (R2 = .01). These associations did not differ by sex (results not reported here).
Table 3.
Results of regression of perceived stress reactivity on linear and quadratic effects of birth weight.
| Model 1 | Model 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Predictor | b | α | p | R 2 | b | α | p | R 2 |
| PSRS-tot | .01 | .20 | |||||||
| Birth weight (linear) | 16.52 | −1.03 | .023 | −13.82 | −0.87 | .042 | |||
| Birth weight (quadratic) | 2.31 | 1.02 | .025 | 2.02 | 0.90 | .036 | |||
| Sexa | 3.78 | 0.23 | < .001 | ||||||
| SES father | 0.76 | 0.04 | .43 | ||||||
| SES current | 1.43 | 0.09 | .07 | ||||||
| Chronic stress | 0.29 | 0.34 | < .001 | ||||||
| PRSC | .02 | .13 | |||||||
| Birth weight (linear) | −5.45 | −1.25 | .006 | −5.17 | −1.18 | .008 | |||
| Birth weight (quadratic) | 0.76 | 1.23 | .007 | 0.74 | 1.19 | .007 | |||
| Sexa | 0.92 | 0.20 | < .001 | ||||||
| SES father | 0.11 | 0.02 | .69 | ||||||
| SES current | 0.20 | 0.04 | .38 | ||||||
| Chronic stress | 0.06 | 0.25 | < .001 | ||||||
| PRFa | .01 | .10 | |||||||
| Birth weight (linear) | −3.09 | −0.98 | .031 | −2.90 | −0.92 | .042 | |||
| Birth weight (quadratic) | 0.44 | 0.99 | .030 | 0.42 | 0.95 | .035 | |||
| Sexa | 0.28 | 0.08 | .09 | ||||||
| SES father | 0.01 | 0.00 | .95 | ||||||
| SES current | −0.01 | 0.00 | .97 | ||||||
| Chronic stress | 0.05 | 0.28 | < .001 | ||||||
Note. PSRS-tot: Perceived Stress Reactivity total score; PRSC: Perceived Reactivity to Social Conflict; PRFa: Perceived Reactivity to Failure
0 = male; 1 = female
Figure 1.
Perceived stress reactivity in 66-to-75-year old adults in relation to their birth weight. The U-shaped solid line represents the association as estimated by model 1 in Table 3.
In a second step, we adjusted the model for early adversity (SES of the father when the participant was born) and current adversity (SES of participant and chronic stress at the assessment time) to control for potential confounding effects of these factors. This adjustment did not change the association between birth weight and perceived stress reactivity (Model 2 in Table 3). As expected, current SES and chronic stress were positively and independently associated with Perceived Stress Reactivity, whereas early adversity was not associated with total Perceived Stress Reactivity. The model explained approximately 15% of the variance in Perceived Stress Reactivity (R2 = .15). Thus, although early and current SES and current chronic stress improved the prediction of stress reactivity, the effect of fetal growth was independent of these factors, suggesting that prenatal adversity influenced stress reactivity independently of early and current adversity.
To examine if birth weight predicted stress reactivity in specific domains, we conducted similar regression analyses with the PSRS subscales as outcomes. We did not find any significant association between fetal growth and the stress reactivity subscales Reactivity to Work Overload, Reactivity to Social Evaluation, and Prolonged Reactivity. However, linear and quadratic components of birth weight significantly predicted stress reactivity in two scales: Perceived Reactivity to Social Conflicts (PRSC) and Perceived Reactivity to Failure (PRFa). Table 2 shows the results of these analyses. Similar to total stress reactivity, the coefficients show an initial inverse association that levels out at average birth weight and increases with increasing birth weight (see Model 1 in Table 2). Again, adjustment for father’s SES and participant’s recent SES and chronic stress did not change these associations (see Model 2 in Table 2), suggesting that prenatal effects were independent of early and more recent adversity. It is notable that the association of birth weight with PRSC was the strongest among the stress reactivity scales.
Discussion
In summary, our findings demonstrate curvilinear associations between birth weight and perceived stress reactivity in a cohort of 66-to-75-year old community-dwelling individuals. Lowest levels of stress reactivity were reported by those participants born at an average birth weight and increased stress reactivity at lower and higher birth weight. As birth weight is a marker of fetal growth, which reflects an adverse fetal developmental environment, our results suggest that prenatal adversity might have long-term consequences for how people typically respond to stress in daily life. Although the effects are small, they are consistent with findings from other studies in younger cohorts (Jones et al., 2006; Nilsson et al., 2001; Schlotz et al., 2007; Wüst et al., 2005) and add to these findings by demonstrating that such effects potentially persist into older age. Our findings are similar to those of another study in the elderly by Kajantie and colleagues who reported curvilinear associations between birth weight and cortisol responses to a standardized psychosocial stressor (Kajantie et al., 2007). Although little is known about the potential mechanisms underlying such long-term associations, animal studies suggest that brain areas involved in stress regulation, particularly limbic system and prefrontal cortical areas, can be affected by prenatal adversity (Charil, Laplante, Vaillancourt, & King, 2010; Fox, Levitt, & Nelson, 2010; Kapoor, Petropoulos, & Matthews, 2008).
Interestingly, the association between fetal growth and perceived stress reactivity in our cohort of older aged adults was clearly U-shaped, with increased stress reactivity at both ends of the fetal growth distribution. The finding of increased stress reactivity at the lower end of the birth weight spectrum is consistent with a number of studies with similar findings for stress reactivity (Nilsson et al., 2001), anxiety (J. Lahti et al., 2010), harm avoidance (Lahti et al., 2008), and distress (Cheung, 2002). This association might reflect a vulnerability factor for psychopathology, as a number of studies also found increased levels of symptoms of psychopathology such as attention deficit/hyperactivity disorder (ADHD) (Lahti et al., 2006; Schlotz, Jones, Godfrey, & Phillips, 2008), other behavioral disorders in children (Kelly, Nazroo, McMunn, Boreham, & Marmot, 2001; Schlotz, Jones, et al., 2008; Wiles et al., 2006), personality disorders in men (M. Lahti et al., 2010), and schizophrenia (Wahlbeck, Forsen, Osmond, Barker, & Eriksson, 2001) at the lower end of the fetal growth spectrum (see Schlotz & Phillips, 2009 for a review). It has also been suggested that increased stress reactivity might be a common risk factor linking fetal adversity and chronic diseases (Phillips, 2007) and its association with fetal adversity might explain the known associations between cardiovascular disease and psychopathology (Goldstein et al., 2011).
However, we also found increased levels of perceived stress reactivity at the higher end of the birth weight spectrum. This is consistent with similar findings of increased levels of stress reactivity (Nilsson et al., 2001), harm avoidance (Lahti et al., 2008), and distress (Cheung, 2002) at above-average fetal growth. One explanation for this effect would suggest that above-average fetal growth could indicate an adverse fetal developmental environment, akin to that reflected by below-average fetal growth. The major risk factors for macrosomia (fetal overgrowth) are maternal pregnancy hyperglycemia and diabetes mellitus (McCance et al., 1994; Metzger et al., 2008). These risk factors might indicate an adverse fetal environment, including increased levels of pro-inflammatory cytokines (intercellular mediators of immune system functions), oxidative stress (overrepresentation of reactive oxygen species that can result in cell damage), and depletion of fetal iron stores (Van Lieshout & Boyle, 2011; Verner et al., 2007), potentially affecting the development of the brain and the stress response system.
Alternatively, the high levels of stress reactivity at both ends of the U-shaped pattern might represent what has been called biological sensitivity to context. According to this theory, stress response systems are subject to developmental plasticity, with increased levels of stress reactivity resulting from both adverse and protective environments (Boyce & Ellis, 2005). This sensitivity to context is assumed to have been favored by natural selection in both, minimally and maximally stressful environments by augmenting vigilance to threats and dangers in a highly stressful environment, and by increasing susceptibility to social resources and ambient support in highly protective environments (Boyce & Ellis, 2005). In support of this theory, increased levels of stress reactivity have been observed in children from supportive, low stressful environments, as well as those from very stressful environments (Ellis, Essex, & Boyce, 2005). Although very speculative, interpreting our results in the framework of this theory would suggest that it applies to the quality of the prenatal environment, with above-average fetal growth reflecting a protective fetal environment.
This U-shape association is consistent with the hypothesized role of stress reactivity as a mechanistic link between fetal growth and chronic disease later in life, as some studies have found an increased risk in both, below and above-average birth weight categories (Huxley et al., 2007; Osmond, Barker, Winter, Fall, & Simmonds, 1993; Schlotz & Phillips, 2009). However, more research clearly is needed to better understand factors and mechanisms underlying this pattern of association between fetal growth and stress reactivity, and its potential consequences for chronic disease.
In addition to the associations of fetal growth with overall stress reactivity, exploratory analyses found that fetal growth was most strongly associated specifically with reactivity to social conflicts and reactivity to failure, whereas associations with reactivity to work overload and to social evaluation were not significant. Although the reasons for these specific associations remain unclear, it could be speculated that they might reflect changes in stress exposure after retirement. As our sample was beyond retirement age at the time of the stress reactivity assessment, it is likely that they were less exposed to work-related stressors such as work overload and social evaluation. Consequently, they would have had fewer opportunities to observe their own responses to such stressors, which might have influenced self-reports of stress reactivity in these domains.
The small effect sizes of variance accounted for in the outcome might be due to a number of reasons. First, the old age of our sample might be a factor. When people get older there are more opportunities for lifetime environmental factors to influence perceived stress reactivity, thereby reducing the variance explained by prenatal factors. Second, birth weight is a rather crude proxy variable of fetal adversity due to putative causal prenatal factors (Schlotz & Phillips, 2009). Therefore, effect sizes are likely to be underestimated. Finally, there are survival biases in the Hertfordshire Cohort Study. It is possible that there was systematic drop-out due to death or non-response in the low birth weight and high stress-reactivity area of the bivariate distribution, with the consequence of attenuated observed effect sizes in older age. However, it should be noted that ‘variance accounted for’ has its limitations as a measure of the importance of effects. A complementary strategy is to demonstrate that an effect is so pervasive that it holds even under inauspicious circumstances (Prentice & Miller, 1992). In that sense our finding of associations between birth weight and perceived stress reactivity in elderly people is meaningful and important because it is consistent with earlier findings in younger cohorts and, most importantly, it holds even with this large interval between predictor and outcome.
The main limitations of our study are due to self-reports being used to assess stress-reactivity, birth weight being used as a marker of prenatal adversity, and a lack of information on genetic factors and early postnatal environmental factors in this cohort study.
In contrast to multi-method assessments of stress reactivity, self-reports of typical stress responses provide an assessment of perceived stress reactivity. Although correlations between acute subjective-emotional stress responses and endocrine and/or cardiovascular responses are moderate (Cohen et al., 2000; Oldehinkel et al., 2011) and associations might be detected only if the different dynamics of the responses systems are taken into account (Schlotz, Kumsta, et al., 2008), perceived stress reactivity is assumed to be a valuable and convenient indicator of stress reactivity. In support of this assumption, PSRS scores showed substantial heritability (Federenko et al., 2006) and predicted cortisol responses to a laboratory stressor (Schlotz, Hammerfald, et al., 2011). Future studies would need to replicate our findings with physiological stress indicators. Although birth weight is influenced by genetic factors, the genetic effect is modest and fetal growth clearly reflects prenatal environmental factors (Brooks, Johnson, Steer, Pawson, & Abdalla, 1995; Lunde, Melve, Gjessing, Skjaerven, & Irgens, 2007; Rice & Thapar, 2010), it does not provide information on potential effects of specific pregnancy exposures such as smoking, maternal stress or nutrition (Schlotz & Phillips, 2009). It is hoped that future birth cohort studies include detailed measures of specific prenatal factors, as that would indicate where interventions might have long-term consequences for stress reactivity.
Although our results are consistent with the notion of long-term effects of prenatal environmental adversity on the development of stress response systems, we cannot rule that the effects found are a consequence of genetic factors or postnatal environmental adversity. While we tried to control for early postnatal and more recent environmental adversity, future studies should include more detailed assessments and genetically sensitive designs in order to disentangle factors and examine potential moderation and mediation effects.
In conclusion, individuals born at a relatively low or high birth weight reported increased levels of stress reactivity in older age. This association was stable when controlling for early and more recent environmental adversity, suggesting that it is not an effect of ongoing (postnatal) adversity. The associations might reflect long-term neurodevelopmental alterations. The exact psychobiological mechanisms underlying the association between prenatal adversity and stress reactivity in older age need to be elucidated in future studies.
Acknowledgements
We are very grateful to the participants and the Hertfordshire Cohort Study team. The cohort study was supported by the Medical Research Council and the University of Southampton, UK. This study was supported by a fellowship grant from Deutsche Forschungsgemeinschaft (DFG) to W.S. (SCHL 1708/1-1).
References
- Barker DJ. The fetal origins of adult disease. Proceedings of the Royal Society of London. Series B: Biological Sciences. 1995;1363;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Brooks AA, Johnson MR, Steer PJ, Pawson ME, Abdalla HI. Birth weight: nature or nurture? Early Human Development. 1995;42(1):29–35. doi: 10.1016/0378-3782(95)01637-i. [DOI] [PubMed] [Google Scholar]
- Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Research Reviews. 2010;65(1):56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Cheung YB. Early origins and adult correlates of psychosomatic distress. Social Science and Medicine. 2002;55(6):937–948. doi: 10.1016/s0277-9536(01)00225-8. [DOI] [PubMed] [Google Scholar]
- Cheung YB, Khoo KS, Karlberg J, Machin D. Association between psychological symptoms in adults and growth in early life: longitudinal follow up study. BMJ. 2002;325(7367):749–752. doi: 10.1136/bmj.325.7367.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. The stability of and intercorrelations among cardiovascular, immune, endocrine, and psychological reactivity. Annals of Behavioral Medicine. 2000;22(3):171–179. doi: 10.1007/BF02895111. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, Manuck SB. Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosomatic Medicine. 2002;64(2):302–310. doi: 10.1097/00006842-200203000-00014. [DOI] [PubMed] [Google Scholar]
- Cohen S, Manuck SB. Stress, reactivity, and disease. Psychosomatic Medicine. 1995;57(5):423–426. doi: 10.1097/00006842-199509000-00002. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Development and Psychopathology. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Jackson JJ, Boyce WT. The stress response systems: Universality and adaptive individual differences. Developmental Review. 2006;26:175–212. [Google Scholar]
- Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Hormones and Behavior. 2009;55(2):292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Federenko IS, Schlotz W, Kirschbaum C, Bartels M, Hellhammer DH, Wust S. The heritability of perceived stress. Psychological Medicine. 2006;36(3):375–385. doi: 10.1017/S0033291705006616. [DOI] [PubMed] [Google Scholar]
- Fox SE, Levitt P, Nelson CA., 3rd. How the timing and quality of early experiences influence the development of brain architecture. Child Development. 2010;81(1):28–40. doi: 10.1111/j.1467-8624.2009.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW. Developmental origins of health and disease. New England Journal of Medicine. 2005;353(17):1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Cherkerzian S, Buka SL, Fitzmaurice G, Hornig M, Gillman M, O’Toole S, Sloan RP. Sex-specific impact of maternal-fetal risk factors on depression and cardiovascular risk 40 years later. Journal of Developmental Origins of Health and Disease. 2011;2(6):353–364. doi: 10.1017/S2040174411000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Steptoe A. Cortisol responses to mental stress and incident hypertension in healthy men and women. Journal of Clinical Endocrinology and Metabolism. 2012;97(1):E29–34. doi: 10.1210/jc.2011-2132. [DOI] [PubMed] [Google Scholar]
- Huxley R, Owen CG, Whincup PH, Cook DG, Rich-Edwards J, Smith GD, Collins R. Is birth weight a risk factor for ischemic heart disease in later life? American Journal of Clinical Nutrition. 2007;85(5):1244–1250. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- Jones A, Godfrey KM, Wood P, Osmond C, Goulden P, Phillips DIW. Fetal growth and the adrenocortical response to psychological stress. Journal of Clinical Endocrinology and Metabolism. 2006;91(5):1868–1871. doi: 10.1210/jc.2005-2077. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Feldt K, Raikkonen K, Phillips DI, Osmond C, Heinonen K, Pesonen AK, Andersson S, Barker DJ, Eriksson JG. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. Journal of Clinical Endocrinology and Metabolism. 2007;92(11):4094–4100. doi: 10.1210/jc.2007-1539. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Research Reviews. 2008;57(2):586–595. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Kelly YJ, Nazroo JY, McMunn A, Boreham R, Marmot M. Birthweight and behavioral problems in children: a modifiable effect? International Journal of Epidemiology. 2001;30:88–94. doi: 10.1093/ije/30.1.88. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Heinonen K, Pesonen AK, Kajantie E, Forsen T, Osmond C, Barker DJ, Eriksson JG. Body size at birth and socio-economic status in childhood: Implications for Cloninger’s psychobiological model of temperament at age 60. Psychiatry Research. 2008;160(2):167–174. doi: 10.1016/j.psychres.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Kajantie E, Heinonen K, Pesonen AK, Jarvenpaa AL, Strandberg T. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. Journal of Child Psychology and Psychiatry. 2006;47(11):1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- Lahti J, Raikkonen K, Pesonen AK, Heinonen K, Kajantie E, Forsen T, Osmond C, Barker DJ, Eriksson JG. Prenatal growth, postnatal growth and trait anxiety in late adulthood - the Helsinki Birth Cohort Study. Acta Psychiatrica Scandinavica. 2010;121(3):227–235. doi: 10.1111/j.1600-0447.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- Lahti M, Raikkonen K, Wahlbeck K, Heinonen K, Forsen T, Kajantie E, Pesonen AK, Osmond C, Barker DJ, Eriksson JG. Prenatal origins of hospitalization for personality disorders: the Helsinki birth cohort study. Psychiatry Research. 2010;179(2):226–230. doi: 10.1016/j.psychres.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Gerin W. Psychophysiological reactivity: mechanisms and pathways to cardiovascular disease. Psychosomatic Medicine. 2003;65(1):36–45. doi: 10.1097/01.psy.0000033128.44101.c1. [DOI] [PubMed] [Google Scholar]
- Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. American Journal of Epidemiology. 2007;165(7):734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- Manuck SB, McCaffery JM. Genetics of stress: Gene-stress correlation and interaction. In: Steptoe A, editor. Handbook of Behavioral Medicine: Methods and Applications. Springer; New York: 2010. pp. 455–478. [Google Scholar]
- McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? BMJ. 1994;308(6934):942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, van Os J, Schwartz JE, Stone AA, Delespaul PA. Emotional reactivity to daily life stress in psychosis. Archives of General Psychiatry. 2001;58:1137–1144. doi: 10.1001/archpsyc.58.12.1137. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Nyberg P, Ostergren P-O. Increased susceptibility to stress at a psychological assessment of stress tolerance is associated with impaired fetal growth. International Journal of Epidemiology. 2001;30(1):75–80. doi: 10.1093/ije/30.1.75. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Ormel J, Bosch NM, Bouma EM, Van Roon AM, Rosmalen JG, Riese H. Stressed out? Associations between perceived and physiological stress responses in adolescents: The TRAILS study. Psychophysiology. 2011;48(4):441–452. doi: 10.1111/j.1469-8986.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307(6918):1519–1524. doi: 10.1136/bmj.307.6918.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? Journal of Internal Medicine. 2007;261(5):453–460. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- Prentice DA, Miller DT. When Small Effects Are Impressive. Psychological Bulletin. 1992;112(1):160–164. [Google Scholar]
- Rice F, Thapar A. Estimating the relative contributions of maternal genetic, paternal genetic and intrauterine factors to offspring birth weight and head circumference. Early Human Development. 2010;86(7):425–432. doi: 10.1016/j.earlhumdev.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W. Stress reactivity. In: Gellman MD, Turner RJ, editors. Encyclopedia of Behavioral Medicine. Springer; New York: in press. [Google Scholar]
- Schlotz W, Hammerfald K, Ehlert U, Gaab J. Individual differences in the cortisol response to stress in young healthy men: Testing the roles of perceived stress reactivity and threat appraisal using multiphase latent growth curve modeling. Biological Psychology. 2011;87(2):257–264. doi: 10.1016/j.biopsycho.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Jones A, Godfrey KM, Phillips DI. Effortful control mediates associations of fetal growth with hyperactivity and behavioural problems in 7- to 9-year-old children. Journal of Child Psychology and Psychiatry. 2008;49(11):1228–1236. [PubMed] [Google Scholar]
- Schlotz W, Jones A, Phillips NM, Godfrey KM, Phillips DI. Size at birth and motor activity during stress in children aged 7 to 9 years. Pediatrics. 2007;120(5):e1237–e1244. doi: 10.1542/peds.2006-3277. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: a question of timing. Psychosomatic Medicine. 2008;70(7):787–796. doi: 10.1097/PSY.0b013e3181810658. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Phillips DI. Fetal origins of mental health: Evidence and mechanisms. Brain, Behavior, and Immunity. 2009;23(7):905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Schulz P. The Short Trier Inventory for Chronic Stress (STICS): School of Psychology. University of Southampton; UK: 2008. [Google Scholar]
- Schlotz W, Yim IS, Zoccola PM, Jansen L, Schulz P. The perceived stress reactivity scale: Measurement invariance, stability, and validity in three countries. Psychological Assessment. 2011;23(1):80–94. doi: 10.1037/a0021148. [DOI] [PubMed] [Google Scholar]
- Schulz P, Jansen LJ, Schlotz W. Stressreaktivität: Theoretisches Konzept und Messung [Stress reactivity: Theoretical concept and measurement] Diagnostica. 2005;51(3):124–133. [Google Scholar]
- Schulz P, Schlotz W. Das Trierer Inventar zur Erfassung von chronischem Streß (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. Diagnostica. 1999;45:8–19. [Google Scholar]
- Schulz P, Schlotz W, Becker P. Das Trierer Inventar zum chronischen Stress (TICS) - Manual. Hogrefe; Göttingen: 2004. [Google Scholar]
- Steptoe A. Stress effects, overview. In: Fink G, editor. Encyclopedia of stress. 2nd ed. Vol. 3. Academic Press; Amsterdam: 2007. pp. 599–600. [Google Scholar]
- Syddall HE, Sayer AA, Dennison EM, Martin HJ, Barker DJ, Cooper C. Cohort Profile: The Hertfordshire Cohort Study. International Journal of Epidemiology. 2005;34(6):1234–1242. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosomatic Medicine. 2003;65(1):46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Boyle MH. Is bigger better? Macrosomia and psychopathology later in life. Obesity Reviews. 2011;12(5):e405–411. doi: 10.1111/j.1467-789X.2010.00816.x. [DOI] [PubMed] [Google Scholar]
- Verner AM, Manderson J, Lappin TR, McCance DR, Halliday HL, Sweet DG. Influence of maternal diabetes mellitus on fetal iron status. Archives of Disease in Childhood. Fetal and Neonatal Edition. 2007;92(5):F399–401. doi: 10.1136/adc.2006.097279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Archives of General Psychiatry. 2001;58(1):48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Peters TJ, Heron J, Gunnell D, Emond A, Lewis G. Fetal growth and childhood behavioral problems: results from the ALSPAC cohort. American Journal of Epidemiology. 2006;163(9):829–837. doi: 10.1093/aje/kwj108. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Peters TJ, Leon DA, Lewis G. Birth weight and psychological distress at age 45-51 years: results from the Aberdeen Children of the 1950s cohort study. British Journal of Psychiatry. 2005;187:21–28. doi: 10.1192/bjp.187.1.21. [DOI] [PubMed] [Google Scholar]
- Wüst S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30(6):591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]