Abstract
Positron emission tomography (PET) imaging uses minute amounts of radiolabeled drug tracers and thereby meets the criteria for clinical microdose studies. The advantage of PET, when compared to other analytical methods used in microdose studies, is that the pharmacokinetics (PK) of a drug can be determined in the tissue targeted for drug treatment. PET microdosing already offers interesting applications in clinical oncology and in the development of central nervous system pharmaceuticals and is extending its range of application to many other fields of pharmaceutical medicine. Although requirements for preclinical safety testing for microdose studies have been cut down by regulatory authorities, radiopharmaceuticals increasingly need to be produced under good manufacturing practice (GMP) conditions, which increases the costs of PET microdosing studies. Further challenges in PET microdosing include combining PET with other ultrasensitive analytical methods, such as accelerator mass spectrometry (AMS), to gain plasma PK data of drugs, beyond the short PET examination periods. Finally, conducting clinical PET studies with radiolabeled drugs both at micro- and therapeutic doses is encouraged to answer the question of dose linearity in clinical microdosing.
Keywords: positron emission tomography (PET), pharmacokinetics, microdosing, radioisotope, small-animal PET, good manufacturing practice (GMP), dose linearity
1. Introduction
In 2004, microdosing was introduced as a powerful tool along the critical path of drug development - an innovative concept of studying the pharmacokinetic (PK) profile of a drug in plasma of human volunteers, after administration of a sub-therapeutic drug microdose (≤100 μg) [1, 2]. In 2006, the US-Food and Drug Administration (FDA) prompted pharmaceutical companies to the conduction of microdose studies, for early drug candidate selection, using ultrasensitive analytical methods such as accelerator mass spectrometry (AMS) and positron emission tomography (PET). Microdosing is part of the exploratory IND (investigational new drug) approach, developed to increase the efficiency of the pharmaceutical drug development process [3]. Because a minimum of safety and toxicology testing is required by regulative authorities before microdose studies can be conducted [2, 3], drug candidates with a favorable PK profile can be selected at an early stage of drug development. In Japan, guidelines for microdosing in clinical trials were released in 2008 [4].
A precondition for extrapolating plasma or tissue concentrations of a drug after administration of a microdose to drug concentrations achieved with a therapeutic dose, is that drug concentrations in plasma and tissue increase linearly, with increasing drug doses administered. Microdose studies have shown dose-independent PK for a number of drugs [5-8]. However, the question of dose linearity is still considered the major drawback to the microdosing concept [9].
A survey on the use of microdosing for early clinical drug development, conducted by the Pharmaceutical Research and Manufacturers of America (PhRMA) revealed that 9 companies out of 16 survey responders (56%) were conducting or planning to conduct microdose studies [10]. Whereas determining in vivo PK of a drug candidate was the main reason for companies to perform microdose studies (50% of responses), a majority of 35% of pharmaceutical companies, who were not conducting microdose studies, declared lack of confidence in extrapolation of PK data to therapeutic doses.
It is crucial that PK models for predicting therapeutic drug behavior from in human microdose data are developed and validated [4], so that microdosing can fulfill its promise to shorten the drug development process. A further challenge in human microdosing, is to combine ultrasensitive analytical methods, for accurately determining plasma and tissue PK of investigational drugs in a single approach, in the same collective of volunteers or patients [11].
2. Principles of positron emission tomography
PET is a non-invasive nuclear imaging technique [12, 13]. After intravenous (iv) administration, radiotracers decay in the human body under the emission of positrons. These travel a few millimeters before combining with an electron. This so-called “annihilation event” generates two collinear photons with an energy of 511 keV each. Inside a PET camera, there are several stacked detector rings, containing scintillation crystals. During a PET examination, subjects are placed supine inside the detector diameter of the PET camera. The generated pairs of photons are recorded simultaneously by detector pairs oriented at 180° to each other (“coincidence detection”). These decay events are reconstructed in order to create a three-dimensional picture, in which the distribution of the positron-emitting molecules in the body is represented according to an intensity scale. Dynamic PET measurements enable repeated measurements of radioactivity distribution in short time intervals. After performance of attenuation correction, radioactivity distribution in body tissues can be quantified in terms of absolute radioactivity concentration units, such as kBq/mL. Modern PET cameras enable a spatial resolution in the order of 2-5 mm and a maximum temporal resolution in the range of a few seconds to minutes.
Molecules labeled with short-lived positron-emitting radionuclides, such as carbon-11 (11C, half-life (t1/2): 20.4 min), fluorine-18 (18F, t1/2: 109.7 min) or gallium-68 (68Ga, t1/2: 67.629 min) are used as radiotracers for PET imaging. The disadvantage of using radionuclides with even shorter half-lives such as oxygen-15 (15O, t1/2: 2.07 min) and nitrogen-13 (13N, t1/2: 9.96 min) is that drug tissue concentrations beyond the initial distribution phase of the drug can not be accurately determined. Radiolabeling is performed at high specific activity (i.e. high ratio of activity to mass), resulting in administration of very small amounts of unlabeled drug (usually <10 μg) [14] and in a high sensitivity for measuring drug tissue concentrations (in the lower femtomole (10-15 mol) range).
With regard to radioactive half-life, 18F is the most attractive PET radioisotope, since it allows for imaging durations of up to 10 hours. A considerable drawback, however, stems from the fact that relatively few drug molecules contain fluorine in their native structure. Consequently, despite the rather short half-life of 11C (20.4 min), the majority of PET microdose studies have relied on 11C-labeled drug molecules. A commonly employed method for the synthesis of 11C-labeled drugs is the methylation of amino, phenolic hydroxyl or carboxyl groups with [11C]methyl iodide or [11C]methyl triflate. However, the field of PET radiochemistry is continuously expanding and new and innovative radiolabeling approaches are being developed, which rely on the use of a wide array of [11C]synthons, such as [11C]carbon monoxide, [11C]phosgene or [11C]cyanide, thus giving the possibility of labeling a range of different functional groups and structures with 11C [14]. Particular challenges in the synthesis of PET radiotracers include time constraints due to the short radioactive half-lives of the employed radioisotopes, the need for automatization of procedures to protect the chemist from radiation exposure and the necessity to produce a radiotracer which meets the quality criteria of a drug for intravenous injection into humans.
The radiation exposure of one PET scan is approximately in the same order as the level of natural background irradiation (1-2 mSv/year). A typically administered activity of 11C-tracer of 400 MBq given iv corresponds to a total effective dose of about 2 mSv [15]. This is less than for a computer tomography investigation.
One limitation of PET imaging is that parent drug cannot be distinguished from radiolabeled metabolites in tissue, because both give the same signal. Consequently, for drugs which are extensively metabolized in vivo, the interpretation of drug tissue PK may be confounded by the presence of radiolabeled metabolites. For providing a quantitative description of PET data, such as the rate constants for transfer of radiolabeled drug between plasma and different tissue compartments, the concentration-time profile of the unmetabolized radiolabeled drug in arterial plasma is required (“arterial input function”). Although approaches have been described to obtain this measure directly from the PET data (“image-derived input function”) [16], the gold standard remains arterial blood sampling, which is an invasive procedure, the applicability of which might be restricted in certain subject groups, such as elderly patients. Arterial blood samples are usually counted for total radioactivity using conventional gamma counting and then processed by assays involving chromatographic separation of parent drug from radiolabeled metabolites. Due to the short radioactive half-lives of PET radionuclides the determination of unchanged parent might be inaccurate, particularly at late time points of the scan session, which might have a considerable impact on the accuracy of quantitative parameter estimates derived from PET data. In fact, the arterial input function determination can be considered as the weakest link in a PET experiment, as this part of the measurement is most likely prone to errors.
3. Use and added value of PET in drug development
3.1. Microdose studies
Radiotracers used for PET imaging usually have a high specific activity, so that the mass of unlabeled drug administered to a subject is low enough to satisfy the definition of a microdose [14]. PET microdosing studies are conducted to determine the distribution of a radiolabeled drug microdose to different organs and tissue over time, including the tissue(s) targeted for therapeutic drug treatment [17, 18]. In addition, the pattern of anatomical distribution of radiolabeled drug might already give first evidence of the drug’s interaction with its pharmacological target [11]. This is because drug tissue concentration levels achieved with radiotracers with high specific activity may be in the same range as a drug’s affinity constant (Ki, Kd) for its pharmacological target - in particular for novel therapeutic drugs with high target site binding affinities (in the nanomolar or subnanomolar range) -, which might cause drug tissue distribution to be governed by binding to its pharmacological target. One such example is the characteristic cerebral distribution pattern of a tracer dose of the 11C-labeled antipsychotic drug raclopride, which is dominated by high-affinity binding to dopamine D2 receptors in the basal ganglia, the brain region with the highest concentration of these receptors [19]. It should be noted, however, that accumulation of radiotracer in tissue is not only a result of its interaction with its pharmacological target, but is influenced by different factors such as delivery of radiotracer to tissue, passive diffusion from blood into tissue, active inward or outward transport of drug by transmembrane transporters and non-specific binding of tracer to tissue components (e.g. lipids, proteins).
3.1.1. Small molecules - PET in clinical oncology
In clinical oncology, PET microdosing has been successfully used to determine the PK of radiolabeled cytotoxic agents, such as [18F]fluorouracil or the 11C-labeled topoisomerase I/II inhibitor N-[2-(dimethylamino)ethyl]acridine-4-carboxamide (DACA), in individual cancer patients, before initiating full-dose treatment [20-26]. PET using radiolabeled substrates of the multidrug efflux transporter P-glycoprotein (P-gp, ABCB1) can be predictive of tumor response to treatment, because over-expression of this transporter protein in tumor cells can lead to multidrug resistance (MDR) [27]. For instance high tumoral uptake of the radiotracer [18F]fluoropaclitaxel in breast cancer cells has been associated with tumor response to therapeutic doses of paclitaxel in mice [28] and humans [29]. Hence, next to providing PK data, PET imaging with tracer amounts of radiolabeled chemotherapeutics can be used to predict drug response, before therapeutic doses of chemotherapeutic drug are administered, based on individual patterns of drug resistance (personalized medicine).
In addition, PET using tracer doses of [18F]fluoro-2-deoxyglucose ([18F]FDG) [30, 31] and [18F]fluorothymidine [32] has been successfully used to assess therapeutic response after full-dose treatment with different cytostatic drugs in lung, breast and gastrointestinal cancer patients. These investigations hold the promise of minimizing patient exposure to ineffective and toxic systemic chemotherapy, because therapeutic response can be assessed earlier than with standard radiographic evaluation.
3.1.2. PET to assess blood-brain barrier penetration of drugs
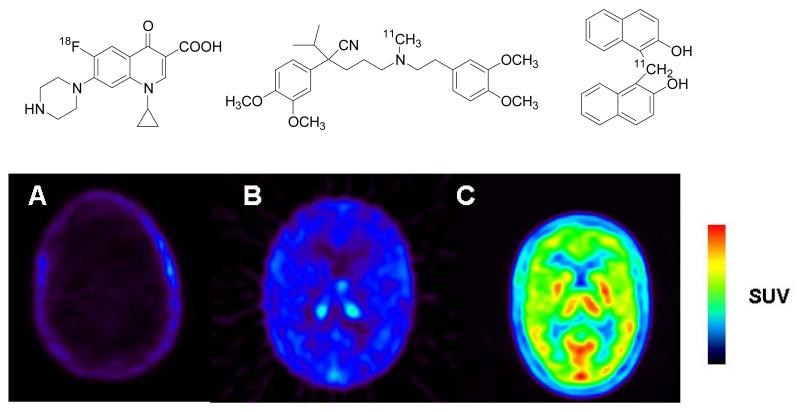
Pharmaceuticals targeted to the central nervous system (CNS) often fail to reach therapeutic concentrations in brain, because the human blood-brain barrier (BBB) selectively filters insufficiently lipophilic drugs or actively removes drugs from the brain, which are substrates of efflux transporters, such as adenosine triphosphate binding cassette (ABC) transporters [33]. PET imaging with radiolabeled tracers of CNS agents can be used to determine BBB penetration in vivo, which preclinical models often fails to predict. In Figure 1, three examples (A-C) are given how PET can be used to study the BBB passage of drug molecules in human subjects. Example A is the 18F-labeled broad spectrum antibiotic [18F]ciprofloxacin which does not penetrate the BBB. This property makes this compound unsuitable for the treatment of cerebral infections but also prevents the occurrence of cerebral side effects which are common for other members of this substance class [34]. Low brain uptake of ciprofloxacin is most likely related to the drug being a substrate of the ABC transporters breast cancer resistance protein (BCRP) and P-gp [35], which are expressed in high concentrations in the capillary endothelium of the BBB and prevent the diffusion of their substrates into brain parenchyma [36]. In example B, the 11C-labeled calcium channel inhibitor [11C]verapamil is shown, which displays low but appreciably higher brain uptake than [18F]ciprofloxacin [37]. The therapeutic target site of verapamil is the human myocardium but in view of the high lipophilicity of the molecule CNS penetration would be expected. Just as in example B, the reason for the low brain uptake of verapamil is related to the fact that this molecule is a high-affinity substrate of P-gp [38]. Example C shows a 11C-labeled investigational drug ([11C]ST1859) which has been developed as a potential antiamyloid drug for the treatment of Alzheimer’s disease [39]. In contrast to the first two examples, the drug displays excellent BBB penetration and sustained brain tissue retention, which constitutes an important prerequisite for the therapeutic applicability of this agent.
Figure 1.
Different degree of blood-brain barrier penetration of three different radiolabeled drugs revealed by PET measurements in humans (A: [18F]ciprofloxacin; B: racemic [11C]verapamil; C: [11C]ST1859). Shown are transaxial PET summation images depicting radioactivity distribution in brain for the duration of the PET scan after iv administration of the radiolabeled drug. The radiation scale is set from 0 to 0.5 (A) and 0 to 2.0 (B, C) standardized uptake value (SUV). PET images were recorded on an Advance PET scanner (General Electrics Medical Systems). The chemical structures of each drug are shown above the PET images.
3.1.3. Macromolecules
To date, the drugs studied with the PET microdosing approach have been small molecules. However, an interesting application of PET microdosing would be to evaluate the PK of macromolecules, such as therapeutic antibodies or peptides. Despite the fact that radiolabeled antibodies (“immuno-PET”) [40] and peptides [41] are increasingly used for diagnostic purposes, no formal microdose studies have been reported so far. Due to the fact that macromolecules generally possess slow in vivo kinetics the use of longer-lived positron-emitters, such as the radiohalogens bromine-76 (76Br, t1/2: 16 h) and iodine-124 (124I, t1/2: 100.2 h) or the radiometal copper-64 (64Cu, t1/2: 12.7 h) is mandatory [42]. Challenges associated with the use of these radionuclides are their restricted availability, high costs, inferior imaging characteristics and higher dosimetry as compared to 11C- or 18F-labeled radiotracers, and often unsatisfactory in vivo stability of macromolecules labeled with such radionuclides [42]. In addition, the introduction of prosthetic groups or chelating moieties is often required to facilitate radiolabeling of macromolecules with long-lived positron-emitters which might affect the bioactivity of these molecules.
3.2. Dose finding studies
PET with validated radiotracers can be applied to select an appropriate clinical starting dose of an investigational drug. Upon co-administration, unlabeled drug and PET tracer compete for binding to the drug’s pharmacodynamic (PD) target. Reduced radioactivity in the region of interest, when increasing doses of unlabeled drug are administered, is an indirect measure for target site occupancy of the drug under investigation [43, 44]. So-called receptor occupancy studies are commonly used in the clinical development of neuropharmaceuticals. In one such example PET together with the radiotracer [18F]SPA-RQ was used to establish a correlation between dose, receptor occupancy in different brain regions and clinical effect for the neurokinin 1 receptor antagonist aprepitant [45]. Because therapeutic drug doses are administered in dose finding studies, they do not qualify as microdose studies and full pre-study toxicological drug evaluation is required.
4. Interplay between small-animal PET and clinical PET in translational research
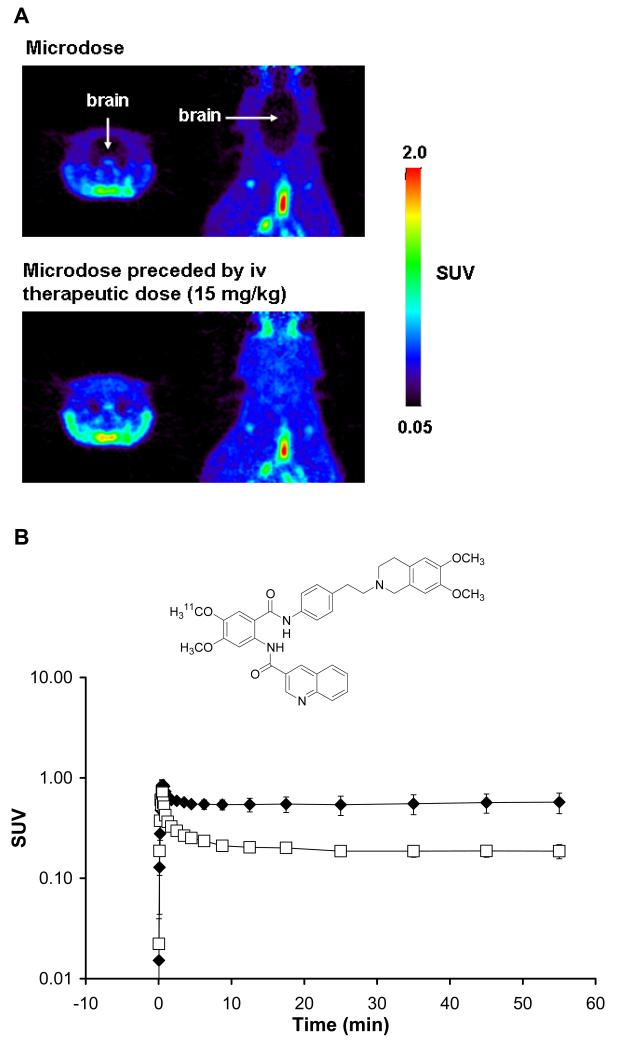
Small-animal PET [46] is increasingly used to study drug PK and PD, before conducting in human PET microdosing studies. The interaction between a radiolabeled drug with its PD target can best be studied by determining radiotracer distribution at baseline, as well as after pretreatment with one or more pharmacological agents that specifically bind at the same PD target. Unlike in human PET studies, tissue biopsy samples can be obtained in preclinical studies to determine if radiolabeled drug metabolites contribute to the PET signal in tissue. Because radiolabeled drug can be administered in vivo together with increasing doses of unlabeled drug, preclinical PET enables assessment of dose linearity of the PK of investigational drugs [47, 48]. Figure 2 shows the brain distribution of the 11C-labeled dual P-gp/BCRP inhibitor tariquidar [49] in Sprague Dawley rats for a microdose (approximately 0.002 mg/kg) as well as for a microdose preceded by an iv therapeutic dose (15 mg/kg), injected 2 h before administration of the microdose, measured with small-animal PET [50]. For the microdose, brain distribution of [11C]tariquidar is governed by high-affinity binding to the drug efflux transporters P-gp and BCRP, which act as gatekeepers at the BBB and thereby prevent brain uptake of the radiolabeled drug. After administration of a therapeutic dose of tariquidar, the high-affinity P-gp/BCRP binding sites are saturated resulting in uptake of [11C]tariquidar into brain tissue. This is a good example of how the biodistribution of a drug can substantially differ between a micro- and macrodose leading to lack of dose linearity. After validating preclinical assumptions with small-animal PET, they can be transferred to human microdose studies in a translational approach. Species differences in drug PK and PD which might be observed may in turn permit to develop improved preclinical models for prediction of human data.
Figure 2.
(A) Coronal and horizontal PET summation images depicting the distribution of the radiolabeled dual P-gp/BCRP inhibitor [11C]tariquidar in rat brain after administration of a microdose (0.002 mg/kg, upper panel) and after administration of a microdose preceded by an iv therapeutic dose (15 mg/kg, given 2 h before radiotracer injection) (lower panel). The radiation scale is set from 0.05 to 2.0 standardized uptake value (SUV). PET images were recorded on a microPET Focus220 scanner (Siemens, Medical Solutions). (B) Time-activity curves (mean SUV±SD) of [11C]tariquidar in whole brain after administration of an iv microdose (open squares) and an iv microdose preceded by an iv therapeutic dose (15 mg/kg) (filled diamonds) in 3 naïve rats. The chemical structure of [11C]tariquidar is shown on top of the graph.
5. Issue of dose linearity in PET microdosing studies
A key question for further exploiting the potential of PET for imaging the PK of radiolabeled drugs is the issue of dose linearity. To date, examples where human PET studies were conducted both at micro- and macrodoses of therapeutic drugs are rare. The putative antidepressant BMS-181101 has been labeled with 11C and administered to healthy volunteers at a microdose as well as after administration of a therapeutic dose to determine drug distribution to brain tissue [51]. Further clinical development of BMS-181101 was abandoned because PET imaging, using both high- and low-specific-activity radiotracer, failed to reveal saturable target site specific binding of this investigational drug.
In another example, distribution of the topoisomerase I/II inhibitor [11C]DACA to tumor and normal tissue was assessed in cancer patients who had never received pharmacological doses of DACA (phase 0) and in patients undergoing a phase 1 trial with the drug [21]. Interestingly, the investigators found an increase in tumor PK parameters (i.e. Cmax, AUC) when [11C]DACA was administered during infusion of unlabeled drug (phase 1 group) as compared to the phase 0 group. A possible explanation for this finding may have been higher free plasma concentrations of the drug in the phase 1 group than in the phase 0 group because of prior exposure to unlabeled DACA in the phase 1 group.
In another study, plasma PK parameters of the radiolabeled cytotoxic agent [18F]fluorouracil were estimated in a cohort of patients who received a therapeutic dose of this agent (380-407 mg/m2) and in a second cohort who only received sub-pharmacological doses (1 mg/m2) [23]. In this study considerable differences were observed in fluorouracil PK parameters between micro- and macrodoses, which might be explained by faster metabolism and higher systemic clearance of the drug microdose.
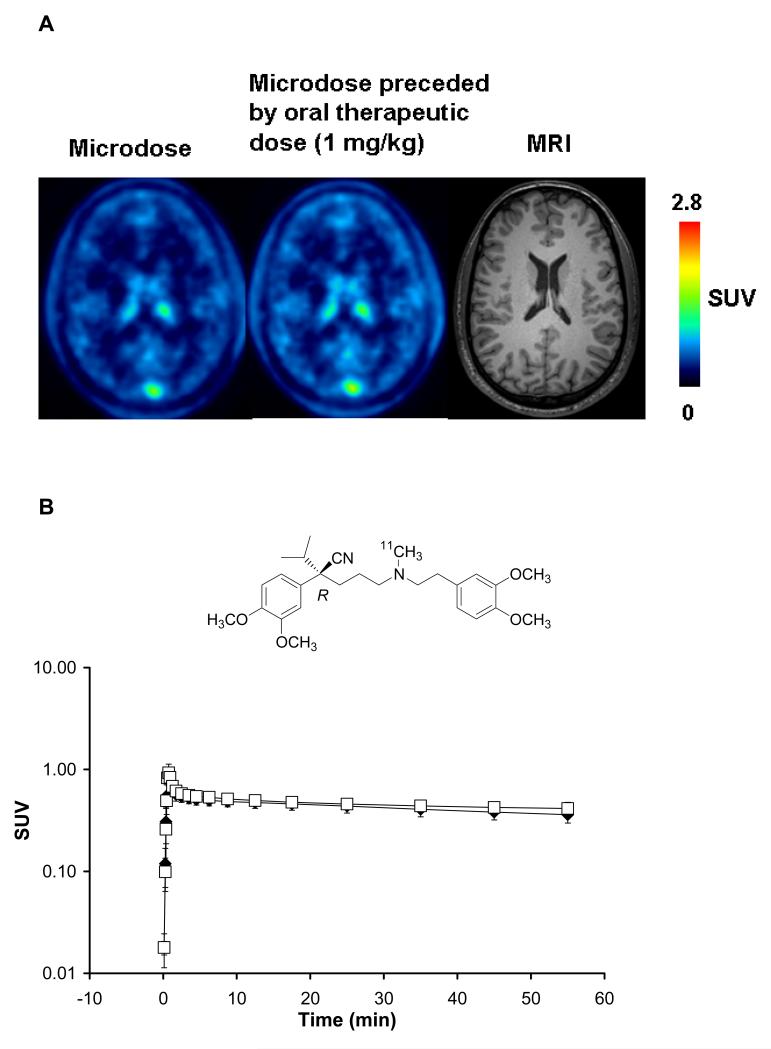
A combined AMS and PET study recently assessed the plasma and brain PK of verapamil at a micro- and therapeutic dose in a randomized, cross-over design [52]. Patients received an iv microdose of verapamil (0.05 mg, dual-labeled (R/S)-[14C], (R)-[11C]verapamil for AMS and PET analysis, respectively) and an iv microdose, preceded by an oral therapeutic dose of unlabeled racemic verapamil (80 mg). For AMS analysis [14C]verapamil was administered as a racemic mixture, whereas for PET imaging the 11C-labeled (R)-enantiomer of verapamil was used. Figure 3 displays mean time-activity curves in brain, measured with PET, at micro- and therapeutic doses of verapamil, which were almost superimposable. No statistically significant differences between the two doses were found for (R)-[11C]verapamil for parameters describing exchange of activity between plasma and brain. In the same way, plasma PK parameters of verapamil as measured by HPLC-AMS analysis were shown to be essentially linear within the employed dose range.
Figure 3.
(A) Transaxial PET summation images depicting radioactivity distribution in brain in one subject after administration of an iv microdose of (R)-[11C]verapamil (left image) and an iv microdose preceded by an oral therapeutic dose (1 mg/kg) of verapamil given 1 h before radiotracer injection (middle image). The right image shows the subject’s brain magnet resonance image (MRI). The radiation scale is set from 0 to 2.8 standardized uptake value (SUV). PET images were recorded on an Advance PET scanner (General Electrics Medical Systems). (B) Time-activity curves (mean SUV±SD) of (R)-[11C]verapamil in whole brain grey matter after administration of an iv microdose (open squares) and an iv microdose preceded by an oral therapeutic dose (1 mg/kg) of racemic verapamil given 1 h before radiotracer injection (filled diamonds) in 6 healthy volunteers. The chemical structure of (R)-[11C]verapamil is shown on top of the graph.
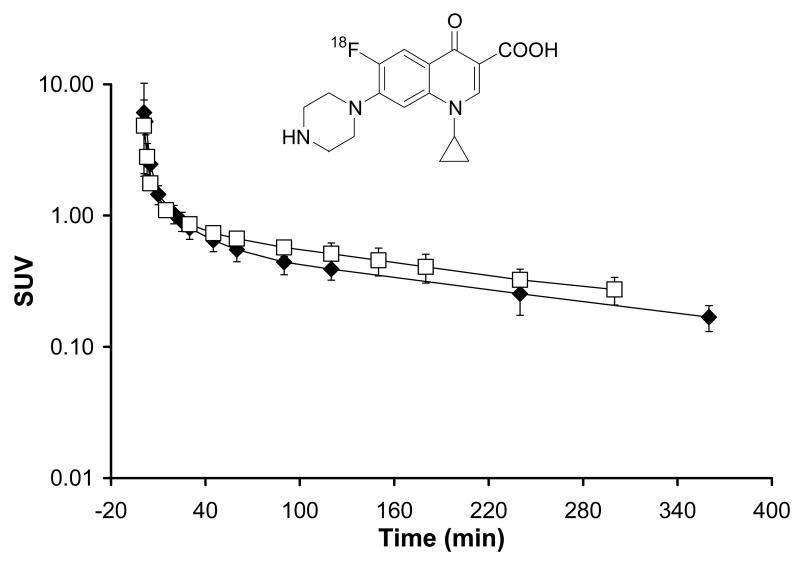
In another example, the antibiotic agent [18F]ciprofloxacin was administered to human subjects both at tracer and therapeutic doses. Figure 4 displays mean plasma concentration-time curves of ciprofloxacin after iv administration of a radiolabeled microdose (approximately 1 mg) of [18F]ciprofloxacin to 4 patients with lower extremity soft tissue infection [53]. Mean plasma concentrations of ciprofloxacin after administration of a radiolabeled iv microdose of [18F]ciprofloxacin preceded by an oral therapeutic dose (250 mg), given 3 h before the iv microdose, in 12 healthy volunteers [34], is displayed in the same graph. In both studies, venous plasma concentrations of [18F]ciprofloxacin were determined by gamma counting. [18F]ciprofloxacin is metabolically stable, so that activity counts in plasma over the time course of the PET experiment only represent unchanged parent, obviating the need for metabolite correction of total activity counts by radio-HPLC analysis [34]. Tissue distribution of radiolabeled ciprofloxacin was simultaneously measured with PET imaging. Figure 4 demonstrates near-to linear plasma PK of ciprofloxacin at micro-and therapeutic dose, with estimated plasma elimination half-lives of 210 min and 225 min, respectively.
Figure 4.
Plasma concentration-time curves (mean SUV±SD) of a radiolabeled iv microdose (1 mg) of [18F]ciprofloxacin given as a bolus over 20 sec to 4 patients with lower extremity soft tissue infection (open squares) and of an iv microdose of [18F]ciprofloxacin preceded by an oral therapeutic dose of ciprofloxacin (250 mg), given 3 h before the iv dose to 12 healthy subjects (filled diamonds). The chemical structure of [18F]ciprofloxacin is shown on top of the graph.
6. Regulatory aspects for using PET in drug development (Europe, USA)
In Europe, the legislative basis for the use of radiopharmaceuticals in clinical studies is essentially determined by two directives of the European Parliament: directive 2001/20/EC on good clinical practice in clinical trials with medicinal products (including radiopharmaceuticals) and directive 2001/83/EC on medicinal products (including radiopharmaceuticals) for human use [54].
Only few radiopharmaceuticals (such as [18F]FDG) have achieved market authorization, whereas most radiotracers used in clinical research are manufactured on-site. The fact that directive 2001/20/EC strictly requires good manufacturing practice (GMP) to the manufacturing of drugs used in clinical studies poses a challenge for using PET in research and development (R&D), especially from academic and scientific associations. Until recently, directive 2001/83/EC specified an exemption of R&D products from full GMP application, which was revised due to the increasing use of PET radiotracers in clinical trials.
Within Europe, national competent authorities, which approve clinical trials, may operate differently, regarding use of radiopharmaceuticals in clinical research. In some countries, such as the UK, full GMP is required for the production of PET tracers for clinical research, whereas in other countries pharmaceutical regulations with respect to the manufacture of PET tracers are not enforced. In the same way, no formal radiation dose limits are defined across the European Union for using radiotracers in clinical research, so that risk-benefit is assessed on a case-by-case basis by national competent authorities. With respect to toxicology assessments, the European Medicines Agency (EMA) position paper on microdose studies states the required preclinical data for the first use of a microdose of a new chemical entity in humans [2].
In the US, rules applying to studies on the development of new medicinal products are less complicated and more uniform across different states [55]. The US-FDA requires Investigational New Drug (IND) submission for radiopharmaceuticals, if the compound, labeled or unlabeled, has never been administered to humans. Whether an exploratory IND or a full IND needs to be submitted, depends on the mass of drug administered. If the administered dose complies with the definition of a microdose, i.e. “less than 1/100th of the dose of a test substance calculated (based on animal data) to yield a pharmacological effect of the test substance with a maximum dose of ≤100 μg or, in the case of biologics, ≤30 nanomoles”, an exploratory IND submission is supported, which - similarly to the EMA position paper - requires reduced preclinical safety and toxicology testing [3]. For radiolabeled compounds, which have been used in humans before, a “Radioactive Drug Research Committee” (RDRC) approval is considered sufficient. The RDRC assesses the pharmacological and radiation dose administered in a clinical study. This usually requires whole-body dosimetry estimations in e.g. non-human primates, prior to initiating a phase 0 PET microdosing study. The FDA has proposed separate GMP guidelines for PET radiopharmaceuticals (PET Drugs - Current Good Manufacturing Practice (CGMP)) [56]. The fundamentals of these guidelines are essentially the same as for conventional drug GMP, except that specific requirements that are not appropriate to PET tracers have been removed and elements specific for PET have been added. In Europe, the European Association of Nuclear Medicine has recently issued a similar GMP guideline for radiopharmaceuticals [57].
7. Comparison of PET with other methods in microdose studies
Human microdose studies are conducted using ultrasensitive analytical methods such as AMS and PET. In a survey on the pharma industry’s view on the utility of microdose studies in clinical drug development, 17% of companies who reported not to be conducting microdosing, specified the requirement of highly sensitive and costly analysis [10]. Conventional liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a cheaper and a more easily available alternative to AMS, that can be used for analyzing drug concentration in biological samples from microdose studies [48]. The assay sensitivity of LC-MS, however, is much lower than that of AMS and PET, which both enable quantification of pico- to femtomolar concentrations of radiolabeled drug in plasma or tissue. The main asset of PET microdosing, when compared to AMS or LC-MS, is that drug PK outside the plasma compartment can be assessed non-invasively in vivo, although AMS has previously been used to quantify drug concentration in peripheral body compartments, by means of tissue biopsy [11]. However, repeated tissue biopsy sampling to gain an impression of drug tissue distribution over time is usually not possible in clinical studies for ethical reasons. A detailed comparison of AMS, PET and LC-MS for use in microdosing studies can be found in previous review articles [11, 58, 59].
8. Combination of different analytical modalities in microdose studies (AMS/PET)
Both AMS and PET provide initial in human PK data and can support decision making in early clinical trials. AMS microdosing allows for obtaining PK over prolonged periods of time after administration of a 14C-labeled drug [60]. A limitation of AMS microdosing is that it mainly provides information on drug distribution to the central blood compartment. This is of limited value as most drugs exert their pharmacological effect in tissue and drug PK in blood is often a poor predictor of drug tissue PK [61]. PET with 11C- or 18F-labeled drugs can be used to study drug tissue distribution and PK. During a PET examination, the plasma PK of an iv administered radiotracer can be also determined by measuring radioactivity counts in blood samples by gamma counting. However, in particular for drugs with slow kinetics it is difficult to derive standard plasma PK parameters in PET microdosing studies, due to the short radioactive half-lives of positron-emitting radionuclides resulting in short sampling periods [23]. Therefore, a combination of AMS and PET imaging in one single study appears to be particularly powerful and informative.
In one recent study, an excellent correlation between AMS analysis and biodistribution measurements, using gamma counter, has been demonstrated for [14C]FDG and [18F]FDG, respectively, in various mouse organs, with particularly high correlation between AMS and gamma counter data in brain and plasma [62]. In this in vivo study, a combined iv dose of [14C]FDG and [18F]FDG was administered to mice, and blood and tissue sampling for AMS analysis and analysis by gamma counting was performed at 30 min, 1 h, 2 h and 4 h after administration in different animals.
The feasibility to combine PET and AMS analysis in humans, has recently been demonstrated when dual 14C- and 11C-labeled verapamil was administered to 6 healthy volunteers, and the PK of verapamil was simultaneously determined using AMS and PET analysis [52]. Next to determining the plasma PK of (R)-[11C]verapamil with gamma counting and HPLC for metabolite correction, brain PK of (R)-[11C]verapamil and its radiolabeled metabolites was measured with PET imaging during 60 min after iv drug administration. In addition, plasma samples were subjected to chiral HPLC followed by AMS analysis to measure plasma concentrations of (R)- and (S)-verapamil separately over 24 h. The set-up used in this clinical pilot study is associated with less risk exposure of the subjects and less costs for the investigator, than when plasma and tissue distribution data of a drug are obtained in two separate clinical studies. Combined AMS and PET studies are particularly useful for drugs, such as a verapamil [63], with a medium to long biological half-life (6-8 h), for which PK analysis by gamma counting is limited by the short radioactive half-lives of PET radionuclides [23], and which distribute outside the blood compartment.
9. Conclusions
PET microdosing has the potential of answering the demand for increased efficiency in selection and development of new drugs. The range of PET imaging in clinical drug development goes from pharmacokinetic evaluation (phase 0) to proof-of concept studies. At present, the range of application of PET microdosing is extending to innovative new medicines. Recent PET microdosing studies have combined PET with other ultrasensitive analytical methods, such as AMS. Small-animal PET provides a translational approach to PET microdosing.
Acknowledgements
O. Langer’s own studies are funded by the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement number 201380 (“Euripides”) and by the Austrian Science Fund (FWF) project “Transmembrane Transporters in Health and Disease” (SFB F35). Martin Bauer (Medical University of Vienna) and Claudia Kuntner (AIT Austrian Institute of Technology GmbH) are gratefully acknowledged for providing PET images for this article.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “New Strategy for Drug Development with Exploratory IND Studies: Scientific Basis and Future Directions”.
References
- [1].Food and Drug Administration, Innovation Stagnation . Challenge and opportunity on the critical path to new medical products. 2004. [Google Scholar]
- [2].European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP) Position paper on non-clinical safety studies to support clinical trials with a single microdose. 2004. CPMP/SWP/2599/02. [Google Scholar]
- [3].Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for Industry, Investigators, and Reviewers. Exploratory IND Studies. 2006 [Google Scholar]
- [4].Sugiyama Y. Effective use of microdosing and Positron Emission Tomography (PET) studies on new drug discovery and development [editorial], Drug Metab. Pharmacokinet. 2009;24:127–9. doi: 10.2133/dmpk.24.127. [DOI] [PubMed] [Google Scholar]
- [5].Yamane N, Tozuka Z, Sugiyama Y, Tanimoto T, Yamazaki A, Kumagai Y. Microdose clinical trial: quantitative determination of fexofenadine in human plasma using liquid chromatography/electrospray ionization tandem mass spectrometry. J. Chromatogr. B. 2007;858:118–28. doi: 10.1016/j.jchromb.2007.08.011. [DOI] [PubMed] [Google Scholar]
- [6].Vuong T, Kopek N, Ducruet T, Portelance L, Faria S, Bahoric B, Devic S. Conformal therapy improves the therapeutic index of patients with anal canal cancer treated with combined chemotherapy and external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:1394–400. doi: 10.1016/j.ijrobp.2006.11.038. [DOI] [PubMed] [Google Scholar]
- [7].Madan A, O’Brien Z, Wen J, O’Brien C, Farber RH, Beaton G, Crowe P, Oosterhuis B, Garner RC, Lappin G, Bozigian HP. A pharmacokinetic evaluation of five H(1) antagonists after an oral and intravenous microdose to human subjects. Br. J. Clin. Pharmacol. 2009;67:288–98. doi: 10.1111/j.1365-2125.2008.03351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lappin G, Kuhnz W, Jochemsen R, Kneer J, Chaudhary A, Oosterhuis B, Drijfhout WJ, Rowland M, Garner RC. Use of microdosing to predict pharmacokinetics at the therapeutic dose: experience with 5 drugs. Clin. Pharmacol. Ther. 2006;80:203–15. doi: 10.1016/j.clpt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- [9].Bertino JS, Jr., Greenberg HE, Reed MD. American College of Clinical Pharmacology position statement on the use of microdosing in the drug development process. J. Clin. Pharmacol. 2007;47:418–22. doi: 10.1177/0091270006299092. [DOI] [PubMed] [Google Scholar]
- [10].Karara AH, Edeki T, McLeod J, Tonelli AH, Wagner JA. PhRMA Survey on the Conduct of First-in-Human Clinical Trials Under Exploratory Investigational New Drug Applications. J. Clin. Pharmacol. 2010 doi: 10.1177/0091270009344987. doi:10.1177/0091270009344987. [DOI] [PubMed] [Google Scholar]
- [11].Wagner CC, Müller M, Lappin G, Langer O. Positron emission tomography for use in microdosing studies. Curr. Opin. Drug Discov. Devel. 2008;11:104–10. [PubMed] [Google Scholar]
- [12].Langer O, Müller M. Methods to assess tissue-specific distribution and metabolism of drugs. Curr. Drug Metab. 2004;5:463–81. doi: 10.2174/1389200043335379. [DOI] [PubMed] [Google Scholar]
- [13].Fischman AJ, Alpert NM, Babich JW, Rubin RH. The role of positron emission tomography in pharmacokinetic analysis. Drug Metab. Rev. 1997;29:923–56. doi: 10.3109/03602539709002238. [DOI] [PubMed] [Google Scholar]
- [14].Bergström M, Grahnen A, Långström B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur. J. Clin. Pharmacol. 2003;59:357–66. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- [15].Lundqvist H, Antoni G, Långström B. Genotoxic hazard of radiopharmaceuticals in humans: chemical and radiation aspects coupled to microdosing. Eur. J. Clin. Pharmacol. 2007;63:641–5. doi: 10.1007/s00228-007-0304-6. [DOI] [PubMed] [Google Scholar]
- [16].Mourik JE, Lubberink M, Schuitemaker A, Tolboom N, van Berckel BN, Lammertsma AA, Boellaard R. Image-derived input functions for PET brain studies. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:463–71. doi: 10.1007/s00259-008-0986-8. [DOI] [PubMed] [Google Scholar]
- [17].Wolf W. Imaging can be much more than pretty pictures. Pharm. Res. 1995;12:1821–2. doi: 10.1023/a:1016258931856. [DOI] [PubMed] [Google Scholar]
- [18].Fischman AJ, Alpert NM, Rubin RH. Pharmacokinetic imaging: a noninvasive method for determining drug distribution and action. Clin. Pharmacokinet. 2002;41:581–602. doi: 10.2165/00003088-200241080-00003. [DOI] [PubMed] [Google Scholar]
- [19].Farde L. The advantage of using positron emission tomography in drug research. Trends Neurosci. 1996;19:211–4. doi: 10.1016/0166-2236(96)40002-9. [DOI] [PubMed] [Google Scholar]
- [20].Saleem A, Yap J, Osman S, Brady F, Suttle B, Lucas SV, Jones T, Price PM, Aboagye EO. Modulation of fluorouracil tissue pharmacokinetics by eniluracil: in-vivo imaging of drug action. Lancet. 2000;355:2125–31. doi: 10.1016/s0140-6736(00)02380-1. [DOI] [PubMed] [Google Scholar]
- [21].Saleem A, Harte RJ, Matthews JC, Osman S, Brady F, Luthra SK, Brown GD, Bleehen N, Connors T, Jones T, Price PM, Aboagye EO. Pharmacokinetic evaluation of N-[2-(dimethylamino)ethyl]acridine-4-carboxamide in patients by positron emission tomography. J. Clin. Oncol. 2001;19:1421–9. doi: 10.1200/JCO.2001.19.5.1421. [DOI] [PubMed] [Google Scholar]
- [22].Saleem A, Charnley N, Price P. Clinical molecular imaging with positron emission tomography. Eur. J. Cancer. 2006;42:1720–7. doi: 10.1016/j.ejca.2006.02.021. [DOI] [PubMed] [Google Scholar]
- [23].Saleem A, Aboagye EO, Matthews JC, Price PM. Plasma pharmacokinetic evaluation of cytotoxic agents radiolabelled with positron emitting radioisotopes. Cancer Chemother. Pharmacol. 2008;61:865–73. doi: 10.1007/s00280-007-0552-2. [DOI] [PubMed] [Google Scholar]
- [24].Harte RJ, Matthews JC, O’Reilly SM, Tilsley DW, Osman S, Brown G, Luthra SJ, Brady F, Jones T, Price PM. Tumor, normal tissue, and plasma pharmacokinetic studies of fluorouracil biomodulation with N-phosphonacetyl-L-aspartate, folinic acid, and interferon alfa. J. Clin. Oncol. 1999;17:1580–8. doi: 10.1200/JCO.1999.17.5.1580. [DOI] [PubMed] [Google Scholar]
- [25].Dimitrakopoulou A, Strauss LG, Clorius JH, Ostertag H, Schlag P, Heim M, Oberdorfer F, Helus F, Haberkorn U, van Kaick G. Studies with positron emission tomography after systemic administration of fluorine-18-uracil in patients with liver metastases from colorectal carcinoma. J. Nucl. Med. 1993;34:1075–81. [PubMed] [Google Scholar]
- [26].Aboagye EO, Saleem A, Cunningham VJ, Osman S, Price PM. Extraction of 5-fluorouracil by tumor and liver: a noninvasive positron emission tomography study of patients with gastrointestinal cancer. Cancer Res. 2001;61:4937–41. [PubMed] [Google Scholar]
- [27].Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical. Annu. Rev. Pharmacol. Toxicol. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- [28].Hsueh WA, Kesner AL, Gangloff A, Pegram MD, Beryt M, Czernin J, Phelps ME, Silverman DH. Predicting chemotherapy response to paclitaxel with 18F-Fluoropaclitaxel and PET. J. Nucl. Med. 2006;47:1995–9. [PubMed] [Google Scholar]
- [29].Kurdziel KA, Kalen JD, Hirsch JI, Wilson JD, Agarwal R, Barrett D, Bear HD, McCumiskey JF. Imaging multidrug resistance with 4-[18F]fluoropaclitaxel. Nucl. Med. Biol. 2007;34:823–31. doi: 10.1016/j.nucmedbio.2007.04.011. [DOI] [PubMed] [Google Scholar]
- [30].Contractor KB, Aboagye EO. Monitoring predominantly cytostatic treatment response with 18F-FDG PET. J. Nucl. Med. 2009;50(Suppl 1):97S–105S. doi: 10.2967/jnumed.108.057273. [DOI] [PubMed] [Google Scholar]
- [31].Lee DH, Kim SK, Lee HY, Lee SY, Park SH, Kim HY, Kang KW, Han JY, Kim HT, Lee JS. Early prediction of response to first-line therapy using integrated 18F-FDG PET/CT for patients with advanced/metastatic non-small cell lung cancer. J. Thorac. Oncol. 2009;4:816–21. doi: 10.1097/JTO.0b013e3181a99fde. [DOI] [PubMed] [Google Scholar]
- [32].Sohn HJ, Yang YJ, Ryu JS, Oh SJ, Im KC, Moon DH, Lee DH, Suh C, Lee JS, Kim SW. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin. Cancer Res. 2008;14:7423–9. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- [33].Reichel A. The role of blood-brain barrier studies in the pharmaceutical industry. Curr. Drug Metab. 2006;7:183–203. doi: 10.2174/138920006775541525. [DOI] [PubMed] [Google Scholar]
- [34].Brunner M, Langer O, Dobrozemsky G, Müller U, Zeitlinger M, Mitterhauser M, Wadsak W, Dudczak R, Kletter K, Müller M. [18F]Ciprofloxacin. Antimicrob. Agents Chemother. 2004;48:3850–7. doi: 10.1128/AAC.48.10.3850-3857.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Alvarez AI, Perez M, Prieto JG, Molina AJ, Real R, Merino G. Fluoroquinolone efflux mediated by ABC transporters. J. Pharm. Sci. 2008;97:3483–93. doi: 10.1002/jps.21233. [DOI] [PubMed] [Google Scholar]
- [36].Löscher W, Potschka H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005;76:22–76. doi: 10.1016/j.pneurobio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [37].Brunner M, Langer O, Sunder-Plassmann R, Dobrozemsky G, Muller U, Wadsak W, Krcal A, Karch R, Mannhalter C, Dudczak R, Kletter K, Steiner I, Baumgartner C, Muller M. Influence of functional haplotypes in the drug transporter gene ABCB1 on central nervous system drug distribution in humans. Clin. Pharmacol. Ther. 2005;78:182–90. doi: 10.1016/j.clpt.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [38].Pauli-Magnus C, von Richter O, Burk O, Ziegler A, Mettang T, Eichelbaum M, Fromm MF. Characterization of the major metabolites of verapamil as substrates and inhibitors of P-glycoprotein. J. Pharmacol. Exp. Ther. 2000;293:376–82. [PubMed] [Google Scholar]
- [39].Bauer M, Langer O, Dal-Bianco P, Karch R, Brunner M, Abrahim A, Lanzenberger R, Hofmann A, Joukhadar C, Carminati P, Ghirardi O, Piovesan P, Forloni G, Corrado ME, Lods N, Dudczak R, Auff E, Kletter K, Müller M. A positron emission tomography microdosing study with a potential antiamyloid drug in healthy volunteers and patients with Alzheimer’s disease. Clin. Pharmacol. Ther. 2006;80:216–27. doi: 10.1016/j.clpt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [40].Wu AM. Antibodies and antimatter: the resurgence of immuno-PET. J. Nucl. Med. 2009;50:2–5. doi: 10.2967/jnumed.108.056887. [DOI] [PubMed] [Google Scholar]
- [41].Lucignani G. Labeling peptides with PET radiometals: Vulcan’s forge. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:209–15. doi: 10.1007/s00259-007-0656-2. [DOI] [PubMed] [Google Scholar]
- [42].Nayak TK, Brechbiel MW. Radioimmunoimaging with Longer-Lived Positron-Emitting Radionuclides: Potentials and Challenges. Bioconjug. Chem. 2009;20:825–41. doi: 10.1021/bc800299f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Waarde A. Measuring receptor occupancy with PET. Curr. Pharm. Design. 2000;6:1593–610. doi: 10.2174/1381612003398951. [DOI] [PubMed] [Google Scholar]
- [44].Lee CM, Farde L. Using positron emission tomography to facilitate CNS drug development. Trends Pharmacol. Sci. 2006;27:310–6. doi: 10.1016/j.tips.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [45].Bergström M, Hargreaves RJ, Burns HD, Goldberg MR, Sciberras D, Reines SA, Petty KJ, Ogren M, Antoni G, Långström B, Eskola O, Scheinin M, Solin O, Majumdar AK, Constanzer ML, Battisti WP, Bradstreet TE, Gargano C, Hietala J. Human positron emission tomography studies of brain neurokinin 1 receptor occupancy by aprepitant. Biol. Psychiatry. 2004;55:1007–12. doi: 10.1016/j.biopsych.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [46].Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur. J. Nucl. Med. Mol. Imaging. 2002;29:98–114. doi: 10.1007/s00259-001-0683-3. [DOI] [PubMed] [Google Scholar]
- [47].Sandhu P, Vogel JS, Rose MJ, Ubick EA, Brunner JE, Wallace MA, Adelsberger JK, Baker MP, Henderson PT, Pearson PG, Baillie TA. Evaluation of microdosing strategies for studies in preclinical drug development: demonstration of linear pharmacokinetics in dogs of a nucleoside analog over a 50-fold dose range. Drug Metab. Dispos. 2004;32:1254–9. doi: 10.1124/dmd.104.000422. [DOI] [PubMed] [Google Scholar]
- [48].Balani SK, Nagaraja NV, Qian MG, Costa AO, Daniels JS, Yang H, Shimoga PR, Wu JT, Gan LS, Lee FW, Miwa GT. Evaluation of microdosing to assess pharmacokinetic linearity in rats using liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 2006;34:384–8. doi: 10.1124/dmd.105.007195. [DOI] [PubMed] [Google Scholar]
- [49].Fox E, Bates SE. Tariquidar (XR9576): a P-glycoprotein drug efflux pump inhibitor. Expert Rev. Anticancer Ther. 2007;7:447–59. doi: 10.1586/14737140.7.4.447. [DOI] [PubMed] [Google Scholar]
- [50].Bauer F, Kuntner C, Bankstahl JP, Wanek T, Bankstahl M, Stanek J, Mairinger S, Dörner B, Löscher W, Müller M, Erker T, Langer O. Synthesis and in vivo evaluation of [11C]tariquidar, a PET radiotracer based on a third-generation P-gp inhibitor. Bioorg. Med. Chem. 2010;18:5489–97. doi: 10.1016/j.bmc.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Christian BT, Livni E, Babich JW, Alpert NM, Dischino DD, Ruediger E, Salazar DE, Ford NF, Fischman AJ. Evaluation of cerebral pharmacokinetics of the novel antidepressant drug, BMS-181101, by positron emission tomography. J. Pharmacol. Exp. Ther. 1996;279:325–31. [PubMed] [Google Scholar]
- [52].Wagner CC, Simpson M, Zeitlinger M, Bauer M, Karch R, Abrahim A, Feurstein T, Schütz M, Kletter K, Müller M, Lappin G, Langer O. A combined accelerator mass spectrometry-positron emission tomography human microdose study with 14C- and 11C-labelled verapamil. Clin. Pharmacokinet. doi: 10.2165/11537250-000000000-00000. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Langer O, Brunner M, Zeitlinger M, Ziegler S, Müller U, Dobrozemsky G, Lackner E, Joukhadar C, Mitterhauser M, Wadsak W, Minar E, Dudczak R, Kletter K, Müller M. In vitro and in vivo evaluation of [18F]ciprofloxacin for the imaging of bacterial infections with PET. Eur. J. Nucl. Med. Mol. Imaging. 2005;32:143–50. doi: 10.1007/s00259-004-1646-2. [DOI] [PubMed] [Google Scholar]
- [54].Salvadori PA. Radiopharmaceuticals, Drug Development and Pharmaceutical Regulations in Europe. Current Radiopharmaceuticals. 2008;1:7–11. [Google Scholar]
- [55].Brocklin HFV. Radiopharmaceuticals for Drug Development: United States Regulatory Perspective. Current Radiopharmaceuticals. 2008;1:2–6. [Google Scholar]
- [56].Food and Drug Administration, Center for Drug Evaluation and Research (CDER) PET Drugs - Current Good Manufacturing Practice (CGMP) 2009. [Google Scholar]
- [57].European Association of Nuclear Medicine . Guidelines on current good Radiopharmacy Practice (cGRPP) in the Preparation of Radiopharmaceuticals. 2007. [Google Scholar]
- [58].Lappin G, Wagner CC, Langer O, van der Merbel N. New ultra-sensitive detection technologies and techniques for use in microdosing studies. Bioanalysis. 2009;1:357–66. doi: 10.4155/bio.09.40. [DOI] [PubMed] [Google Scholar]
- [59].Bauer M, Wagner CC, Langer O. Microdosing studies in humans: the role of positron emission tomography. Drugs R D. 2008;9:73–81. doi: 10.2165/00126839-200809020-00002. [DOI] [PubMed] [Google Scholar]
- [60].Lappin G, Stevens L. Biomedical accelerator mass spectrometry: recent applications in metabolism and pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2008;4:1021–33. doi: 10.1517/17425255.4.8.1021. [DOI] [PubMed] [Google Scholar]
- [61].Müller M, de la Peña A, Derendorf H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents. II: tissue distribution. Antimicrob. Agents Chemother. 2004;48:1441–53. doi: 10.1128/AAC.48.5.1441-1453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Minamimoto R, Hamabe Y, Cheng C, Shimoda M, Oka T, Inoue T. Correlation analysis of measurement result between accelerator mass spectrometry and gamma counter. Ann. Nucl. Med. 2010;24:45–52. doi: 10.1007/s12149-009-0327-4. [DOI] [PubMed] [Google Scholar]
- [63].McTavish D, Sorkin EM. Verapamil. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension. Drugs. 1989;38:19–76. doi: 10.2165/00003495-198938010-00003. [DOI] [PubMed] [Google Scholar]